SUMMARY
Allograft rejection remains a major limitation to successful solid organ transplantation. Here, we investigated the biosynthesis and bioactions of the pro-resolving mediators lipoxin A4 and resolvin E1 in host responses to organ transplantation. In samples obtained during screening bronchoscopy after human lung transplantation, bronchoalveolar lavage fluid levels of lipoxin A4 were increased in association with the severity of allograft rejection that was graded independently by clinical pathology. Lipoxin A4 significantly inhibited calcineurin activation in human neutrophils, and lipoxin A4 stable analogs prevented acute rejection of vascularized cardiac and renal allografts. Transgenic animals expressing human lipoxin A4 receptors revealed important sites of action in host tissues for lipoxin A4’s protective effects. Resolvin E1 displays counter-regulatory actions for leukocytes, in part, via increased lipoxin A4 biosynthesis, yet RvE1 administered (1 µg, iv) to donor (days −1 and 0) and recipient mice (day −1, 0 and +4) was even more potent than a lipoxin stable analog (1 µg, iv) in prolonging renal allograft survival (median survival time = 74.0 days with RvE1 and 37.5 days with a LXA4 analog). Together, these results highlight the potential for pro-resolving mediators in prolonging survival of solid organ transplants.
INTRODUCTION
Allograft rejection remains a major obstacle to the long-term success of organ transplantation [1]. Transplanted tissues are subject to surgical trauma, ischemia-reperfusion injury and insult produced by the immune response [2]. The interaction of infiltrating leukocytes with resident cells of the transplanted tissues stimulates parenchymal cells to generate cytokines and incite an inflammatory response. Neutrophils (PMNs) and innate immune responses play pivotal roles in early allograft survival and the development of chronic rejection of transplanted lung in the form of bronchiolitis obliterans syndrome (BOS) [3, 1]. In the kidney, tubular epithelial cells are a rich source of cytokines and chemokines that can promote renal inflammation. Together, infiltrating PMNs and epithelial cells determine the extent of interstitial inflammation and local tissue damage (reviewed in [4]).
Lipid mediators are potent regulators of leukocyte responses [5] and leukotriene B4 (LTB4) is linked to the pathophysiology of BOS in chronic allograft rejection [6]. Lipoxins (LXs) are a distinct class of lipoxygenase-derived eicosanoids biosynythesized in human and murine lung [7, 8] and kidneys [9]. Unlike LTB4, LXs promote resolution of acute inflammation and injury [10]. LXs were the first compounds identified in a new genus of mediators that carry both anti-inflammatory and pro-resolving actions that now also include the resolvins and protectins (reviewed in [10]). Because immune-mediated rejection remains a major impediment to the long-term success of transplanted solid organs, we sought to determine whether LXs were present in transplanted tissues and if either LXs or resolvins could decrease local tissue inflammatory responses that can limit the success of allografts.
MATERIALS AND METHODS
Materials
Lipoxin A4 (LXA4, 5S,6R,15S-trihydroxy-7E,9E,11Z,13E-eicosatetraenoic acid) was from Calbiochem (La Jolla, CA) and resolvin E1 (RvE1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid) was from Cayman Chemical (Ann Arbor, MI).
Lipoxin A4 in BALFs
Bronchoalveolar lavage fluids (BALFs) were obtained under a protocol approved by the Brigham and Women’s Hospital Committee for the Protection of Human Subjects in Research from bronchoscopies performed on lung transplant recipients for screening or diagnostic purposes based on clinical criteria (n=21, d=59). LXA4 was quantitated (pg/mg protein) in BALF cell-free supernatants (200g, 10 min, 4°C) by ELISA (Neogen, Lexington, KY) (as qualified in [11]).
Human PMN Activation of Calcineurin and Inflammatory Mediator Release
Peripheral blood (~60 ml) was obtained by venipuncture from healthy volunteers who denied taking any medications for at least 2 weeks and had given written informed consent to a protocol approved by Brigham and Women’s Hospital’s Human Research Committee. PMN were isolated from whole blood (as described [12]) and PMN (10–20×106 in HBSS + 1.6 mM CaCl2) were incubated (15min, 37°C) with LXA4 (100nM), cyclosporine-A (CSA, 200nM, Biomol, Plymouth Meeting, PA) or vehicle (0.1% ethanol) prior to exposure to TNF-α (50ng/ml, BD Pharmingen, San Diego, CA) for 10min at 37°C. Cellular calcineurin (PP2B) phosphatase activity was measured using a colorimetric assay that detected free-phosphate release by Malachite green assay (Biomol). Results were calculated in pmoles of PO4/ µg of protein and expressed as % inhibition of TNF-α-induced calcineurin activity. For release of inflammatory mediators, PMN (5×106/reaction) were resuspended in RPMI-1640 medium (Invitrogen Corporation), 1% heat-inactivated FBS (Sigma Aldrich, St Louis, MO), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Invitrogen, Carlsbad, CA), and exposed (37°C, 5% CO2, 15min) to vehicle (medium alone), LXA4 (100nM) or CSA (200nM) prior to addition of TNF-α (20ng/ml). After 6h, cell-free supernatants were collected and ELISAs performed for IL-1β (Cayman Chemical), IL-8 (Diaclone, Stamford, CT), and LTB4 (Oxford Biomedical Research, Oxford, MI).
Animals
All studies were reviewed and approved by the Harvard Medical Area Animal IRB. Mice were maintained under specific pathogen-free conditions in sterilized micro-isolator cages, and received autoclaved feed and autoclaved, acidified drinking water. Human LXA4 receptor transgenic animals (ALX-tg) were generated in FVB mice as described [8]. Eight- to 12-wk-old male mice, including human ALX-tg and Non-tg littermate FvB and BALB/cByJ (BALB/c) (Charles River Labs, Wilmington, MA) were used as donors and recipients in heterotopic heart transplantation experiments. Eight- to 12-wk-old male B6 and BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were used in renal transplantation experiments. Select murine cohorts received a LXA4 stable analog (ATLa, 15-epi-16-para-fluoro-phenoxy-LXA4-methyl ester) that is both chemically more stable than native LXA4 and resists rapid inactivation [13].
Vascularized heterotopic cardiac transplantation
Murine hearts were transplanted heterotopically as previously described [14]. Hearts were harvested and donor aorta anastamosed to recipient vena cava. All surgical procedures were completed within 60 min. Clinical allograft function was assessed by the presence of a palpable heartbeat. Donor hearts that did not beat immediately after reperfusion or that stopped beating within 24h after transplantation were excluded from analysis (>95% of all grafts functioned at 24h). Upon loss of a palpable heartbeat, allografts were harvested and fixed in 10% neutral buffered formalin for histopathology. After dehydration and paraffin embedding, multiple 5–6 µm-thick sections were immunostained for the leukocyte common antigen CD45 (1:1000, BD Bioscience, San Diego, CA) or chemically stained with Naphthol AS-D chloroacetate esterase (Sigma Chemical Co.). Area and number of positively staining cells was measured using NIH Image software (original magnification, ×200).
Renal Transplantation
Kidneys of BALB/c mice were transplanted into fully allogeneic C57BL/6 recipients as in [15]. Briefly, the donor left kidney was isolated by ligating its branch of vessels and the kidney, ureter, and bladder were harvested en bloc, including the renal artery, with a small aortic cuff, and the renal vein, with a small cava cuff. The graft was perfused in situ with 0.2–0.4 ml cold Ringer’s lactate containing heparin. After removal of the left native kidney of each recipient, anastomosis was made between the vascular cuffs of donor kidney and the recipient’s abdominal aorta and vena cava using end-to-side suture technique. The total ischemic time averaged 30–40 min. Finally, for anastomosis of the ureter, the recipient bladder was pierced with a 21-gauge needle as in [16], the ureter was pulled through, and the periureteral tissue was stitched to the exterior wall of the bladder. The donor ureter was allowed to retract inside the bladder. The right native kidney was removed after anastomosis. Kidney graft function was followed by daily measurement of blood serum creatinine and blood urea nitrogen (Mega Diagnostics, Los Angeles, CA) and by evaluation of overall animal health.
Statistical analysis
Results are expressed as the mean ± s.e.m. of more than 3 independent experiments with n ≥ 3 mice or blood donors in each group. Statistical significance of differences was assessed by a one tailed unpaired Student’s t-test and a one-way ANOVA with Bonferroni’s multiple comparison post test to compare selected pairs. P <0.05 was set as the level of significance. Survival rate data were analyzed using Fishers exact test and Kaplan Meir survival analyses.
Results
Lipoxin A4 in human lung transplant rejection
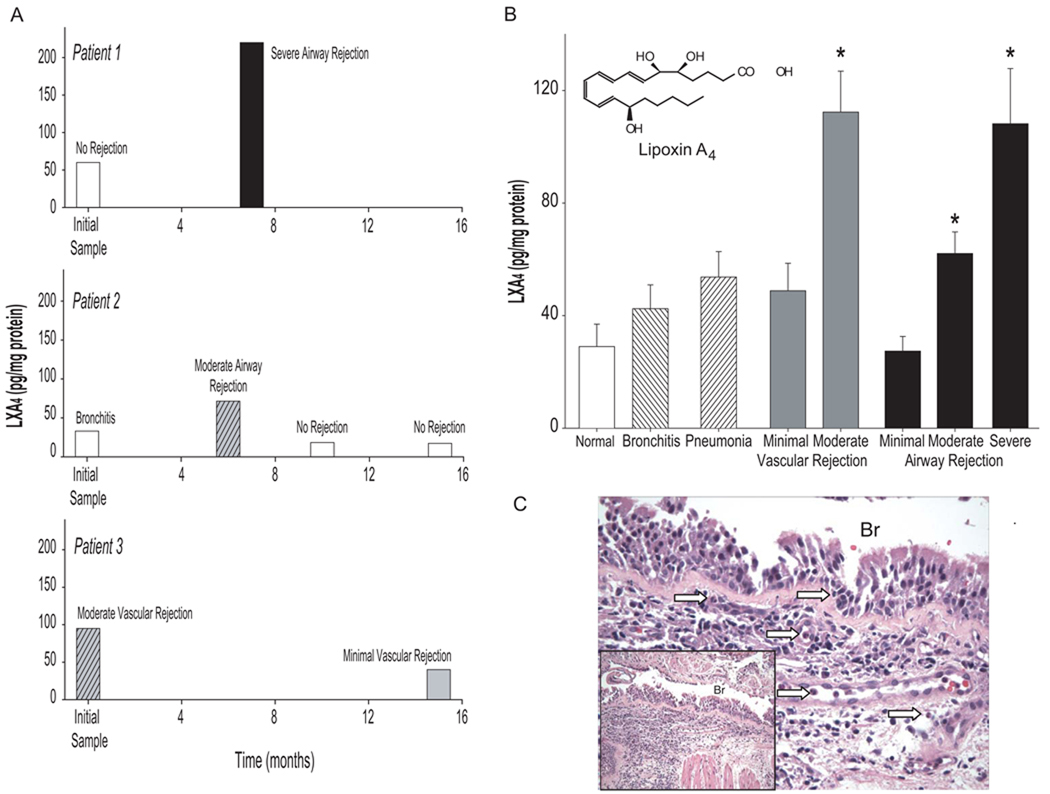
To determine whether LX production was related to the airway inflammation of rejection and/or infection, LXA4 was monitored (pg/mg protein) in bronchoalveolar lavage fluids (BALFs) from lung transplant recipients (n=21, d=59) that underwent screening or diagnostic bronchoscopy. Patients were assigned a diagnosis based on clinical criteria and the histology of transbronchial lung biopsies performed at the time of BAL. For some subjects, multiple samples were available from bronchoscopies performed after transplantation, so LXA4 generation could be measured over an interval of several months as their clinical condition changed. Lipoxin A4 levels were significantly increased during allograft rejection of a moderate or severe degree with either a vascular or airway focus (Figure 1). The increases in LXA4 during rejection were new, transient and linked to severity (Figures 1A and 1B). Modest elevations in LXA4 were also present during bronchitis and pneumonia (42.5 +/− 8.5 and 53.7 +/− 9.0 pg LXA4/mg protein, respectively; mean +/− s.e.m.) (Figure 1B). Similar to earlier reports, histological analyses of transbronchial lung biopsies revealed that in addition to adaptive immune responses, the pathobiology of airway and vascular allograft rejection was also characterized by PMN infiltration (Figure 1C). These results indicate that LXA4 was present in transplant recipients and may participate in the local tissue pathophysiology of allograft rejection.
Figure 1. Lipoxin A4 levels increase during lung allograft rejection.
(a) LXA4 levels in bronchoalveolar lavage fluids performed for clinical indication after lung transplant and (b) correlated with clinical diagnosis. Values represent the mean +/− S.E. for n≥3, d=2. *P<0.05 by Student’s t-test compared to the absence of pathology. (c) Representative histopathology for severe airway rejection from Patient 1. Arrows denote representative PMN.
LXA4 and Cyclosporine A regulate human PMN activation via distinct mechanisms
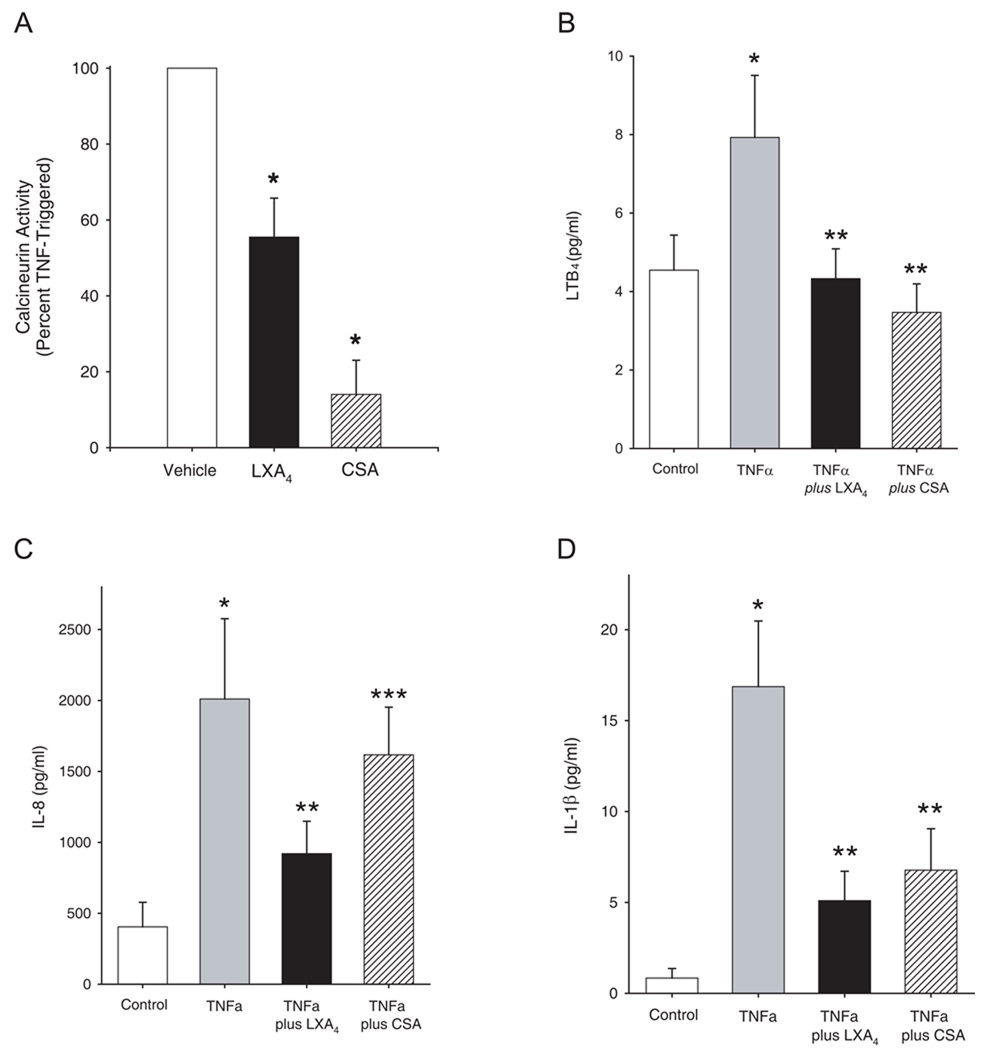
Immunophilins, such as cyclosporine A (CSA), are potent, yet incomplete inhibitors of PMN responses and allograft rejection, as neither acute nor chronic rejection can be entirely prevented with CSA [17]. To determine whether LXA4 shared CSA’s inhibition of calcineurin activity, we exposed freshly isolated human PMNs from healthy subjects to either LXA4 (100 nM) or CSA (200 nM) prior to activation with TNFα (see Methods). At these concentrations, both LXA4 and CSA were potent inhibitors of PMN calcineurin activity, leading to approximately 50% and 80% inhibition of calcineurin, respectively (Figure 2A). We next compared the activity of LXA4 (100 nM) and cyclosporine A (CSA) (200 nM) as inhibitors of TNFα-initiated mediator release by human PMNs. LXA4 and CSA significantly inhibited LTB4, IL-8, and IL-1β production (Figure 2B–2D). However, the magnitude of inhibition for TNFα-initiated IL-8 release was significantly less with CSA than LXA4 (Figure 2C). These results indicate that LXA4 and CSA have distinct activity relationships in PMN for calcineurin activity and generation of pro-inflammatory mediators, suggesting that LXA4’s reduction in PMN activation is transduced via both calcineurin–dependent and independent signaling pathways.
Figure 2. Regulation of human PMN activation by lipoxin A4 involves calcineurin dependent and independent pathways.
Freshly isolated human PMN from healthy donors were exposed (5 min, 37°C) to LXA4 (100nM), cyclosporin A (200 nM) or vehicle (0.1% ethanol) prior to TNFα (10 ng/ml) and (a) calcineurin activity and (b–d) cytokine release were determined (see Methods). Values reported are the mean ± S.E. for n ≥ 3 separate PMN donors. *P < 0.01 (as compared to vehicle), **P < 0.05 (as compared to TNFα) and ***P < 0.05 (for CSA compared to LXA4).
LXA4 receptor (ALX/FPR2) influences cardiac allograft survival
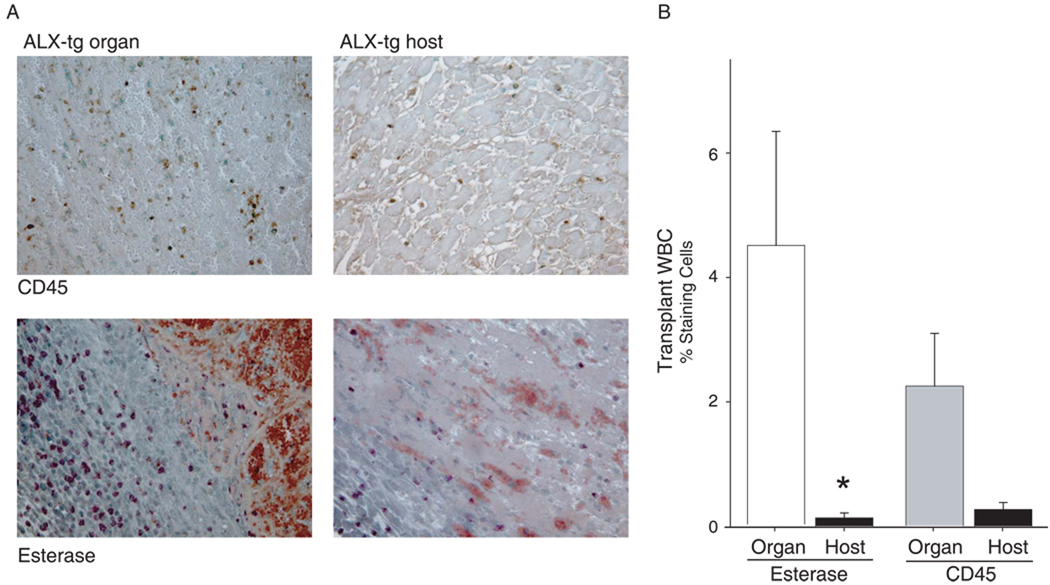
To determine if the increased LXA4 levels identified during allograft rejection had protective and pro-resolving actions, we investigated the impact of lipoxin signaling in two independent and well-characterized murine models of solid organ transplant rejection - vascularized cardiac and renal transplantation. For cardiac transplantation, allograft survival after allogeneic transplant of FVB and BALB/c strains was approximately 9 days after surgery (Table 1). Transgenic FVB mice that express human LXA4 receptors (ALX/FPR2) were prepared earlier (denoted ALX-tg) [8]. When FVB ALX-tg animals served as donors, there was no significant change in allograft survival. In sharp contrast, when FVB ALX-tg mice served as recipients for BALB/c donor organs, there was a significant increase in mean allograft survival (Table 1). Some animals received a metabolically stable analog of the aspirin-triggered-15-epi-LXA4 (ATLa, 10 µg) [13] that was used to perfuse the donor organ and then administered (iv) on days 0 (immediately after transplantation) and 1. Over one week after perioperative exposure to ATL, allograft survival was increased by 30% in ALX-tg compared to non-transgenic animals. Allograft survival was also significantly longer in this cohort compared to ALX-tg animals that had not received the LXA4 analog (Table 1). Of note, the ALX transgene was targeted to myeloid cells by a component of the CD11b promoter[8, 18] and the beneficial effects for the ALX-tg recipients were not similarly evident for experimental groups with ALX-tg donor hearts. Histological examination of the organs at the time of rejection revealed a marked decrease in leukocytes from ALX-tg mice, in particular PMNs, in cardiac tissues, as determined by immunostaining for CD45 and chemically staining for PMN esterase activity (Figure 3). Together, these results indicate that the LX/ALX axis modulates innate immune responses, such as PMN activation and trafficking, to prolong allograft survival.
Table 1.
ALX-tg recipients have increased cardiac allograft survival
| Donor Strain | Recipient Strain | Lipoxin A4 Analog | Allograft Survival (d) | Mean +/− SE |
|---|---|---|---|---|
| FVB | BALB/c | − | 8,8,9,9,9 | 8.6 +/− 0.3 |
| FVB ALX-tg | BALB/c | − | 9,9 | 9.0 |
| FVB ALX-tg | BALB/c | + | 8,9,10 | 9.0 +/− 0.6 |
| BALB/c | FVB | − | 7,9,9,10,10 | 9.0 +/− 0.6 |
| BALB/c | FVB ALX-tg | − | 10,10,11,11,11 | 10.6 +/− 0.3* |
| BALB/c | FVB ALX-tg | + | 11,12,12 | 11.7 +/− 0.3*,† |
Heterotopic cardiac transplants were performed as described in Materials and Methods. Allograft survival was assessed as the number of days that a pulse could be palpated in the allograft. Mean survival time in days of allografts in each experimental group.
P<0.05 for FVB ALX-tg compared to FVB organ recipient and
P<0.05 for comparison between ALX-tg with and without LXA4 analog (ATLa).
Figure 3. Lipoxin Receptor signaling dampens neutrophil accumulation in cardiac allograft rejection.
Heterotopic cardiac transplants were performed (see Methods). When a transplanted heart pulse was no longer palpable, allografts were removed. (a) Representative heart tissue sections (magnification: ×200) were obtained from formalin-fixed, paraffin-embedded tissue, prepared and stained for CD45 (top row) or esterase (bottom row). (b) Tissue morphometric analyses were performed to determine the impact of ALX-tg expression on PMN accumulation in cardiac tissues. Values reported are the mean ± S.E. for more than 3 independent experiments with n ≥ 3 mice in each group. *P < 0.05 (ALX-tg host vs. donated organ)
Pro-resolving mediators influence kidney allograft rejection
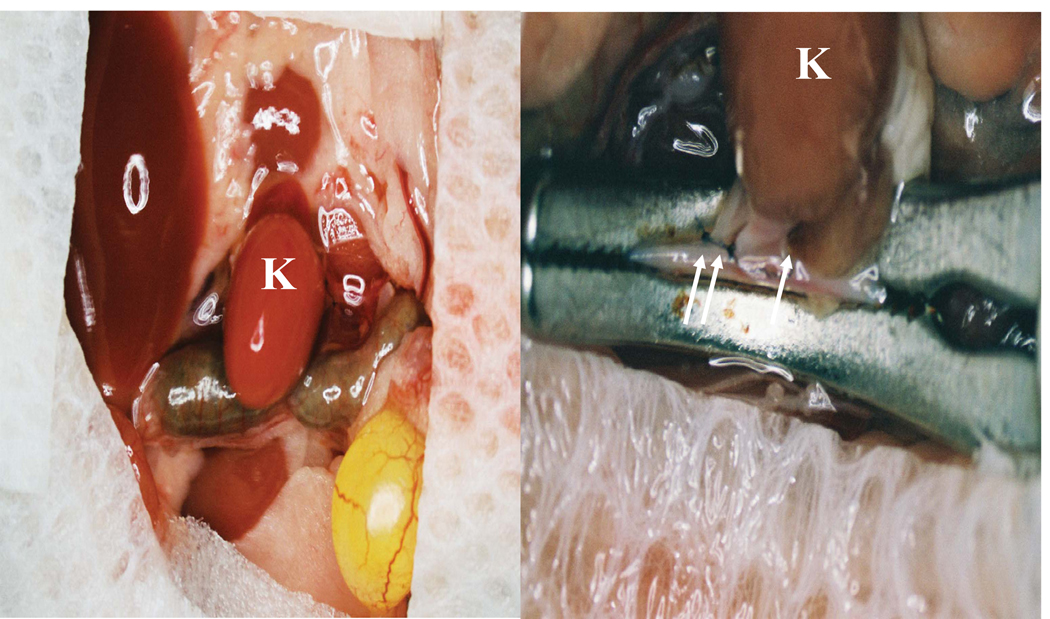
In addition to cardiac transplantation, the effects of ATLa on kidney transplantation were determined in a fully-MHC disparate model (BALB/c kidney into C57BL/6 (B6) recipient). The donor kidney was transplanted with subsequent ligation of the kidney's arteries, veins and ureter (Figure 4), and the recipients' kidneys were both nephrectomized. Transplantation of allogeneic BALB/c kidneys into unmodified 8–10-week old B6 mice results in a medium survival time (MST) of 30.5 days (Figure 5). Pretreatment of BALB/c donor mice with 50mg/kg of LPS i.p. on day −1, results in a significant acceleration of allograft rejection in transplanted kidneys from these mice (MST=8, p<0.002) (Figure 5), emphasizing the importance of inflammation within donor organs for organ rejection.
Figure 4. Kidney transplantation in mice.
A syngeneic B6 or allogeneic BALB/c kidney was transplanted into B6 recipients. The transplantation procedure involved transplantation of the donor kidney (K) and ligation (arrows) of the kidney's arteries, veins and ureter. In the same procedure the recipients' kidneys were both nephrectomized.
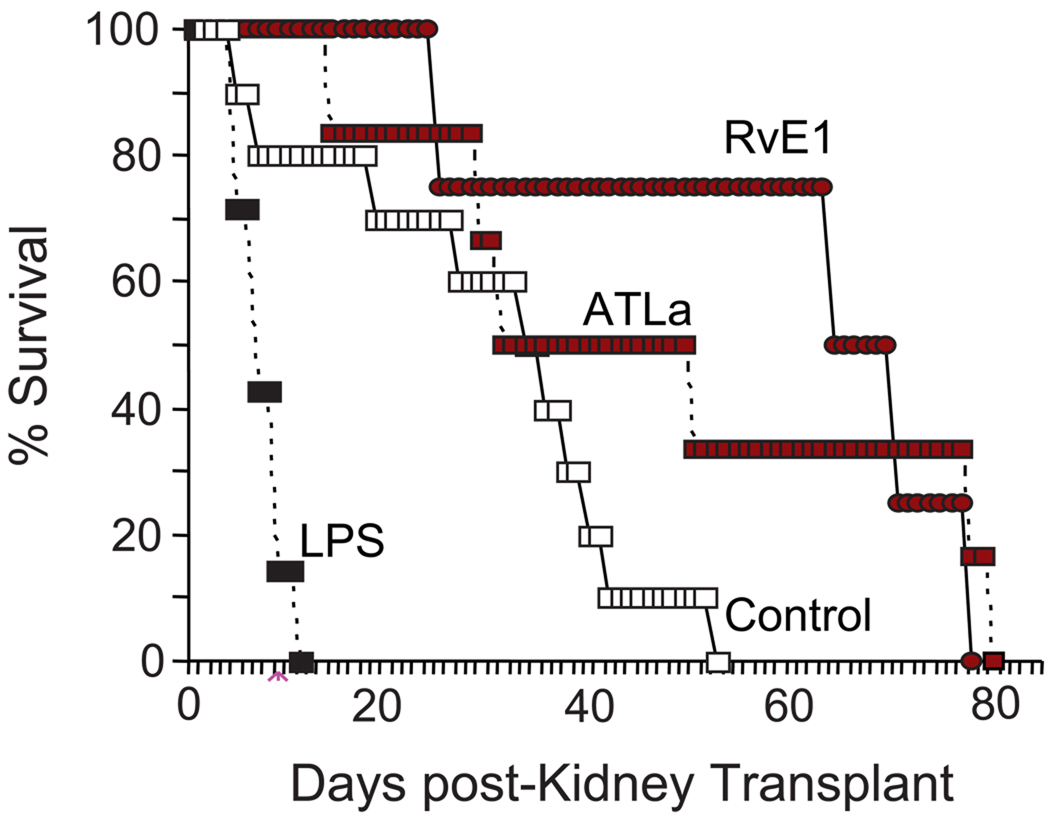
Figure 5. LXA4 and RvE1 enhance the survival of renal allografts.
Allogeneic BALB/c kidneys were transplanted into B6 mice. The groups of mice received vehicle (Control, white squares), LPS (50 mg/kg) pretreatment (LPS, n=7, black squares), LXA4 stable analog on days −1, 0 and +4 (ATLa, red squares, dashed line) or RvE1 on days −1, 0 and +4 (RvE1, red circles) and allograft survival was determined (See Methods). Survival of allografts was prolonged when donors and recipients were treated with ATL (n=6) or RvE1 (n=6) before or at the early stage of transplantation compared with the untreated control group (n=10, p<0.01) and significant by Kaplan Meir multiple comparisons survival analysis (P<0.01).
In order to evaluate potential protective roles for pro-resolving mediators on the transplanted organs, ATLa or RvE1 was administered (1 µg/per mouse, iv) to both donor BALB/c and host C57BL/6 mice on days −1 and 0 prior to allogeneic kidney transplantation. The transplanted recipients received an additional tail vein injection on day +4 after kidney transplantation. Similar to the changes with heterotopic cardiac allograft rejection (Table 1), administration of ATLa led to a modest increase in the mean time to kidney rejection (MST=37.5, p<0.072) (Figure 5). Of interest, approximately 50% of the ATLa-exposed animals displayed a marked increased in survival compared to control animals (Figure 5). RvE1 is a potent inhibitor of PMN chemotaxis, tissue accumulation and activation [19–22] and can increase LXA4 generation in tissue inflammation (39), so here RvE1’s actions on renal allograft survival were determined for comparison to the LXA4 analog, ATLa. RvE1 led to a significant increase in the time to donor kidney rejection (MST=74, p<0.001) that was nearly double that seen with ATLa. These findings were significant by Kaplan Meir survival analysis (P<0.01) (Figure 5).
DISCUSSION and CONCLUSIONS
Here, we provide the first evidence for LXA4 within the lungs of transplant patients during allograft rejection. Endogenous levels of LXA4 correlated with the severity of both airway and vascular rejection, suggesting a pivotal role for this counter-regulatory lipid mediator in regulating immune-mediated attack of the transplanted organ. LXA4 is a potent regulator and stop signal for PMN migration and activation [23, 24] and here decreased TNFα-initiated activation, which is pivotal to early events in organ rejection [14]. In addition, an LXA4 stable analog and ALX-tg mice provided the means for investigating roles for the LXA4–ALX axis in vivo during solid organ transplant rejection. Current murine models of lung transplant rejection do not adequately replicate the human condition, so we turned to two independent and well-characterized models of vascularized allograft rejection. Myeloid targeted expression of human ALX/FPR2 significantly decreased PMN accumulation and prolonged allograft survival with additional protection provided by concomitant administration of a LXA4 stable analog. Similar to cardiac transplantation, ATLa increased the MST after allogeneic kidney transplantation. This organ protection was enhanced further by the pro-resolving mediator RvE1. Together, these findings indicate that LXA4 is generated during host responses to transplant rejection and both LXA4 and RvE1 can decrease innate immune responses that give rise to allograft rejection.
There is increasing evidence that PMNs play important roles in host rejection of transplanted solid organs by immune as well as non-immune mechanisms [25–27]. Inhibition of calcineurin activity by CSA and other immunophilins markedly enhance allograft tolerance and can inhibit PMN activation [28, 29]. In contrast to CSA, LXA4 was a less potent inhibitor of calcineurin activity, yet equivalent or more potent in blocking inflammatory mediator release from activated human PMNs. Interleukin-8, IL-1β and LTB4 have been implicated in the pathophysiology of lung allograft rejection [27]. In addition, TNFα signaling is an important mediator of acute and chronic cardiac allograft rejection [14]. TNFα increases leukocyte calcineurin activity and activates PMN [30]. LXA4 and RvE1 display potent anti-inflammatory and pro-resolving actions for PMN enriched tissue inflammation [12, 31–33, 22], and decrease TNFα initiated PMN trafficking, exudate formation and mediator release [30, 19]. In addition to anti-inflammatory properties, lipoxins and resolvins also promote resolution via enhanced macrophage clearance of apoptotic PMNs, chemokines, cytokines and microbial products [34, 35, 33]. LXs and RvE1 are potent inhibitors of LTB4-initiated PMN activation[24, 20], and, independent of calcineurin regulation, LXs can block polyisoprenyl phosphate remodeling [24] and leukocyte-specific protein-1 phosphorylation[36]. RvE1 does not bind to ALX, but can serve as a receptor level antagonist for LTB4 at its BLT1 receptor[20]. In view of important roles for LTB4 and TNFα in allograft rejection[14, 6], LXs and resolvins may serve as pivotal local counter-regulatory mediators that carry the added benefit of not increasing the risk for bacterial infection [37, 38, 22].
Resolvin E1 is an anti-inflammatory and pro-resolving lipid mediator derived from eicosapentaenoic acid that displays potent activities in several mucosal organs, including ocular, oral, and gastrointestinal tissues (reviewed in [10]). In a murine model of asthma, RvE1 accelerated the resolution of allergic airway responses [39]. RvE1’s pro-resolving actions are linked, in part, to inhibition of IL-23, IL-6, and IL-17, as well as stimulating endogenous LXA4 generation [39, 40]. Here, RvE1 displayed even greater protection than ATLa in prolonging renal allograft survival. IL-17 is a pivotal mediator for allograft rejection and vasculopathy[41]. Given the important roles that BLT1 receptors serve in the transduction of LTB4-initiated signals for allograft inflammatory responses[6], RvE1’s receptor level antagonism of BLT1 [20] may provide this compound with increased potency for organ protection after allogeneic transplantation. Thus, RvE1 can promote allograft survival via several mechanisms, including inhibition of IL-17 and BLT1 signaling and by stimulating endogenous LXA4 production. Moreover, the organ protection provided independently by RvE1 and a LXA4 analog suggests a broader role for mediators that posses both anti-inflammatory and pro-resolving activities in preventing the host inflammatory responses of allograft rejection.
Lipoxin A4 and its bioactive stable analogs act on ALX/FPR2 receptors (reviewed in [10]) to protect in experimental models of graft versus host disease after bone marrow transplantation [42]. Heterotopic cardiac transplantation with hALX-tg mice indicated that the critical effectors for organ protection were provided by the host as the hALX-tg hearts did not display increased survival time. The host factors are likely to be leukocytes, given the inclusion in the transgene construct of a component of CD11b to direct expression of hALX to murine myeloid cells [18]. ALX receptor signaling markedly inhibits granulocyte locomotion and activation. Important roles for PMN-mediated inflammation are emphasized by the LPS-induced acceleration of renal allograft rejection. Activated neutrophils release cytokines, reactive oxygen species, proteases, myeloperoxidase and other enzymes that can lead to bystander tissue damage [43]. LTB4 is a potent PMN attractant and secretagogue [5], and LT inhibition improves allograft function and survival [44, 6]. Together, these findings point to important protective roles for pro-resolving mediators in allograft rejection by leukocyte activation.
In summary, the present results demonstrate that LXA4 formation and actions are linked to the local tissue pathophysiology of allograft rejection. The LXA4/ATLa stable analog administered in perfusate and by intravenous routes at the time of transplantation regulated immune-mediated events relevant to organ rejection in two separate and independent experimental models of vascularized transplantation with a mode of therapeutic immunomodulation that is distinct from the currently clinical strategy of calcineurin inhibition. The actions of RvE1 were even more potent than ATLa in these transplant systems, highlighting the potential for anti-inflammatory and pro-resolving mediators to serve important regulatory roles in solid organ transplant rejection. Thus, the potent counter-regulatory actions of specialized pro-resolving mediators and their receptors open a novel therapeutic strategy for solid organ transplant rejection that does not involve immune suppression.
ACKNOWLEDGEMENTS
This work was supported by DK067940 (BN), HL68669 (BDL), DE016191 (BDL, CNS), DK48549 (MAA) and DK07448 (CNS, BN), by The Patricia Welder Robinson Young Investigator Grant of the National Kidney Foundation (BN) and a fellowship from La Fondation de la Recherche Medicale (CB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
BDL is a co-inventor on patents on the use of lipoxins and resolvins in lung disease that are assigned to Brigham and Women’s Hospital and have been licensed for clinical development. CNS is inventor on composition of matter patents owned by BWH and licensed for clinical development. CNS is also a founder of Resolvyx Pharmaceuticals and retains founder stock.
References
- 1.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation--how much of the promise has been realized? Nat Med. 2005;11(6):605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 2.Murray JE. Human organ transplantation: background and consequences. Science. 1992;256(5062):1411–1416. doi: 10.1126/science.1604314. [DOI] [PubMed] [Google Scholar]
- 3.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166(4):440–444. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V, Abbas AK, Fausto N, Aster J. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Saunders; 2010. [Google Scholar]
- 5.Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. pp. 153–174. [Google Scholar]
- 6.Medoff BD, Seung E, Wain JC, et al. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202(1):97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee TH, Crea AE, Gant V, et al. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990;141(6):1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 8.Levy BD, De Sanctis GT, Devchand PR, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8(9):1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 9.Brady HR, Lamas S, Papayianni A, Takata S, Matsubara M, Marsden PA. Lipoxygenase product formation and cell adhesion during neutrophil-glomerular endothelial cell interaction. Am J Physiol. 1995;268(1 Pt 2):F1–F12. doi: 10.1152/ajprenal.1995.268.1.F1. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 11.Levy BD, Bertram S, Tai HH, et al. Agonist-induced lipoxin A4 generation: detection by a novel lipoxin A4-ELISA. Lipids. 1993;28(12):1047–1053. doi: 10.1007/BF02537069. [DOI] [PubMed] [Google Scholar]
- 12.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 13.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101(4):819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKee CM, Defina R, He H, Haley KJ, Stone JR, Perkins DL. Prolonged allograft survival in TNF receptor 1-deficient recipients is due to immunoregulatory effects, not to inhibition of direct antigraft cytotoxicity. J Immunol. 2002;168(1):483–489. doi: 10.4049/jimmunol.168.1.483. [DOI] [PubMed] [Google Scholar]
- 15.Filep JG, Zouki C, Petasis NA, Hachicha M, Serhan CN. Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94(12):4132–4142. [PubMed] [Google Scholar]
- 16.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16(2):103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 17.Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J. 2004;23(1):159–171. doi: 10.1183/09031936.03.00039203. [DOI] [PubMed] [Google Scholar]
- 18.Devchand PR, Arita M, Hong S, et al. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003;17(6):652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- 19.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 21.Campbell EL, Louis NA, Tomassetti SE, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21(12):3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 22.Seki H, Fukunaga K, Arita M, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184(2):836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92(1):75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a 'stop' signaling switch for aspirin-triggered lipoxin A4. FASEB J. 1999;13(8):903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 25.Beeh KM, Kornmann O, Lill J, Buhl R. Induced sputum cell profiles in lung transplant recipients with or without chronic rejection: correlation with lung function. Thorax. 2001;56(7):557–560. doi: 10.1136/thorax.56.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devouassoux G, Drouet C, Pin I, et al. Alveolar neutrophilia is a predictor for the bronchiolitis obliterans syndrome, and increases with degree of severity. Transpl Immunol. 2002;10(4):303–310. doi: 10.1016/s0966-3274(02)00074-6. [DOI] [PubMed] [Google Scholar]
- 27.Wilkes DS, Egan TM, Reynolds HY. Workshop on Lung Transplantation: Opportunities for Research and Clinical Advancement. Am J Respir Crit Care Med. 2005;14:14. doi: 10.1164/rccm.200501-098WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendey B, Klee CB, Maxfield FR. Inhibition of neutrophil chemokinesis on vitronectin by inhibitors of calcineurin. Science. 1992;258(5080):296–299. doi: 10.1126/science.1384129. [DOI] [PubMed] [Google Scholar]
- 29.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377(6544):75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 30.Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189(12):1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 33.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164(4):1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 35.Ariel A, Fredman G, Sun YP, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7(11):1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohira T, Bannenberg G, Arita M, et al. A stable aspirin-triggered lipoxin A4 analog blocks phosphorylation of leukocyte-specific protein 1 in human neutrophils. J Immunol. 2004;173(3):2091–2098. doi: 10.4049/jimmunol.173.3.2091. [DOI] [PubMed] [Google Scholar]
- 37.Canny G, Levy O, Furuta GT, et al. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci U S A. 2002;99(6):3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22(10):3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devchand PR, Schmidt BA, Primo VC, et al. A synthetic eicosanoid LX-mimetic unravels host-donor interactions in allogeneic BMT-induced GvHD to reveal an early protective role for host neutrophils. FASEB J. 2005;19(2):203–210. doi: 10.1096/fj.04-2565com. [DOI] [PubMed] [Google Scholar]
- 43.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 44.Spurney RF, Ibrahim S, Butterly D, Klotman PE, Sanfilippo F, Coffman TM. Leukotrienes in renal transplant rejection in rats. Distinct roles for leukotriene B4 and peptidoleukotrienes in the pathogenesis of allograft injury. J Immunol. 1994;152(2):867–876. [PubMed] [Google Scholar]