Abstract
Salvage biosynthesis of nicotinamide adenine dinucleotide (NAD+) from nicotinamide (NAM) lowers NAM levels and replenishes the critical molecule NAD+ after it is hydrolyzed. This pathway is emerging as a regulator of multiple biological processes. Here we probe the contribution of the NAM-NAD+ salvage pathway to muscle development and function using C. elegans. C. elegans males with mutations in the nicotinamidase pnc-1, which catalyzes the first step of this NAD+ salvage pathway, cannot mate due to a spicule muscle defect. Multiple muscle types are impaired in the hermaphrodites, including body wall muscles, pharyngeal muscles and vulval muscles. An active NAD+ salvage pathway is required for optimal function of each muscle cell type. However, we found surprising muscle-cell-type specificity in terms of both the timing and relative sensitivity to perturbation of NAD+ production or NAM levels. Active NAD+ biosynthesis during development is critical for function of the male spicule protractor muscles during adulthood, but these muscles can surprisingly do without salvage biosynthesis in adulthood under the conditions examined. The body wall muscles require ongoing NAD+ salvage biosynthesis both during development and adulthood for maximum function. The vulval muscles do not function in the presence of elevated NAM concentrations, but NAM supplementation is only slightly deleterious to body wall muscles during development or upon acute application in adults. Thus, the pathway plays distinct roles in different tissues. As NAM-NAD+ biosynthesis also impacts muscle differentiation in vertebrates, we propose that similar complexities may be found among vertebrate muscle cell types.
Keywords: nicotinic acid, niacin, NAMPT, visfatin, vitamin B3
Introduction
Nicotine adenine dinucleotide (NAD+) is a critically important biomolecule that acts as a coenzyme in energy metabolism and as a cosubstrate for NAD+ consumers such as sirtuins and PARPs (Belenky et al., 2007). These NAD+ consumers hydrolyze NAD+, releasing nicotinamide (NAM) and using ADPribose in a variety of reactions that impact vital biological processes such as longevity, stress response, glucose homeostasis, DNA repair, immunity, and muscle differentiation (for recent reviews see Galli et al., 2010; Kirkland, 2010; Sauve, 2008; Vinciguerra et al., 2010). As NAD+ consumers deplete NAD+, organisms must have the means to replenish this critical molecule via de novo or salvage synthesis (Rongvaux et al., 2003). C. elegans likely uses only salvage synthesis (Altschul et al., 1997; Vrablik et al., 2009). In invertebrates and fungi, nicotinamidases convert the NAM released by NAD+ consumers to nicotinic acid as the first step in a NAM to NAD+ salvage pathway (Magni et al., 1999). Vertebrates use a distinct pathway initiated by nicotinamide phosphoribotransferase (NAMPT) to salvage NAM for NAD+ biosynthesis. Because the vertebrate and the invertebrate NAM-NAD+ salvage pathway each reduce NAM, an inhibitor of at least some NAD+ consumers, and promote biosynthesis of NAD+, the cosubstrate of NAD+ consumers, these pathways impact consumer activity, thereby regulating the varied biological functions of consumers (Sauve, 2008). The relative importance of preventing NAM accumulation versus promoting NAD+ biosynthesis to the regulation of NAD+ consumers is still unclear, and the relative importance of the NAM-NAD+ salvage pathway in regulating NAD+ consumers versus synthesizing NAD+ for other purposes is also understudied.
We are broadly interested in how perturbations in NAM-NAD+ salvage biosynthesis affect metazoan development and are using the invertebrate C. elegans model in our studies. Though enzymatically distinct, NAMPT and nicotinamidases are considered functionally equivalent enzymes because they both consume NAM and promote NAD+ biosynthesis (Yang et al., 2006). Illustrating this functional conservation and the relevance of the C. elegans model to higher organisms, we previously found that NAMPT can partially substitute for the nicotinamidase PNC-1 in C. elegans (Vrablik et al., 2009).
Recent studies suggest that NAMPT is an important regulator of myogenesis in vertebrates (Fulco et al., 2008; van der Veer et al., 2005). NAMPT expression is increased in differentiated smooth muscle cells, and smooth muscle differentiation is promoted by over-expression of NAMPT in culture (van der Veer et al., 2005). NAMPT is also highly expressed in the skeletal muscle of multiple vertebrates including humans, rodents, and chickens (Krzysik-Walker et al., 2008; Revollo et al., 2007; Samal et al., 1994). NAMPT mRNA expression increases during skeletal muscle development in rodents (Tang et al., 2000), and NAMPT protein levels increase in skeletal muscle with exercise in humans (Costford et al., 2010), suggesting that NAMPT would be associated with promotion of skeletal muscle myogenesis as well. However, differentiation of mammalian skeletal muscle cells in culture is associated with a decreased NAD+/NADH ratio, and NAMPT mediates blockage rather than promotion of mature myotube formation under conditions of nutrient deprivation in a tissue culture model (Fulco et al., 2008; Fulco et al., 2003).
Because the NAD+ salvage pathway has robust effects on muscle physiology in vertebrates, we hypothesized that the pathway would do the same in invertebrates. C. elegans offers an anatomically simple model to explore the consequences of perturbing NAM levels and NAD+ biosynthesis on muscle development and physiology with cellular resolution. We have revealed roles for PNC-1 nicotinamidase in C. elegans muscle, confirming our hypothesis, and we have explored the relative contributions of NAM and NAD+ levels to the development and function of a variety of muscles in C. elegans, demonstrating specificity between muscle types in their response to these metabolites.
Materials and methods
C. elegans culture
CB1490 him-5(e1490) and N2 are the wild-type strains. We performed all assays with the putative null allele pnc-1(pk9605) (Vrablik et al., 2009), and some assays with pnc-1(ku212) as well. All strains were grown on standard NGM culture plates (+/− additives, see below) at 20–22°C with E. coli OP50 as the food source (Brenner, 1974). To prepare dead food, we incubated culture plates spotted with 250 µL of OP50 until a thick lawn was present. Then we irradiated half the plates for 10 minutes using a Bio Rad GS Gene Linker (Hercules, CA) UV-cross linker. We streaked random plates to check culture viability. Gravid hermaphrodites were bleached on plates with killed or live food, the resulting progeny were transferred to a fresh plate of the same condition and scored as appropriate for the phenotype.
Pharmacology
Plates were supplemented with filter-sterilized stocks of nicotinamide (Alfa Aesar, Ward Hill, MA) or nicotinic acid (Calbiochem, San Diego, CA) (NA solution adjusted to pH 5.4 with 10 N NaOH for solubility) by adding the appropriate concentration to the NGM media immediately prior to pouring the plates or to the top of the established OP50 lawn. Gravid hermaphrodites were placed on supplemented plates, and progeny were assayed for the indicated phenotype. To assay for developmental or acute pharmacological effects, hermaphrodites were moved to the new condition at L4 and allowed to acclimate for 24 hours before the assay. Males were isolated as L4s and then transferred to the new condition as recently molted adults. Males were allowed to acclimate for 24 or 48 hours prior to the assays, as specified.
NAD+-supplemented plates were prepared by adding dilutions of a 15 mM NAD+ stock solution (Sigma-Aldrich, St. Louis, MO) to the top of killed OP50 plates 2 hours prior to using the plates. Gravid hermaphrodites were bleached on the plates, and L1 progeny were moved to a freshly prepared plate the next day and then scored at the appropriate developmental stage.
Cross-progeny production assay
One L4 male was mated to one L4 unc-51(bc369) hermaphrodite on an NGM plate spotted with 100 µL of OP50. Plates were screened for the presence of non-Unc cross progeny after 3–4 days. We discarded plates if the male wandered and desiccated on the side of the plate.
Male-mating behavior assay
Plates were spotted with 10 µL of OP50 1–2 days before the assay. Five mid-L4 unc-51(bc369) hermaphrodites were aged 24 hours and then evenly spaced on one plate just prior to the assay. We placed a single virgin, adult male (aged 24 hours from L4 molt) in the middle of the food source and recorded his behavior for 10 minutes. Failure to make contact with a hermaphrodite within 10 minutes was deemed no response. We scored both the ability to complete a sub-behavior as well as the time the behavior was noted. We report the percentage of males that successfully complete a behavior. Completion of a behavior implies the ability to complete all previous behaviors.
Spicule morphology assay
We scored copulatory spicule morphology in young adult males (<24 hours post-molt) using DIC microscopy. We report percentage of males with two normal spicules. There was no difference observed in the penetrance of crumpled spicules between virgin and mated males.
Spicule prodding assay
unc-51(bc369) mid-L4 hermaphrodites were aged 24 hours and placed on thin NGM plates that had been spotted with 1 µL OP50 and aged 24 hours. We cut a ~1 cm2 region from the plate and placed it on a microscope slide, added a single virgin male and a cover slip. Using an Olympus Fluorview microscope with a DIC filter and a 40× objective, we made video recordings at 12 frames/sec when the male tail paused at the vulva. The rates of individual prodding attempts were averaged and the total amount of time observed prodding is indicated. Sample sizes indicate number of animals scored. The prodding rate we observed for wild-type males was lower than previously reported values (Garcia et al., 2001). This difference is likely due to limitations in the speed of the camera. Because the same camera and settings were used for all samples, this underestimate of prodding rate would not alter our conclusions and may even underestimate the pnc-1 prodding defect.
Spicule protraction assay
L4 males were segregated from hermaphrodites for 24 hours prior to the assay. 25 µL of levamisole (Sigma-Aldrich, St. Louis, MO), freshly diluted in water from a 100 mM stock solution), was placed on a depression well slide and 5–15 males were added to the solution and observed for 3 minutes for prolonged protraction of spicules (>10 sec) using a Nikon SMZ1500 stereoscope. Males with obviously crumpled spicules were excluded from the protraction assay.
Levamisole-induced egg-laying assay
mid-L4 hermaphrodites were aged 24–28 hours prior to the assay. We added 20 µL of levamisole (freshly diluted to 0.5 mM with M9) or M9 and a single hermaphrodite to each well of a 96-well plate. We counted the eggs laid by each animal after 60 minutes at room temperature,
Pharyngeal pumping assay
mid-L4 hermaphrodites were aged 23–25 hours at 20° C prior to the assay. To count grinder contractions, we recorded pharyngeal pumping at room temperature for 20–60 seconds using a Photometrics Turbo 1394 camera on a SMZ1500 microscope. Only animals that stayed on the bacterial lawn during the assay were included.
Thrashing assay
mid-L4 hermaphrodites were aged for 23–25 hours on plates supplemented with 100 mg/L FUDR (5-Fluoro-2-deoxyuridine, Sigma, F0503). An animal was gently placed in a drop of M9 solution on the surface of an unspotted NGM plate and acclimated for 1 minute at room temperature. Thrashes (a maximum bend in one direction) were counted for 1 minute using a Nikon SMZ1500 stereoscope.
Results
pnc-1 males fail to produce cross-progeny
Our previous finding that PNC-1 promotes proper temporal development of the gonad in C. elegans hermaphrodites (Vrablik et al., 2009), led us to investigate the timing of gonad development in males to see if this was a general function of pnc-1. Using two methods to assess gonad timing, we found that pnc-1 males consistently had delayed gonad development (Supp. Fig. 1). Thus, nicotinamidase activity is important for temporal regulation of gonadogenesis in both sexes. During genetic crosses to analyze the pnc-1 mutants, we observed that pnc-1 mutant males failed to produce cross progeny. We quantified this defect by mating males with paralyzed unc-51 mutant hermaphrodites and scoring for non-paralyzed progeny. pnc-1(ku212) and pnc-1(pk9605) males produce cross-progeny in only 12% (n=116) and 26% (n=135) of crosses respectively, which is significantly less than wild type (90% n=157). This is a new role for nicotinamidase activity in male development and/or physiology.
pnc-1 male mating attempts fail at spicule insertion
Male mating is one of the most complex behaviors of C. elegans, providing a sensitive system for assaying neuromuscular function. In order for males to successfully produce offspring, they must properly develop the germline and mating structures, as well as have the neuro-muscular coordination to locate the hermaphrodite and execute a complex series of mating behaviors. Germline defects are unlikely to cause the pnc-1 cross-progeny defect because, despite slow development of the male gonad, adult pnc-1 males have a morphologically normal gonad with wild-type numbers of spermatids that can be activated in vitro (not shown). So to determine if neuro-muscular defects might prevent pnc-1 males from producing cross-progeny, we performed a detailed analysis of pnc-1 male mating behavior.
Male mating consists of a stereotyped series of sub-behaviors: response to the hermaphrodite, backing with the sensory tail pressed against the body, turning upon reaching the ends of the hermaphrodite body, vulva location, insertion of copulatory spicules into the vulva and finally ejaculation (Barr and Garcia, 2006). The percentage of mutant and wild-type males that responded to hermaphrodites was not significantly different in a 10 min. mating assay (p = 0.18). However, pnc-1 males took longer than wild-type males to initiate mating, 222 seconds compared to 85 seconds (p = 0.002). This suggests that pnc-1 males have a mild sensory defect, but since most males eventually initiate mating, this cannot explain the defect in cross-progeny production. Additionally, pnc-1 males successfully execute backing, turning and vulva location behaviors as frequently as wild-type males (Fig. 1), and there was no significant, observable difference between the quality of backing or turning of pnc-1 males (not shown). However, pnc-1 males fail to insert their copulatory spicules into the hermaphrodite vulva. Only 11% of pnc-1 males successfully inserted their spicules, which is significantly less than those that could locate the vulva. Of the pnc-1 males that successfully inserted their spicules, most could successfully ejaculate (Fig. 1 and not shown). Thus pnc-1 males specifically fail at the mating step of spicule insertion. Since insertion of spicules into the vulva serves to anchor the male tail and facilitate sperm transfer, we conclude that pnc-1 males cannot produce cross-progeny because they cannot insert their copulatory spicules.
Figure 1. pnc-1 males cannot complete the spicule insertion step of mating behavior.
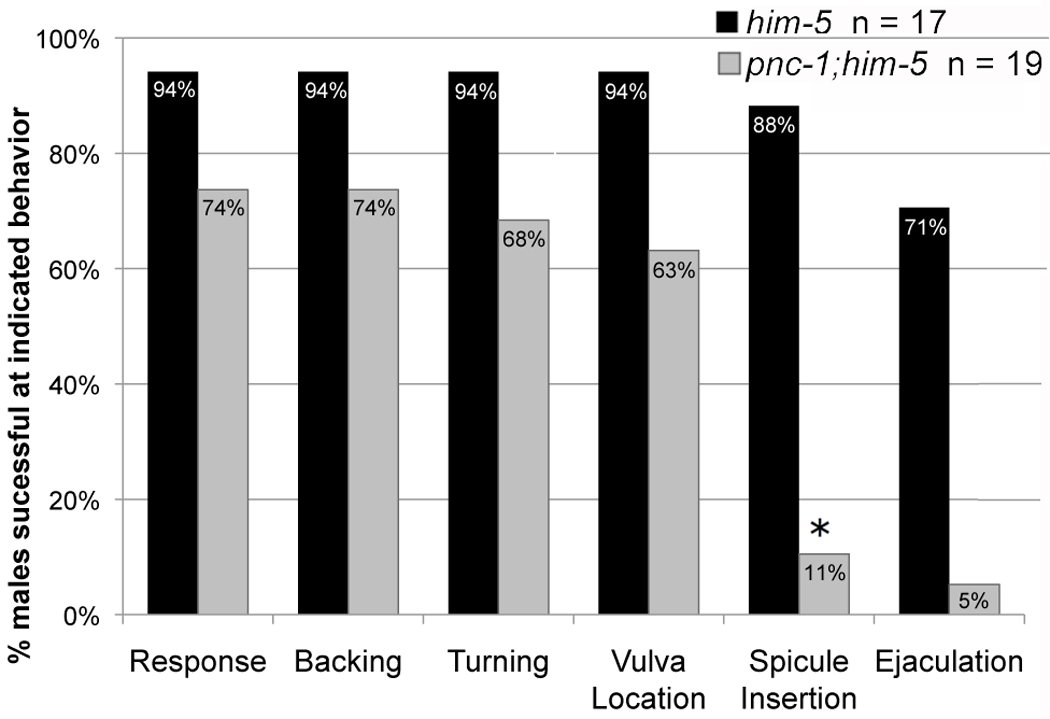
Columns indicate the percentage of males that successfully completed the indicated mating behavior. Black columns represent him-5 males and gray bars represent pnc-1(pk9605);him-5 males. Successful completion of a mating behavior implies the ability to complete all previous behaviors, e.g. if a male located the vulva without having to turn, it was scored as competent for both behaviors. The population of pnc-1 males that could insert their spicules was significantly lower than those that could perform the previous mating behaviors. n= number of males assayed. Asterisk indicates a p value < 0.05, calculated using Fisher’s Exact test.
pnc-1 spicule muscles are impaired
Morphogenesis and function of the spicules depends on the activity of the spicule retractor and protractor muscles. The spicule retractor muscles are required for spicule elongation during development; when either the blast cell that gives rise to all male sex-specific muscles is ablated or when the spicule retractor muscles are ablated prior to spicule morphogenesis, the spicules have a crumpled morphology (Jiang and Sternberg, 1999; Sulston et al., 1980). Spicule insertion during mating involves rapid contractions of the spicule protractor muscles to ‘prod’ the vulva followed by sustained contraction to insert the spicules through the vulva (Garcia et al., 2001).
A closer look at the spicules of pnc-1 males revealed that ~25% have at least one spicule that is morphologically crumpled (Fig. 2A, B), suggesting defects in spicule muscle function involving at least the retractors. Those males with crumpled spicules failed to mate. However, some males with normal spicules also failed to mate (not shown), as expected since the spicule insertion defect is more penetrant than the spicule morphology defect. Thus, we hypothesize that a neuro-muscular defect affecting the spicule protractor muscles may underlie the spicule insertion defect.
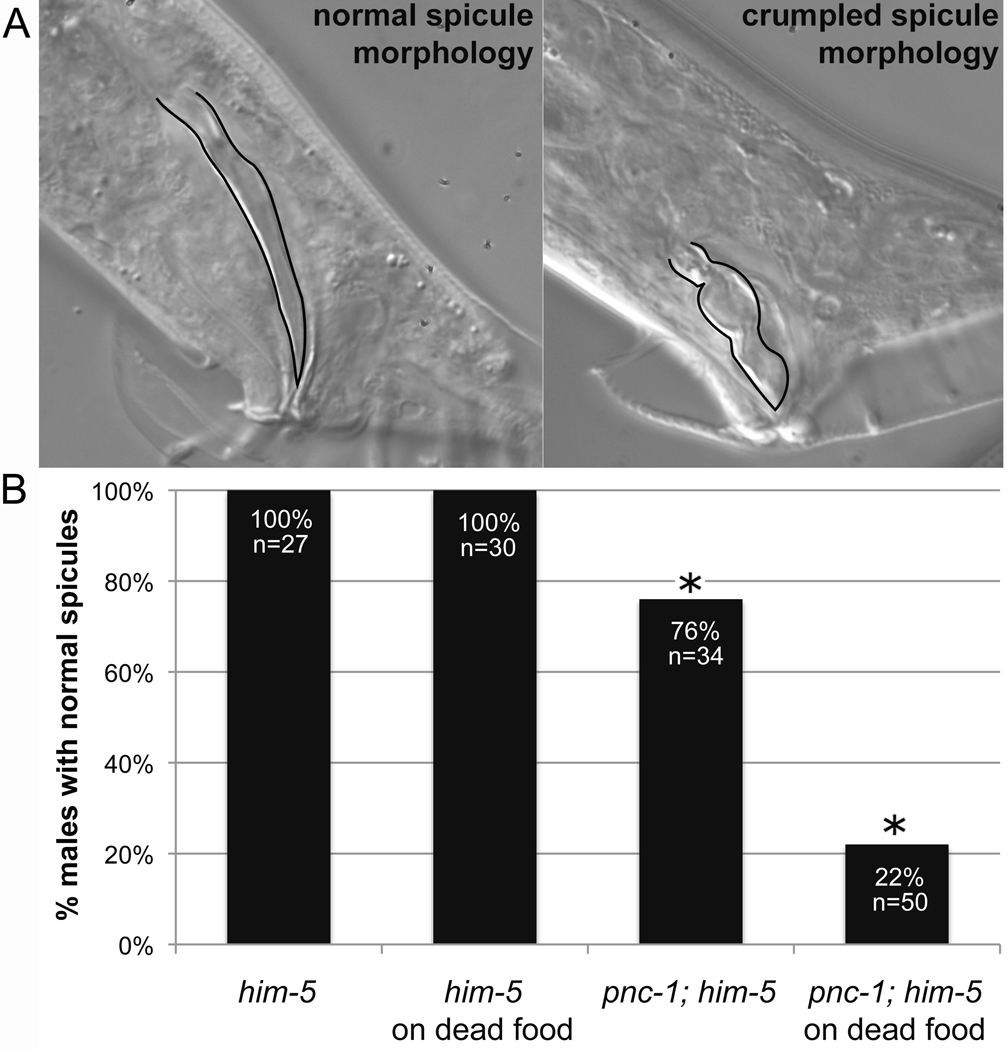
Figure 2. pnc-1 males have aberrant spicule morphology.
(A) DIC photomicrographs of a male with a normal spicule morphology (left) and a pnc-1 mutant male with crumpled spicule morphology. Spicules are outlined in black. (B) Columns indicate the percentage of males in which both spicules have a normal morphology. No effect was seen from raising ‘wild-type’ him-5 males on dead food, but dead food induced more penetrant spicule crumpling in pnc-1 males. n= number of males examined. Asterisks indicate a p value < 0.05, calculated using Fisher’s Exact test.
To test this hypothesis, we directly assayed spicule protractor muscle activity by quantifying the rate of prodding at the vulva in wild-type and mutant males. The average prodding rate of pnc-1 males is only 2 sec−1, which is less than half the rate of wild-type males, 4.4 sec−1 (Fig. 3). Thus, pnc-1 males have impaired function of the spicule protractor muscles, and this is the most likely explanation for the spicule insertion defects. However, it is still unclear if the reduced function results from impaired muscle or from disrupted neuronal signaling to the muscle. Spicule protractor muscles respond to the neurotransmitter acetylcholine (Garcia et al., 2001). Levamisole (LEV) is a potent cholinergic agonist that directly binds to and activates acetylcholine receptors (Rand, 2007). We tested the function of spicule protractor muscles in pnc-1 males using the acetylcholine agonist LEV and found that it induces spicule protraction in wild-type males with an EC90 of 3.1 µM, in agreement with previous reports (Garcia et al., 2001). However, we could not determine the EC90 of LEV-induced protraction in pnc-1 males because the maximum percentage of pnc-1 males with protracted spicules plateaus at under 60% (Fig. 4A). Almost half of pnc-1 males never respond to LEV. While we cannot exclude a neuronal defect, the LEV assay indicates that even with abundant neurotransmitter signaling, spicule protractor muscle response is impaired in pnc-1 males.
Figure 3. The prodding rate of pnc-1 male spicules is reduced and can be rescued by NA supplementation during development.
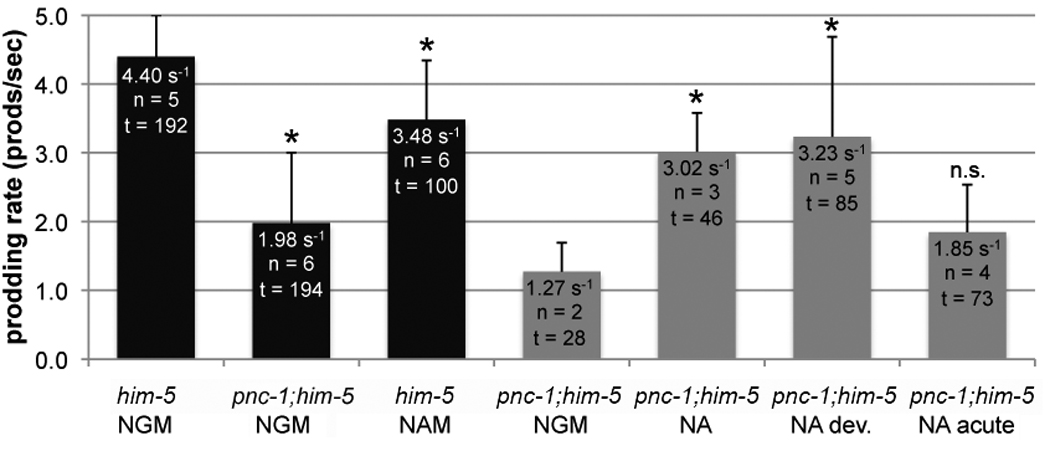
Columns represent the rate of prodding. Culture conditions are indicated below the genotype on the x-axis. NGM is normal media. Cultures were supplemented with 25 mM NA and NAM as indicated. The black columns indicate males that were aged 24 hours past the adult molt. Gray columns indicate males that were acclimated for 48 hours post L4 molt to fully separate developmental and acute effects. n = indicates the number of animals observed. t = total time of all prodding attempts in seconds. Bars indicate standard deviation of individual prodding attempts and asterisks indicate a p value < 0.05, calculated using the Mann-Whitney test.
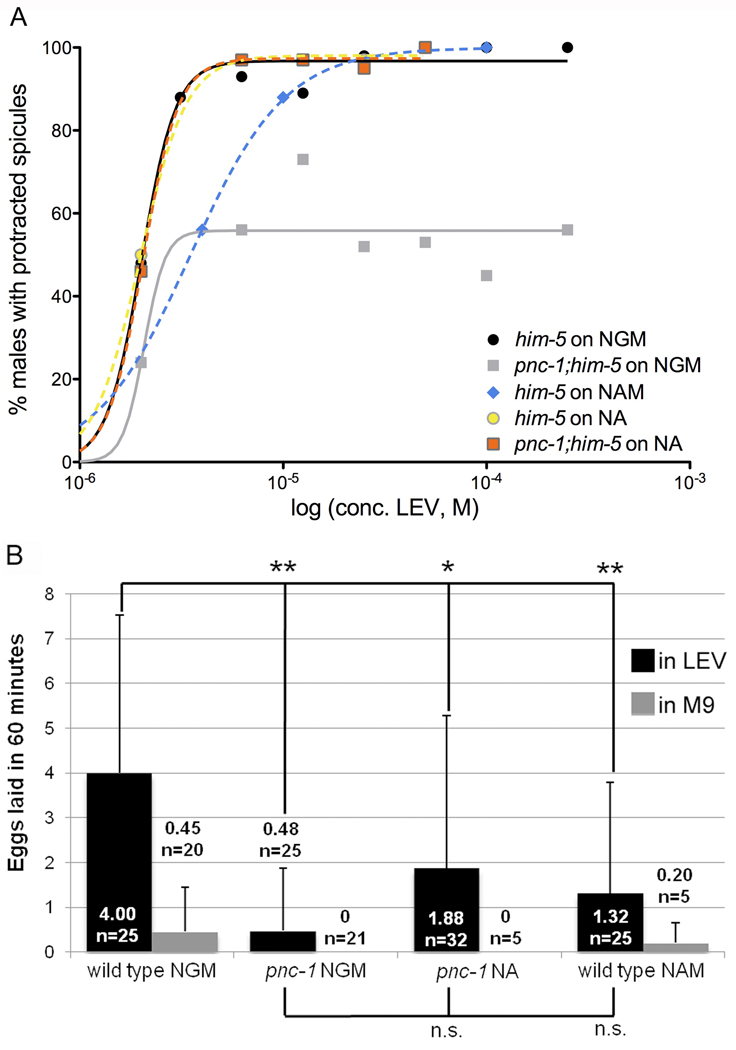
Figure 4. pnc-1 animals have a reduced response to the acetylcholine agonist levamisole.
(A) Spicule protractor muscles do not respond to levamisole. The percentage of males with prolonged spicule protraction (>10 sec) in response to levamisole at the indicated concentration is plotted. Spicule protraction was scored for 16–132 males at each concentration and five to seven concentrations were tested for each strain/condition. Solid lines indicate normal culture conditions, with wild-type males in black and pnc-1 males in gray. Dashed lines indicate pharmacological treatment: the blue line represents wild-type males raised with 25 mM NAM, orange and yellow lines respectively representing pnc-1 and wild-type males raised with 25 mM NA. Curves were fit and the EC50 and maximal responses were determined using the GraphPad Prism software. (B) Vulval muscles do not respond to levamisole. Black columns indicate the number of eggs laid within 60 minutes per hermaphrodite in response to 0.5 mM levamisole and gray columns indicate response in buffer. NAM and NA indicate animals cultured in the presence of 25 mM nicotinamide or nicotinic acid. n= number of animals examined. Bars indicate standard deviation and **: p<0.01, *: p<0.05 (calculated using Student's t-test).
pnc-1 vulval, pharyngeal and body wall muscles are impaired
We next asked if PNC-1 nicotinamidase activity contributed to the function of other muscles. We previously reported an egg-laying defect in pnc-1 hermaphrodites. This egg-laying defect cannot be explained by the gonad timing defect (Vrablik et al., 2009) or a separate phenotype affecting the viability of the uterine-vulva 1 cells (Johnson et al., 2009). Thus, we hypothesized that the egg-laying defect may result from impaired activity of the vulval muscles. To test this hypothesis, we quantified the egg-laying response to LEV. Egg laying is inhibited by M9 buffer, and exposure to LEV can overcome this inhibition to stimulate egg-laying in wild-type C. elegans (Trent et al., 1983; Weinshenker et al., 1995). Treatment with 0.5 mM LEV induces wild type to lay an average of 4 eggs in one hour, up from an average of under 0.5 in M9 (Fig. 4B). pnc-1 hermaphrodites have a weak response, if any, to LEV treatment. They lay no eggs in M9 and an average of less than 0.5 eggs upon LEV treatment (Fig. 4B). This indicates that vulval muscle function is impaired, and thus pnc-1 activity is necessary for the proper function of the sex muscles in both males and hermaphrodites.
To assay non-sex muscle function, we tested two fundamental behaviors of C. elegans, locomotion and feeding, which are controlled by the body wall muscles and pharyngeal muscles, respectively. While pnc-1 animals have grossly normal locomotion on agar plates, a more demanding assessment of body wall muscle function with a liquid thrashing assay revealed that function is impaired in pnc-1 animals. When a worm is transferred to liquid, it thrashes for a long period of time (over 90 minutes for wild type) before it alternates between quiescence and thrashing (Ghosh and Emmons, 2008). We use the liquid thrashing rate at the long initial swimming phase as an indication of body wall muscle function. Since pnc-1 hermaphrodites have an egg-laying defect, we prevented internal hatching of eggs by feeding the DNA synthesis inhibitor FUDR to worms beginning at the L4 stage (Hosono, 1978). pnc-1 hermaphrodites exhibit a 30% reduction in thrashing rate compared to wild type (Fig. 5A).
Figure 5. Pharyngeal pumping and thrashing are slow in pnc-1 mutants.
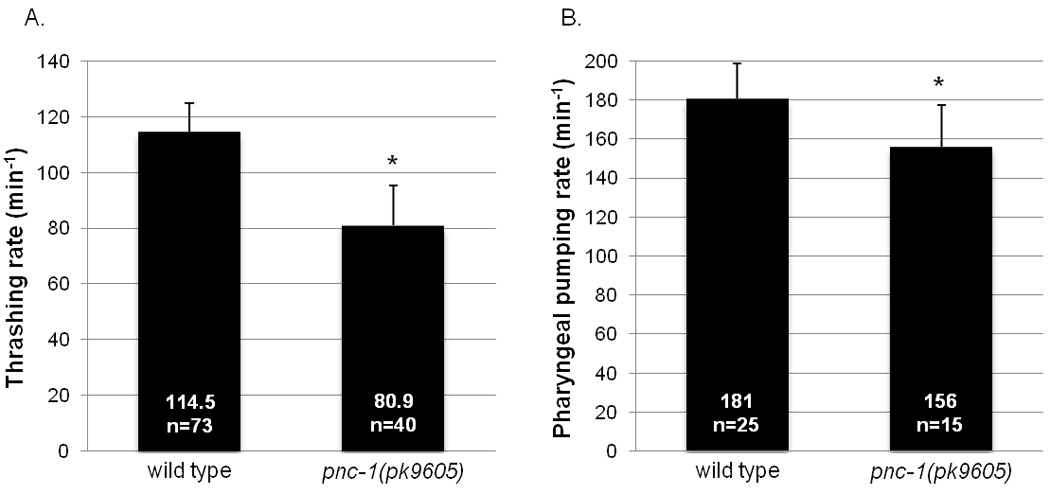
(A) Columns indicate the average number of thrashes per minute. (B) Columns indicate the average number of pharyngeal pumps per minute. n = the number of animals scored. The rates of pnc-1 mutant and wild-type animals were compared, asterisks indicate a p value < 0.05, calculated using Student’s t-test. Bars indicate standard deviation.
C. elegans feeding behavior consists of pharyngeal pumping and isthmus peristalsis (Riddle et al., 1997). Each pump is a near-simultaneous contraction of pharyngeal muscles followed by a near simultaneous relaxation. Pharyngeal pumping rate is used as an indication of pharyngeal muscle function. We did not observe any difference between pnc-1(pk9605) and wild type in pharyngeal pumping rates when the worms are raised on normal culture conditions (data not shown). However, some pnc-1 phenotypes are exacerbated when mutants are raised on UV-irradiated/dead E. coli as the food source (Vrablik et al., 2009). We found that pnc-1 animals raised on dead OP50 E. coli showed a moderate but significant decrease of pharyngeal pumping rate compared to wild type (Fig. 5B). Because contractions can continue in the absence of neuronal input (Avery and Horvitz, 1989), we conclude that the decreased pharyngeal pumping rate of pnc-1 animals is likely due to impaired muscle. The reduced rates of prodding, egg-laying, thrashing and pharyngeal pumping suggest that pnc-1 is broadly required for muscle function.
NA acts as an NAD+ precursor to rescue both spicule morphology and gonad timing defects
We are interested in determining the relative importance of NAD+ biosynthesis versus NAM consumption to the biological functions of the NAM-NAD+ salvage biosynthesis pathway. Our previous work demonstrated that nicotinamidase regulation of substrate and product levels separately impact distinct aspects of hermaphrodite development (Vrablik et al., 2009). Thus, we investigated the effects of substrate accumulation and product depletion on muscle function in pnc-1 mutants. The male gonad-timing and spicule morphology defects are rescued by raising pnc-1 males on media supplemented with the nicotinamidase product, nicotinic acid (NA) (not shown and Fig. 6A). We hypothesize that NA acts as a usable NAD+ precursor because a different NAD+ precursor, nicotinamide mononucleotide, rescues gonad timing in pnc-1 hermaphrodites (Vrablik et al., 2009). Consistent with this interpretation, we found that direct supplementation with NAD+ robustly rescued both the gonad timing and spicule morphology defects (Fig. 6B). This supports our conclusion that NA rescues pnc-1 defects by serving as an NAD+ precursor. We continue to use supplemental NA as a tool to restore NAD+ biosynthesis.
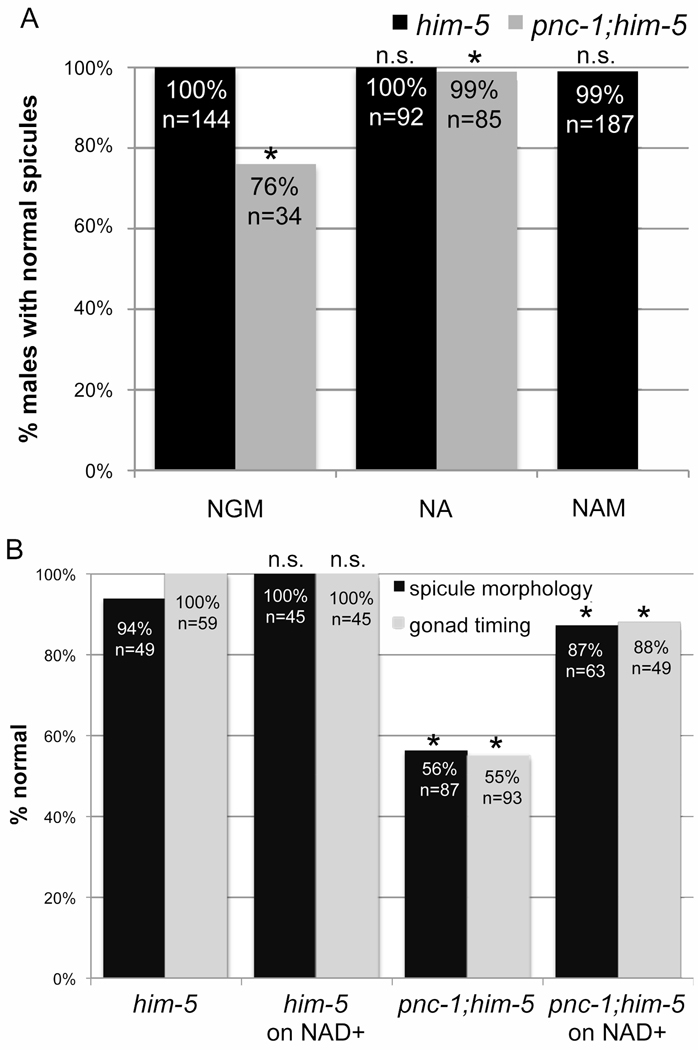
Figure 6. NAD+ supplementation can directly rescue gonad timing and spicule morphology.
(A) NA supplementation rescues the spicule morphology of pnc-1 males, and NAM does not cause a spicule morphology defect. Black columns indicate wild-type males and gray columns indicate pnc-1 males. Males were cultured on normal media or media supplemented with 25 mM NA or NAM as indicated. Significance was determined for comparisons of mutant or wild-type males on normal media to those on NA/NAM. (B) Black columns indicate the percentage of males with normal spicule morphology. Gray columns indicate the percentage of hermaphrodites with normal gonad timing. Significance was calculated for pnc-1 compared to wild type on normal media and for unsupplemented pnc-1 or wild type to their counterparts supplemented with 1 mM NAD+. n= number of animals scored. Asterisks in both panels indicate a p value < 0.05, calculated using Fisher’s Exact test.
Both NA and NAD+ rescue the pnc-1 spicule morphology defect, while exogenous NAM substrate had no affect on spicule morphology of wild-type males (Fig. 6A). This suggests that male spicule morphology is more sensitive to changes in the concentration of NAD+ than NAM. When pnc-1 males were cultured with UV-irradiated/dead E. coli as the food source, the penetrance of the spicule morphology defect dramatically increased (Fig. 2B). Presumably further decreases in NAD+ levels in pnc-1 increase the penetrance of the spicule morphology defect. We suggest that live E. coli supplements NAD+ or usable NAD+ precursors to suppress the pnc-1 spicule morphology defect.
Muscle groups are differentially sensitive to NAM and NAD+ levels
Since spicule morphology is dependent on spicule retractor muscles, the pharmacology results suggest that spicule retractor muscles may be specifically sensitive to perturbations in the synthesis of NAD+, but insensitive to NAM accumulation. We assayed prodding behavior and LEV-induced spicule protraction to directly probe the effects of changes in NAM levels versus changes in NAD+ biosynthesis on spicule protractor muscle function. Supplementing pnc-1 males with NA significantly increased their prodding rate: pnc-1 males raised on NA prodded at a rate of 3 sec−1 compared to pnc-1 males raised on NGM at 1.3 sec−1 (p < 0.005) (Fig. 3). Interestingly, we found that exogenous NAM caused a slight but significant decrease in the prodding rate of him-5 males to 3.5 sec−1 when raised on NAM relative to 4.4 sec−1, when raised on NGM (p < 0.0001) (Fig. 3A).
We observed similar results in the LEV-induced protraction assay. NA-treated pnc-1 males respond to LEV with an EC90 of 3.2 µM LEV, the same as wild-type males. Raising wild-type males on NAM mildly impaired response to LEV, EC90 11 µM, an approximately 4-fold increase relative to wild type (Fig 5A). We conclude that restoration of NAD+ biosynthesis, robustly rescues spicule muscle function by all three measures: morphology, prodding and response to LEV, whereas elevated NAM mildly disrupts spicule protractor muscle function selectively.
In contrast to the spicule protractor muscle defect, which is relatively insensitive to NAM accumulation, exogenous NAM induces an egg-laying defect in wild-type hermaphrodites, and supplementation of pnc-1 with NA does not rescue egg-laying (Vrablik et al. 2009). Impaired vulval muscle function likely causes the pnc-1 egg-laying defect (Fig. 4B). We next tested how perturbations in substrate and product accumulation affect vulval muscles using the LEV assay. Consistent with the results of the egg-laying assay, wild-type worms raised on NAM supplemented plates have a dramatically reduced response to LEV (Fig. 4B). Supplementing pnc-1 mutants with NA mildly increases egg-laying response to LEV relative to pnc-1 hermaphrodites raised on NGM, but the difference was not statistically significant (Fig. 4B). Therefore, elevated NAM levels induce a vulval muscle defect, although we do not rule out an important role for NAD+ biosynthesis in the muscle with these experiments.
Intriguingly, body wall muscle function is affected similarly by perturbations in both NAD+ biosynthesis and NAM accumulation. The thrashing defect is partially rescued by supplementing pnc-1 mutants with NA (Fig. 7A), and thrashing is reduced in wild-type animals by excess NAM (Fig. 7B). A likely explanation for this incomplete rescue by NA supplementation is that elevated NAM and reduced NAD+ biosynthesis in pnc-1 mutant body wall muscles cause distinct defects, preventing normal function upon restoration of only NAD+ biosynthesis. These effects of NAM and NA on thrashing are specific as no effects were observed by supplementing wild-type worms with NA or pnc-1 mutants with NAM (not shown).
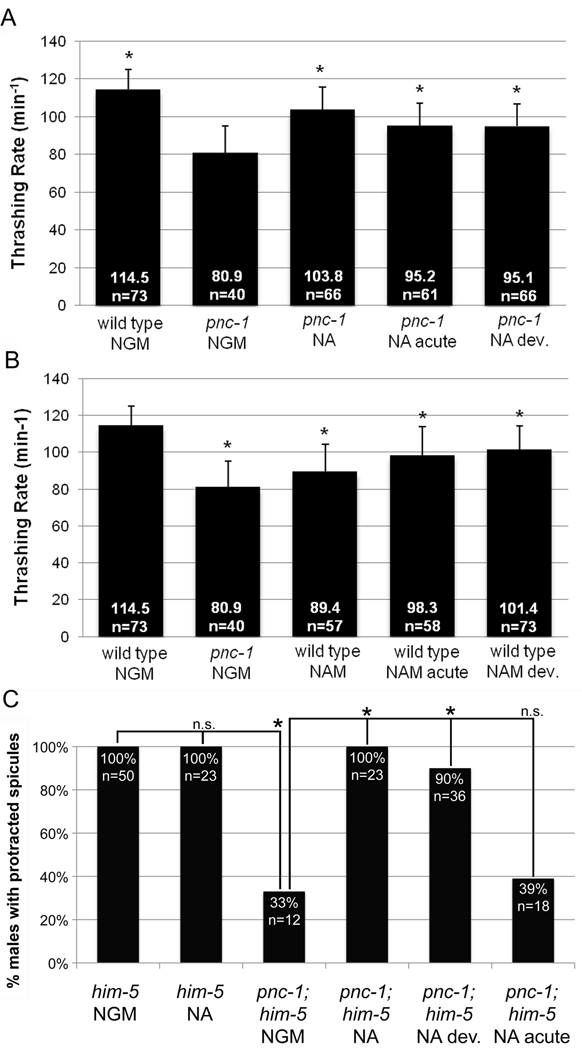
Figure 7. Developmental and acute perturbations in NAM or NAD+ levels have muscle-specific effects on function.
(A) NA has developmental and acute effects on body wall muscle. Columns indicate the thrashing rate. Culture conditions are indicated below the genotype on the x-axis. NGM is normal media. Cultures were supplemented with 37.5 mM NA as indicated. Thrashing rate was rescued to the same extent with 25 mM and 37.5 mM NA (not shown), ruling out the explanation that insufficient NA for full rescue of the thrashing defect may have been supplied. For statistical analysis, the thrashing rate of wild-type animals cultured on normal media and pnc-1 mutants cultured on NA-supplemented media for all or part of their lifetime were each compared to pnc-1 mutants cultured on normal media. Bars indicate standard deviation and asterisks indicate a p value < 0.05, calculated using Student’s t-test. (B) NAM has developmental and acute effects on body wall muscle. Columns indicate the thrashing rate. For statistical analysis, the thrashing rate of pnc-1 mutants cultured on normal media and wild-type animals cultured on 37.5 mM NAM-supplemented media for all or part of their lifetime were each compared to wild-type animals cultured on normal media. Bars indicate standard deviation and asterisks indicate a p value < 0.05 and n.s. indicates not significant, calculated using Student’s t-test. (C) Nicotinic acid has developmental effects on spicule protractor muscles. Columns indicate the percentage of males with protracted spicules in response to 50 µM levamisole. NAM and NA indicate animals cultured in the presence of 25 mM nicotinamide or nicotinic acid. n= number of animals observed in each panel. Asterisks indicate a p value < 0.05 and n.s. indicates not significant, calculated using Fisher’s Exact test.
Thus, in the muscle groups examined, we saw differential sensitivity to changes in NAM levels. NAM is detrimental to muscle function in all but perhaps the spicule retractor muscle, but the vulval muscles are more sensitive to NAM than other muscle types. NAD+ biosynthesis defects contribute to the functional defects of each muscle that could be tested.
Muscles-specific responses to developmental and acute fluctuations in NAM/NAD+ levels
Muscle activity consumes large amounts of energy, and NAD+ has a crucial role in delivering electrons to the electron transport chain for oxidative phosphorylation. Thus, we hypothesized that limited energy availability might underlie some of the muscle function deficits and that restoration of NAD+ biosynthesis would have acute beneficial effects on muscle function in a pnc-1 mutant. We raised pnc-1 on either normal (NGM) or NA-supplemented media and then acclimated them to the opposite condition for 24 or 48 hours (NGM to NA and NA to NGM). We then tested spicule muscle and body wall muscle function because both are significantly disrupted by blocked NAD+ biosynthesis. By both measures of spicule protractor muscle function, prodding rate (Fig. 3) and LEV-induced spicule protraction (Fig. 7C), we found that NA rescued spicule muscle function when supplied during development only but not when supplied only in adulthood. We used the thrashing assay to test body wall muscles. Supplementing pnc-1 mutants with NA during development or only after the L4 stage can both partially rescue the thrashing rate of pnc-1 mutants (Fig. 7A), but this rescue is not as effective as constant supplementation with NA.
We conclude that the lack of NAD+ biosynthesis during development results in defective formation of spicule and body wall muscles with suboptimal functionality in adulthood. The body wall muscles also require ongoing NAD+ biosynthesis in adulthood for optimal muscle activity. Our results suggest that the spicule muscles are relatively insensitive to the presence of ongoing NAD+ biosynthesis in young virgin males since the level of rescue when NA is supplied developmentally and withdrawn 48 hours before the assay is the same as when NA is supplied throughout development and adulthood (Fig. 3).
Since the mechanism(s) whereby NAM affects body wall muscle function are unknown, we investigated if the effects of NAM were developmental or acute. Wild-type animals that had been raised on NGM or NAM-supplemented media were moved to the new condition (e.g. NGM to NAM) at mid-L4. We scored the thrashing rate 24 hours later. NAM supplementation to wild-type worms during development or acutely as adults both partially decrease thrashing rate of wild type, but this decrease is not as severe as constant supplementation with NAM (Fig. 7B). Thus, appropriately low levels of NAM are required developmentally and acutely to ensure proper function of body wall muscles in adulthood.
Discussion
Understanding the biological effects of the molecules NAM and NAD+ (and other NAD+ metabolites/ precursors) is required in order to determine how NAD+ salvage pathways impact metabolism, NAD+ consumer activity or other biological activity. We found that muscles are differentially sensitive to in vivo perturbations of NAM concentration versus NAD+ biosynthesis by examining both striated (body wall muscle) and non-striated (sex muscles) muscle in C. elegans. In the muscles that are sensitive to NAD+ biosynthesis, NA-supplementation is beneficial, and in muscles that are sensitive to NAM, NAM supplementation is detrimental. Thus, PNC-1 and the NAM-NAD+ salvage biosynthesis pathway consistently play a positive role in promoting muscle development and function in C. elegans, and NAD+ salvage impacts development of muscle in invertebrates as well as vertebrates.
The exclusively positive role of PNC-1 in promoting development and function of distinct muscle types contrasts with studies in vertebrates. First, NAMPT directly affects the differentiation of specific muscle types in culture systems, whereas PNC-1 is not strictly required for muscle differentiation in vivo. Nonetheless, negative effects due to NAM accumulation and compromised NAD+ biosynthesis in C. elegans do occur during development, suggesting that muscle differentiation in pnc-1 mutants results in muscles with suboptimal functionality. Moreover, a positive role for NAMPT in skeletal muscle function after differentiation has not been excluded. NAMPT promotes cell survival under conditions of stress in cardiac muscle (Hsu et al., 2009), but its acute affects on skeletal muscle function are unknown. Second, while PNC-1 consistently exerts positive effects on distinct muscle types, NAMPT has opposite effects in differentiation of vertebrate smooth muscle and skeletal muscle (Fulco et al., 2008; van der Veer et al., 2005).
As repeated contraction of muscle is an energetically costly process, one can readily envision that biosynthesis of NAD+, because of its critical role in oxidative phosphorylation, would promote muscle activity. However, the fact that NAM accumulation and NAD+ deficiency affect a developmental event(s) that is manifested in adult muscle suggests that the muscle defects are not solely the passive result of reduced energy availability, although this could contribute. Instead, at least some of the effects are likely to arise from perturbation of specific developmental events. The NAD+ consumer SIRT1 mediates the effects of NAMPT on skeletal muscle differentiation, and evidence suggests that sirtuins may mediate the effects of NAMPT in smooth muscle differentiation (Fulco et al., 2008; van der Veer et al., 2005). What detrimental effects might NAM have on muscle development and activity in C. elegans? If the observed effects of NAM are caused by inhibition of a sirtuin, we would expect the sirtuin to have a positive role in muscle development and function. However, we have failed to detect any evidence of muscle defects (assayed by egg laying and thrashing) in sirtuin mutants, including the SIRT1 homolog sir-2.1(ok434) mutant (unpublished). It remains to be seen if regulation of other NAD+ consumers may underlie the muscle phenotypes. The relative importance of promoting NAD+ biosynthesis versus consuming NAM to positively regulate NAD+ consumers has been debated (Anderson et al., 2003; Lin et al., 2004). These results and our previous work (Vrablik et al., 2009) suggest that the most relevant role of the NAD+ salvage pathway in regulating NAD+ consumers or any other function is likely to be cell type specific. Furthermore, we suggest that deficiencies in NAD+ production or accumulation of NAM during development can have subtle effects on muscle function in adulthood.
Because of their key role in promoting longevity and other vital biological processes, NAD+ consumers and the pathways that regulate them are appealing therapeutic targets. Similar to our rescue experiments with NA, glucose-stimulated insulin secretion defects in NAMPT−/+ heterozygous mice can be corrected by supplementation with nicotinamide mononucleotide (NMN), the NAMPT product (Revollo et al., 2007). Based on these experiments, therapeutic manipulation of the biological effects of NAD+ consumers via dietary supplementation with NAD+ biosynthesis intermediates has been suggested (Imai, 2010). This has led to the proposal that age-related declines in NAMPT-mediated biosynthetic activity (Imai, 2010; Ramsey et al., 2008) may be ameliorated by supplementation with NMN. Given that NAMPT activity is likely to be broadly required in many cells and tissues, it seems important to keep in mind that while promoting NAD+ biosynthesis may alleviate some issues associated with declining NAMPT activity during aging, it does nothing to prevent potential damaging effects of substrate (NAM) accumulation that may accompany such declines.
Supplementary Material
Acknowledgment
We thank David J. Moore for his work on cross progeny assays, Andy Singson for advice on sperm assays, and Rene Garcia for advice on mating assays. We also thank Melissa Rolls and other members of the Hanna-Rose lab for helpful discussions. This research was supported by grants to W.H-R. from the National Science Foundation (IOS-0718675) and the NIH (R01-GM086786).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989;3:473–485. doi: 10.1016/0896-6273(89)90206-7. [DOI] [PubMed] [Google Scholar]
- Barr MM, Garcia LR. Male mating behavior. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.78.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–19. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab. 2010;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 Regulates Skeletal Muscle Differentiation as a Potential Sensor of the Redox State. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. doi: 10.1158/0008-5472.CAN-09-2465. [DOI] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW. Regulation of Distinct Muscle Behaviors Controls the C. elegans Male's Copulatory Spicules during Mating. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Emmons SW. Episodic swimming behavior in the nematode C. elegans. J Exp Biol. 2008;211:3703–3711. doi: 10.1242/jeb.023606. [DOI] [PubMed] [Google Scholar]
- Hosono R. Sterilization and growth inhibition of Caenorhabditis elegans by 5-fluorodeoxyuridine. Experimental Gerontology. 1978;13:369–373. doi: 10.1016/0531-5565(78)90047-5. [DOI] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S. A possibility of nutriceuticals as an anti-aging intervention: activation of sirtuins by promoting mammalian NAD biosynthesis. Pharmacol Res. 2010;62:42–47. doi: 10.1016/j.phrs.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LI, Sternberg PW. Socket Cells Mediate Spicule Morphogenesis in Caenorhabditis elegans Males. Developmental Biology. 1999;211:88–99. doi: 10.1006/dbio.1999.9293. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Liu LY, Hanna-Rose W, Chamberlin HM. The Caenorhabditis elegans heterochronic gene lin-14 coordinates temporal progression and maturation in the egg-laying system. Developmental Dynamics. 2009;238:394–404. doi: 10.1002/dvdy.21837. [DOI] [PubMed] [Google Scholar]
- Kirkland JB. Poly ADP-ribose polymerase-1 and health. Exp Biol Med (Maywood) 2010;235:561–568. doi: 10.1258/ebm.2010.009280. [DOI] [PubMed] [Google Scholar]
- Krzysik-Walker SM, Ocon-Grove OM, Maddineni SR, Hendricks GL, III, Ramachandran R. Is Visfatin an Adipokine or Myokine? Evidence for Greater Visfatin Expression in Skeletal Muscle than Visceral Fat in Chickens. Endocrinology. 2008;149:1543–1550. doi: 10.1210/en.2007-1301. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182. doi: 10.1002/9780470123195.ch5. xi. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB. Acetylcholine. WormBook. 2007:1–21. doi: 10.1895/wormbook.1.131.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Rongvaux A, Andris F, Van Gool F, Leo O. Reconstructing eukaryotic NAD metabolism. Bioessays. 2003;25:683–690. doi: 10.1002/bies.10297. [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA. NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther. 2008;324:883–893. doi: 10.1124/jpet.107.120758. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: Postembryonic development of nongonadal structures. Dev. Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Tang H, Cheung WMW, Ip FCF, Ip NY. Identification and Characterization of Differentially Expressed Genes in Denervated Muscle. Molecular and Cellular Neuroscience. 2000;16:127–140. doi: 10.1006/mcne.2000.0864. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer E, Nong Z, O'Neil C, Urquhart B, Freeman D, Pickering JG. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34. doi: 10.1161/01.RES.0000173298.38808.27. [DOI] [PubMed] [Google Scholar]
- Vinciguerra M, Fulco M, Ladurner A, Sartorelli V, Rosenthal N. SirT1 in muscle physiology and disease: lessons from mouse models. Dis Model Mech. 2010;3:298–303. doi: 10.1242/dmm.004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrablik TL, Huang L, Lange SE, Hanna-Rose W. Nicotinamidase modulation of NAD+ biosynthesis and nicotinamide levels separately affect reproductive development and cell survival in C. elegans. Development. 2009;136:3637–3646. doi: 10.1242/dev.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas J. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.