Abstract
The AE3 Cl−/HCO3− exchanger is abundantly expressed in the sarcolemma of cardiomyocytes, where it mediates Cl−-uptake and HCO3−-extrusion. Inhibition of AE3-mediated Cl−/HCO3− exchange has been suggested to protect against cardiac hypertrophy; however, other studies indicate that AE3 might be necessary for optimal cardiac function. To test these hypotheses we crossed AE3-null mice, which appear phenotypically normal, with a hypertrophic cardiomyopathy mouse model carrying a Glu180Gly mutation in α–tropomyosin (TM180). Loss of AE3 had no effect on hypertrophy; however, survival of TM180/AE3 double mutants was sharply reduced compared with TM180 single mutants. Analysis of cardiac performance revealed impaired cardiac function in TM180 and TM180/AE3 mutants. TM180/AE3 double mutants were more severely affected and exhibited little response to β-adrenergic stimulation, a likely consequence of their more rapid progression to heart failure. Increased expression of calmodulin-dependent kinase II and protein phosphatase 1 and differences in methylation and localization of protein phosphatase 2A were observed, but were similar in single and double mutants. Phosphorylation of phospholamban on Ser16 was sharply increased in both single and double mutants relative to wild-type hearts under basal conditions, leading to reduced reserve capacity for β-adrenergic stimulation of phospholamban phosphorylation. Imaging analysis of isolated myocytes revealed reductions in amplitude and decay of Ca2+ transients in both mutants, with greater reductions in TM180/AE3 mutants, consistent with the greater severity of their heart failure phenotype. Thus, in the TM180 cardiomyopathy model, loss of AE3 had no apparent anti-hypertrophic effect and led to more rapid decompensation and heart failure.
Keywords: Slc4a3, decompensated, force frequency response, congestive heart failure
1. Introduction
Anion exchanger isoform 3 (AE3, gene symbol Slc4a3) is one of at least four Cl−/HCO3− exchangers in cardiac myocytes. It consists of two protein variants, a longer full-length form (AE3fl) that is expressed in brain and other tissues and a cardiac-specific form (AE3c) that is expressed at very high levels in heart [1–3]. In addition to AE3, the heart contains moderate levels of both AE1 (Slc4a1) and AE2 (Slc4a2) and high levels of PAT1 (Slc26a6), which can mediate both Cl−/HCO3− and Cl−/OH− exchange [4,5]. Why the heart requires such a high capacity for Cl−/HCO3− exchange is unclear; however, it contributes to regulation of intracellular pH (pHi) [6]. In addition, by operating in concert with Na+-dependent acid extrusion mechanisms such as Na+/H+ exchange or Na+ -HCO3− cotransport, it can contribute to pHi-neutral Na+ uptake [7,8].
Inhibition of Cl−/HCO3− exchange in heart has been suggested as a possible cardioprotective strategy in treatment of hypertrophy [9], ischemia-reperfusion (I/R) injury [10,11], and arrhythmias [2]. There is evidence that AE3-mediated Cl−/HCO3− exchange in cardiac myocytes is increased during hypertrophy and that AE3 operates in concert with the NHE1 Na+/H+ exchanger [8]. However, because of the lack of specific inhibitors and the abundance and diversity of Cl−/HCO3− exchangers in heart, it is unclear whether AE3 or one or more of the other isoforms in heart is coupled with NHE1. Although NHE1 operating by itself leads to cytosolic alkalinization, which in turn would limit its activity, extrusion of HCO3− by AE3 and/or other Cl−/HCO3− exchangers would provide a balancing acidification mechanism, potentially enhancing NHE1 activity and facilitating Na+-loading. With both transporters operating together, uptake of Na+ and Cl− would occur with little change in pHi, and increased subsarcolemmal Na+ could affect Ca2+ homeostasis via modulation of Na+/Ca2+ exchange.
Loss of NHE1 activity is cardioprotective both in hypertrophy and in I/R injury [13], so if loss of AE3-mediated Cl−/HCO3− exchange reduces NHE1 activity it could be cardioprotective. On the other hand, in studies of the spontaneously hypertensive rat using an inhibitory antibody directed against AE3, no reduction in hypertrophy was observed [9]. Also, genetic ablation of AE3 in mouse had no protective effect on cardiac I/R injury as observed in the Langendorff heart model [14] and the combined loss of AE3 and the NKCC1 Na+-K+-2Cl− cotransporter, neither of which by itself impaired cardiac performance, led to reduced contractility [14]. Thus, even if inhibition of Cl−/HCO3− exchange could serve cardioprotective functions under some conditions, the specific role of AE3-mediated Cl−/HCO3− exchange is unclear and it is possible that one or more of the other Cl−/HCO3− exchanger isoforms in heart might be responsible.
To better understand the functions and importance of AE3 in the heart and to assess whether loss of its activity might be cardioprotective, we analyzed double mutant mice carrying null mutations in AE3 and harboring a Glu180Gly mutation in α-tropomyosin (TM180) [15]. Previous studies indicated that loss of AE3 alone had no adverse effects on cardiac performance [14]. The mutant α-tropomyosin transgenic mice, which serve as a model for a familial hypertrophic cardiomyopathy that occurs in humans [16], develop cardiac hypertrophy and begin dying at about 4 months of age [15]. Although the degree of hypertrophy in TM180 mutants was unaffected by the loss of AE3, double mutants exhibited severe deficits in cardiac performance, β-adrenergic responses, and Ca2+ handling, and they developed heart failure much more rapidly than TM180 single mutant mice. These results show that AE3 plays an important role in maintaining cardiac function in the TM180 model of familial hypertrophic cardiomyopathy and that ablation of its activity does not have any apparent cardioprotective effects in this model.
2. Methods
2.1. Generation of mutant mice
Gene-targeted AE3 heterozygous mutant (AE3+/−) mice [14] and transgenic mice (line 57) carrying a Glu180Gly mutation (TM180) in α-tropomyosin [15], both on an inbred FVB/N genetic background, were used for the generation of mice used in these experiments. All procedures conformed to guidelines published by the National Institutes of Health (Guide for the Care and Use of Laboratory Animals; Publication No. 86-23, revised 1996) and were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
2.2. Evaluation of cardiac function and force-frequency analysis in vivo
Analysis of cardiovascular function was performed as described previously [17,18]. Mice were anesthetized with ketamine and inactin (50 and 100 μg/g bodyweight), the right femoral artery was cannulated with a catheter connected to a pressure transducer for measurement of mean arterial pressure (MAP) and the right femoral vein was cannulated for delivery of drugs. A high fidelity pressure transducer (Millar Instruments, Houston, TX), advanced into the left ventricle (LV) via the right carotid artery and ascending aorta, was used to analyze LV function. A PowerLab data-acquisition system (ADInstruments, Colorado Springs, CO) connected to a Macintosh computer was used to record and analyze data. For force-frequency analyses, mice were anesthetized, surgically instrumented as described above, and heart rates were electrically paced using a 1.0 F bipolar pacing wire advanced into the right atrium via the jugular vein [19].
2.3. Immunoblot analyses
Mice were anesthetized with 2.5% Avertin (15 μl/g bodyweight) and allowed to stabilize for 10 minutes on a thermally controlled heating pad. Ventricles were collected, snap-frozen in liquid nitrogen, and stored at −80 ºC for future processing. In studies designed to analyze phosphorylation of phospholamban (PLN) in response to β-adrenergic stimulation, ventricles were collected from mice that had been anesthetized with ketamine and inactin and surgically instrumented as described in the previous section. Ventricular homogenates were prepared in 10 mM Tris-HCl (pH 7.4) buffer containing 1 mM EDTA, 2 mM DTT, 0.25% Nonidet P-40, 0.5% Triton X-100, and both protease and phosphatase inhibitors (Sigma). Extraction of myofibrillar fractions was performed as reported before [14]. Protein was estimated using a modified Bradford assay (Thermo Scientific), separated by SDS-PAGE, transferred onto nitrocellulose or PVDF membranes, incubated with primary antibodies followed by the corresponding secondary antibodies, and protein signals were obtained using the KPL LumiGlo chemiluminescent substrate system (KPL, Gaithersburg MD, USA). The primary antibodies for SERCA2a, PLN, phospho-Ser16 and phospho-Thr17 of PLN, the NCX1 Na+/Ca2+ exchanger, protein phosphatase 1 catalytic subunit (PP1), and the non-methylated form of protein phosphatase 2A catalytic subunit (PP2A) were the same as published before [14]. Analysis of total PP2A after cold base treatment to demethylate the C-terminus was as described before [14]. Also used were antibodies against a common epitope of the full-length and cardiac variants of AE3 [2], the L-type Ca2+ channel α2 subunit (catalog number MA3-921; Thermo Scientific), the ryanodine receptor (catalog number MA3-916; Thermo Scientific), and calmodulin-dependent kinase II (CamKII) (a pan-CamKII antibody that recognizes δ and other isoforms; catalog number 611292; BD Transduction Laboratories).
2.4. Preparation of cardiomyocytes and analysis of Ca2+ transients
Ventricular myocytes were prepared from adult mouse hearts mounted in a Langendorff apparatus as described previously [20]. The hearts were perfused for 5 min at 37 ºC with a modified Krebs-Henseleit buffer solution containing (in mM): NaCl 113, KCl 4.7, KH2PO4 0.6, MgSO4.7H2O 1.2, NaHCO3 10, HEPES 10, taurine 30, 2,3-butanedione monoxime (BDM) 10 and glucose 5.5 (pH 7.46). The perfusion buffer was then switched to buffer containing Liberase Blendzyme IV (Roche) at 0.25 mg/mL and trypsin (Invitrogen) at 0.14 mg/mL with no BDM. After 8–12 min of perfusion, hearts became swollen and flaccid and ventricles were dissected and dispersed by gently pipetting through a plastic pipette. Isolated cardiomyocytes were sieved to remove debris and Ca2+ was gradually introduced to 1.2 mM final concentration.
Cells were loaded with Fura2-AM (Molecular Probes Inc.; Eugene, OR) at a final concentration of 2 μM for 15-20 min at room temperature in the dark, with 0.5 mM probenecid (Molecular Probes) as an inhibitor of Fura-2AM efflux, and then washed for 20 min in perfusion buffer (containing 1.2 mM Ca2+ but not BDM). Fluorescence measurements were obtained with a dual-beam spectrofluorophotometer (PTI International, Birmingham, NJ) at room temperature (22 ± 1 ºC) after field stimulation at 0.5 Hz and Ca2+ transient amplitudes were obtained by calculating the fluorescence ratios at 340-to-380 nm (R340/380). Data were acquired using Felix 3.01 acquisition software (PTI International) and analyzed using Ionoptix Ionwizard Analysis Software (IonOptix LLC, Milton, MA).
2.5. Statistics
Values are presented as means ± standard error (SE). Survival analysis was performed by the Kaplan-Meier procedure, with statistical significance determined by log-rank analysis. For other procedures, one-way analysis of variance (ANOVA) was used along with two-sided Student's t-test, and a P-value of < 0.05 was considered significant.
3. Results
3.1 Effects of AE3 ablation on survival and hypertrophy in TM180 mice
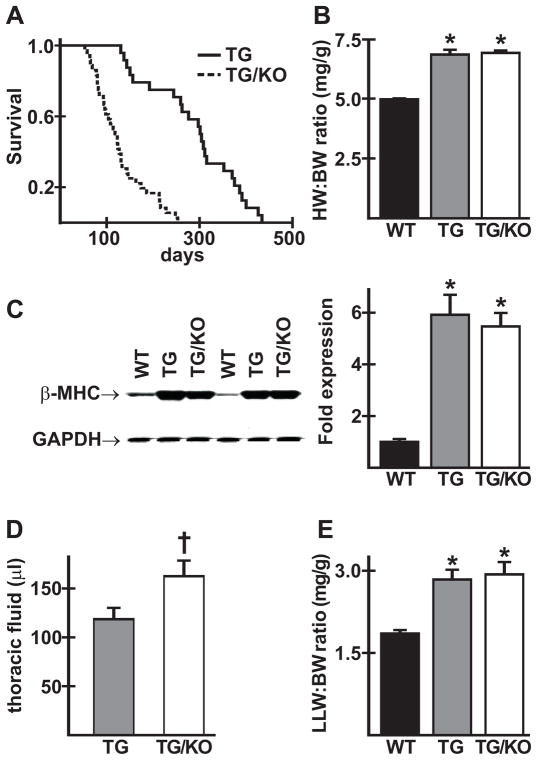
AE3 heterozygous (AE3+/−) female mice were bred with AE3+/− males harboring the TM180 mutation. Offspring of the expected genotypes, including TM180/AE3 double mutants, were born in normal Mendelian ratios, indicating that the mutations caused no increase in embryonic or fetal death rates. When TM180 single mutants and TM180/AE3 double mutants were aged, double mutants appeared sicker than single mutants at 2–3 months of age, as indicated by rough coats, labored breathing, and reduced activity, and males appeared to be more severely affected than females. Survival analysis (Fig. 1A) showed a dramatic reduction in survival of TM180/AE3 double mutants when compared with TM180 single mutants. However, hypertrophy, as measured by heart weight/body weight ratios, was the same in both single and double mutants (Fig. 1B) and increased expression of the β-myosin heavy chain, a marker of hypertrophy, was the same in both mutants (Fig. 1C). Thoracic fluid, which was negligible in wild-type (WT) mice, was significantly greater in TM180/AE3 double mutants than in TM180 single mutants (Fig. 1D). Compared with WT controls, left lung weight/body weight ratios were elevated to the same degree in both single and double mutants (Fig. 1E).
Fig. 1.
TM180/AE3 mutant mice exhibit reduced survival but no change in hypertrophy compared to TM180 single mutants. (A) Survival curves of transgenic TM180 mice (TG) and TG mice with AE3 knocked out (TG/KO); n = 26 TG and 38 TG/KO male mice; p < 0.0001 by Kaplan-Meier log-rank analysis. (B) Heart weight/body weight ratios for 2.5-month-old male and female wild-type (WT), TG, and TG/KO mice showed similar hypertrophy in TG (6.84 ± 0.02 mg/g) and TG/KO (6.91 ± 0.01) compared with WT (4.97 ± 0.04); n = 20 WT, 22 TG, and 23 TG/KO mice; *p < 0.001 vs WT. (C) Immunoblot analysis showed upregulation of β-myosin heavy chain (β-MHC) in ventricles of 2.5- or 3-month-old TG and TG/KO mice; n = 6 males of each genotype; *p < 0.01 vs WT. (D) Accumulation of thoracic fluid was higher in 2.5-month-old TG/KO than in TG mice; n = 17 TG and 14 TG/KO male and female mice; †p < 0.05 vs TG. (E) Left lung weight/body weight ratios (LLW/BW in mg/g) were similarly elevated in 2.5-month-old male and female TG and TG/KO mice; n = 9 WT, 8 TG and 10 TG/KO; *p < 0.01 vs WT.
3.2 Immunoblot analysis of NHE1 and AE3 levels in wild-type and mutant hearts
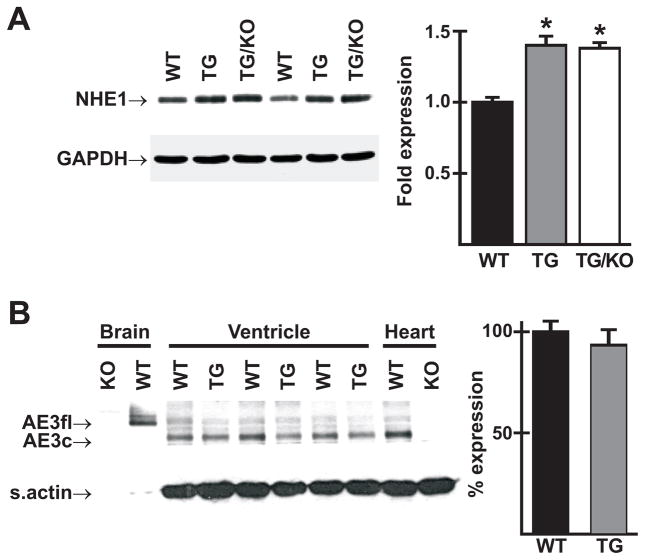
NHE1, which is known to be upregulated in hypertrophic hearts, was increased to the same degree in both single and double mutants (Fig. 2A). mRNA encoding the full-length form of AE3 was reported to be upregulated in hypertrophic hearts of the spontaneously hypertensive rat (SHR), with a corresponding reduction in levels of the cardiac AE3 mRNA [12]. Using an antibody that identifies both the full-length and cardiac variants of AE3, high expression of AE3fl was observed in WT brain, with no expression of AE3c (Fig. 2B). In contrast, high levels of AE3c but only low levels of AE3fl were expressed in ventricles of WT and TM180 single mutant hearts. AE3 expression in ventricles of hypertrophied TM180 hearts was almost identical to that seen in WT ventricles (Fig. 2B). Similar levels were seen in whole heart homogenates of WT mice, whereas no expression was detected in AE3-null hearts.
Fig. 2.
NHE1 and AE3 protein expression in ventricles of mutant and wild-type mice. (A) Immunoblot analysis revealed increased expression of NHE1 in ventricles of 3-month-old TM180 transgenic (TG) and TM180/AE3 double mutant (TG/KO) mice; n = 6 male mice of each genotype; *p < 0.001 vs WT. (B) Immunoblotting using an AE3 antibody that identifies both full length (AE3fl) and cardiac (AE3c) forms of AE3 revealed no significant change in ventricles of TM180 single mutant (TG) vs. WT male mice. Note high expression of AE3fl and AE3c in WT brain and whole heart, respectively, and absence of these variants in KO brain and whole heart.
To determine whether expression of any of the other cardiac Cl−/HCO3− exchangers might be altered in response to the loss of AE3, RT-PCR analysis was performed using RNA samples from WT, AE3-null, TM180, and TM180/AE3 hearts (Supplementary Fig. 1). mRNA encoding AE2, but not AE1 or Slc26a6, was significantly increased in AE3-null relative to WT hearts. There was no significant difference in the expression of any of these anion exchanger mRNAs between TM180 single and TM180/AE3 double mutant hearts; however, both Slc26a6 and AE1 mRNAs were decreased in TM180 and TM180/AE3 hearts relative to WT hearts.
3.3 Force-frequency relationships in vivo
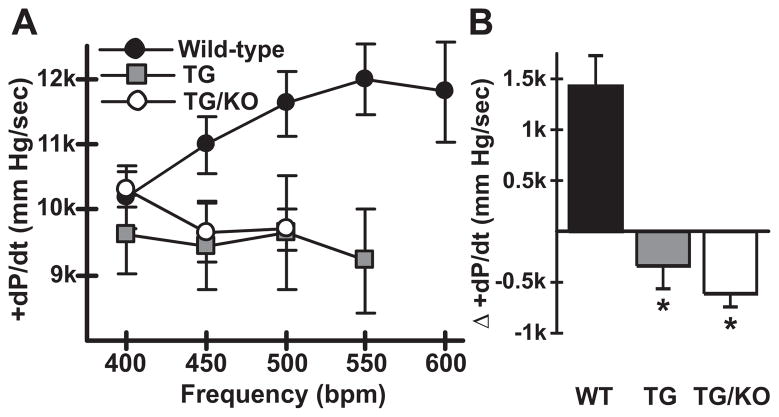
The data in Fig. 1 suggested that loss of AE3 in TM180 mutant mice caused a worsening of the heart failure phenotype. As heart failure is associated with a negative force-frequency response (FFR), in vivo pacing studies were performed using WT and both single and double mutant mice. In WT mice, an increase in heart rate from 400 to 550 beats per min (bpm) led to the expected increase in +dP/dt (from 10189 ± 470 to 11986 ± 542 mm Hg/sec; p<0.05), with a slight reduction occurring as heart rate was further increased to 600 bpm (Fig. 3A). In contrast, hearts of TM180/AE3 and TM180 mice did not exhibit an increase in contractility as heart rate was increased and could not be efficiently paced beyond 500–550 bpm. TM180/AE3 mice appeared to be less able than TM180 single mutants to achieve or sustain heart rates at the higher frequencies. When the differences between +dP/dt at 400 and 500 bpm were calculated, a positive FFR was apparent in WT mice, whereas both TM180 and TM180/AE3 mutants exhibited a negative FFR (Fig. 3B). The results were essentially the same when differences in +dP/dt at 40 mm Hg (dP/dt40) between 400 and 500 bpm were calculated (data not shown).
Fig. 3.
TM180 (TG) and TM180/AE3 (TG/KO) mutants exhibit a negative force-frequency response. Hearts of anesthetized surgically-instrumented 3-month-old mice were subjected to atrial pacing beginning at 400 beats per minute (bpm) and contractile parameters were measured. n = 5 WT, 4 TG, and 5 TG/KO mice, with 2 males and either 2 or 3 females of each genotype. WT mice could be paced to 550 and 600 bpm but some TG and TG/KO mice could not. If fewer than 3 mice could achieve a given frequency, such as TG/KO at 550 bpm, the data were not plotted. (A) A positive FFR with respect to maximum +dP/dt (mm Hg/sec) was observed in WT mice but not in TG and TG/KO mice. (B) Difference in +dP/dt at 400 bpm and 500 bpm revealed a negative FFR in TG and TG/KO mice. *p < 0.02 vs WT.
3.4 Effects of AE3 ablation on cardiac performance in TM180 mice
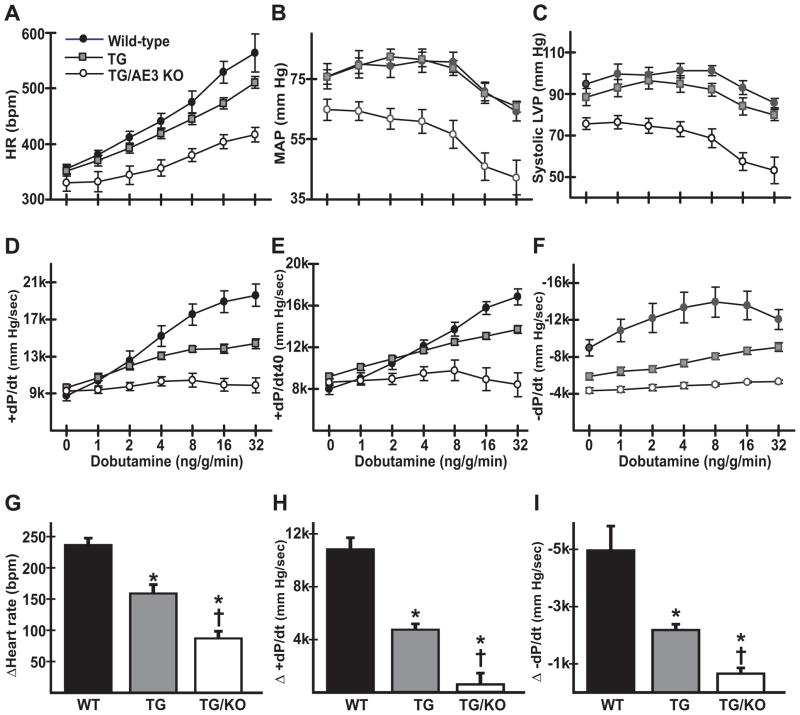
Cardiovascular performance of anesthetized WT, TM180, and TM180/AE3 mice was analyzed, with measurements conducted under both basal conditions and following β-adrenergic stimulation via infusion of dobutamine (Fig. 4). Previous studies of WT and AE3-null mice using the same protocols showed that loss of AE3 by itself caused no significant changes in heart rate, MAP, or cardiac performance [14]. During β-adrenergic stimulation, heart rate (Fig. 4A) was reduced in TM180/AE3 mutants compared with WT and TM180 single mutants (TM180/AE3, 417 ± 15 bpm; TM180, 510 ± 9 bpm; WT, 563 ± 8 bpm; p<0.01), and MAP was also reduced in double mutants under both basal and stimulated conditions (Fig. 4B). Systolic left ventricular pressure was lower in double mutants relative to both TM180 and WT mice under basal conditions (TM180/AE3, 79.8 ± 3.2 mm Hg; TM180, 93.4 ± 3.4 mm Hg; WT, 98.8 ± 4.6 mm Hg; p<0.05) and after β-adrenergic stimulation (Fig. 4C). β-adrenergic stimulation of contractility, as indicated by measurements of maximum +dP/dt and +dP/dt40, and relaxation, as measured by minimum −dP/dt, were significantly reduced in TM180/AE3 mutants relative to both WT and TM180 mice (Fig. 4D-F). When differences between values obtained under basal conditions and after maximum β-adrenergic stimulation were calculated, significant differences in stimulation of heart rate, contractility, and relaxation were observed between all three genotypes (Fig. 4G-I), with TM180/AE3 double mutants being more severely affected than TM180 single mutants.
Fig. 4.
β-adrenergic stimulation of cardiovascular performance is severely reduced in TM180/AE3 double mutants. Pressure measurements were recorded using transducers in the left ventricle and right femoral artery of anesthetized 2.5-month-old WT, TM180 (TG), or TM180/AE3 (TG/KO) mice of each genotype under basal conditions and in response to β-adrenergic stimulation (intravenous infusion of increasing doses of dobutamine). Heart rate (A), mean arterial pressure (B), systolic left ventricular pressure (C), maximum +dP/dt in mm Hg/sec (D), +dP/dt at 40 mm Hg (E), and minimum −dP/dt in mm Hg/sec (F) are shown for WT, TG, and TG/KO mice. Differences between basal and maximum values during β-adrenergic stimulation are shown for heart rate (G), +dP/dt (H), and -dP/dt (I). n = 7 WT (4 female, 3 male), 9 TG (5 female, 4 male), and 6 TG/KO (4 female, 2 male) mice. *p < 0.05 vs WT, †p < 0.05 vs TG.
3.5. Expression of proteins involved in Ca2+ handling
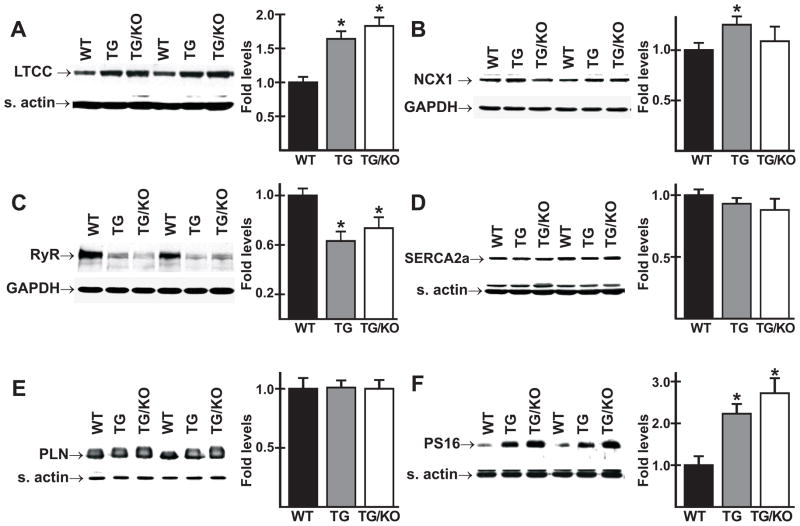
Alterations in proteins involved in Ca2+ handling can have a major impact on cardiac performance. The primary mechanisms for influx and extrusion of Ca2+ across the sarcolemma are the L-type Ca2+ channel (LTCC) and the NCX1 Na+/Ca2+ exchanger. Immunoblot analysis revealed increased expression of the α2 subunit of the LTCC in cardiac homogenates of both TM180/AE3 and TM180 mice compared with those of WT mice (Fig. 5A). Relative to levels in WT hearts, expression of NCX1 was increased modestly but significantly in TM180 hearts, but not in TM180/AE3 hearts (Fig. 5B). Expression of the ryanodine receptor, the Ca2+ release channel of the sarcoplasmic reticulum (SR), was reduced in both mutants (Fig. 5C).
Fig. 5.
Expression of proteins involved in Ca2+ handling. Immunoblot analysis was performed using homogenates of ventricles from 2.5- or 3-month-old male WT, TM180 (TG), and TM180/AE3 (TG/KO) mice. Representative immunoblots and relative expression levels are shown for the L-type Ca channel, (A), NCX1 Na+/Ca2+ exchanger (B), ryanodine receptor (C), SERCA2a Ca2+ pump (D), phospholamban (E), and PLN phosphorylated on Ser16 (PS16) (F). n = at least 6 mice of each genotype, except for panel E, in which n = 3 of each genotype; *p < 0.05 vs WT.
Sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2a (SERCA2a) is the SR Ca2+ pump, and phospholamban (PLN) is the major regulator of SERCA2a activity [21]. When PLN is in the non-phosphorylated form it inhibits SERCA2a activity, but these inhibitory effects are alleviated when PLN is phosphorylated on Ser16 and/or Thr17 [21]. Immunoblot analysis of cardiac homogenates from WT, TM180, or TM180/AE3 mice revealed no significant differences in either SERCA2a (Fig. 5D) or PLN (Fig. 5E) expression. However, levels of PLN phosphorylated on Ser16 (Fig. 5F) were sharply upregulated in TM180 and TM180/AE3 hearts when compared to WT hearts. Slightly higher levels of phosphorylation were observed in TM180/AE3 hearts than in TM180 hearts, but the differences between double and single mutants were not statistically significant.
3.6. Expression or localization of CamKII and protein phosphatases 1 and 2A
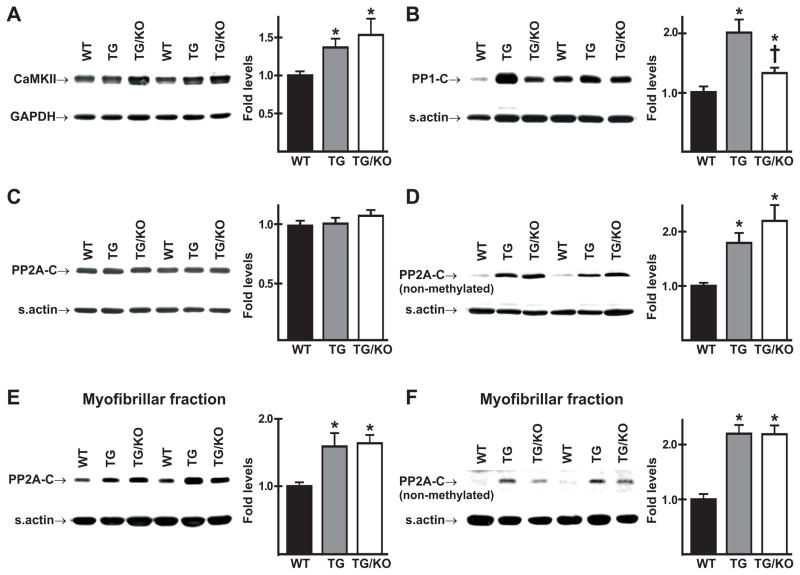
Immunoblot analysis showed that CamKII, which is known to be upregulated in heart failure [22], was increased in cardiac homogenates of both mutants, with slightly higher levels in double mutants (Fig. 6A). The catalytic subunit of protein phosphatase 1 (PP1-C), which plays a major role in dephosphorylation of Ser16 of PLN [23], was increased in both mutants when compared to WT controls (Fig. 6B), with significantly higher levels in TM180 hearts than in TM180/AE3 hearts. In contrast, no differences in total levels (carboxymethylated and nonmethylated) of the catalytic subunit of protein phosphatase 2A (PP2A-C) in cardiac homogenates were observed between any of the genotypes (Fig. 6C). However, the levels of nonmethylated-PP2A-C were significantly increased in cardiac homogenates of both TM180 and TM180/AE3 hearts, with slightly higher levels in TM180/AE3 hearts (Fig. 6D), and both total PP2A-C and nonmethylated PP2A-C were increased in the myofibrillar fractions of TM180 and TM180/AE3 hearts (Figs. 6E and F).
Fig. 6.
Expression of Ca2+-calmodulin-dependent protein kinase II (CaMKII) and catalytic subunits of protein phosphatases PP1 and PP2A (PP1-C and PP2A-C). Immunoblot analysis was performed using homogenates of ventricles (A-D) or myofibrillar fractions (E,F) from 2.5-month-old male WT, TM180 (TG), and TM180/AE3 (TG/KO) mice. Representative immunoblots and relative expression levels are shown for (A) CamKII, (B) PP1-C, (C) total PP2A-C (non-methylated and methylated), (D) non-methylated form of PP2A-C, (E) total (non-methylated and methylated) PP2A-C associated with the myofribrillar fraction, (F) non-methylated PP2A-C associated with the myofribrillar fraction. n = at least 6 male mice of each genotype. *p < 0.05 vs WT; †p < 0.05 vs TG.
3.7. Analysis of reserve capacity for β-adrenergic stimulation of PLN phosphorylation in vivo
The studies in Fig. 5 showed that phosphorylation of PLN on Ser16, which is the major mechanism by which β-agonists stimulate contractility [24], is increased 2.2 ± 0.2-fold in TM180 hearts and 2.7 ± 0.4-fold in TM180/AE3 hearts under basal conditions. This suggests that they have a reduced reserve capacity for β-adrenergic stimulation, which could be involved in their impaired cardiac performance in response to dobutamine (Fig. 4). To test this hypothesis, we analyzed the phosphorylation of PLN in hearts of WT mice under both basal and stimulated conditions and in mice of all three genotypes after maximum β adrenergic stimulation.
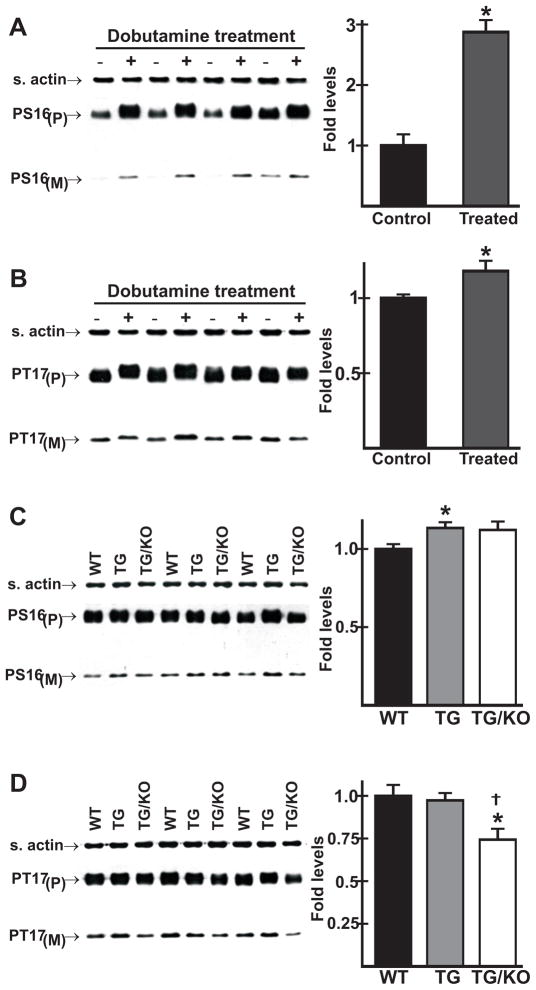
In a control experiment, we first compared Ser16 phosphorylation under basal conditions in ventricular homogenates from WT mice anesthetized as in Fig. 5 with samples prepared using the same surgical and anesthesia conditions used in Fig. 4. Ser16 phosphorylation was the same in hearts of avertin-treated or ketamine and inactin-treated surgically-instrumented mice, indicating that the surgical treatment did not affect Ser16 phosphorylation (data not shown). In surgically-instrumented WT mice, dobutamine treatment led to a 2.8-fold increase in phosphorylation of Ser16 (Fig. 7A), but only an 18 % increase in phosphorylation of Thr17 (Fig. 7B), indicating that Thr17 phosphorylation was near maximum in WT mice under basal conditions. We then compared PLN phosphorylation in WT, TM180, and TM180/AE3 mice after β-adrenergic stimulation. Phosphorylation of Ser16 was only slightly higher in single (p < 0.05) and double (p = 0.12) mutants relative to WT controls (Fig. 7C). Given the high levels of Ser16 phosphorylation in mutant hearts under basal conditions (Fig. 5E), it is clear that there is a sharp reduction in reserve capacity for Ser16 phosphorylation under maximally stimulated conditions. Phosphorylation of Thr17 was essentially the same in WT and TM180 single mutants, but was significantly lower in TM180/AE3 double mutants (Fig. 7D).
Fig. 7.
Effects of β-adrenergic stimulation in vivo on phosphorylation of phospholamban in WT and mutant mice. Mice were anesthetized and surgically-instrumented as in Fig. 3. Ventricles were collected under basal conditions or after maximum stimulation with dobutamine, and immunoblot analysis of homogenates was performed using antibodies that recognize phosphoserine 16 (PS16) or phosphothreonine 17 (PT17) of PLN, with P and M designating pentameric and monomeric forms. (A) PS16 and (B) PT17 levels in WT ventricles under both basal (− dobutamine) and stimulated (+ dobutamine) conditions; *p < 0.05 vs control. (C) PS16 and (D) PT17 levels in WT, TM180 (TG), and TM180/AE3 (TG/KO) ventricles following maximum β-adrenergic stimulation; *p < 0.05 vs WT; †p < 0.05 vs TG. n = 4 (A and B) or 3 (C and D) 2.5-month-old male mice of each genotype.
3.8. Effects of AE3 ablation on Ca2+ handling in isolated myocytes of TM180 mice
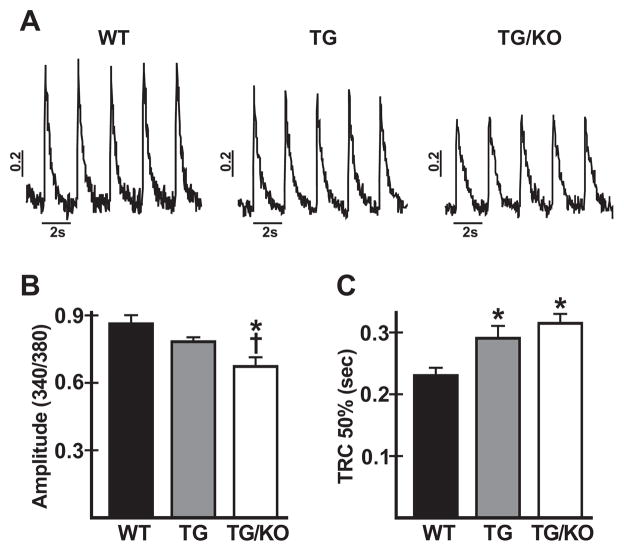
The above studies showed that TM180 and TM180/AE3 hearts have a reduced reserve capacity for β-adrenergic stimulation of PLN phosphorylation. To determine whether there are differences in Ca2+ handling among the three genotypes, ventricular myocytes were isolated from WT, TM180, and TM180/AE3 mice and Ca2+ transients were analyzed after loading with Fura-2AM. The amplitude of the Ca2+ transient (Fig. 8A and B) was significantly reduced in myocytes of TM180/AE3 mutants when compared with either TM180 or WT cells, and was modestly reduced in TM180 myocytes relative to WT (340/380nm fluorescence ratios: WT, 0.86 ± 0.04; TM180, 0.78 ± 0.02; TM180/AE3, 0.67 ± 0.04). Moreover, time to 50% decay of the Ca2+ transient (Fig. 8C) was significantly greater in TM180/AE3 and TM180 myocytes than in WT myocytes (0.315 ± 0.015, 0.291 ± 0.020, and 0.230 ± 0.013 seconds, respectively). Thus, both mutants exhibit impaired Ca2+ handling, with double mutants being more severely affected.
Fig. 8.
Analysis of Ca2+ transients in cardiac myocytes from WT and mutant mice. Myocytes were isolated from 3-month-old WT, TM180 (TG), and TM180/AE3 (TG/KO) mice and loaded with Fura-2AM. Ca2+ transients were analyzed during stimulation at 0.5 Hz. (A) Representative tracings of Ca2+ transients for the three genotypes, determined as fluorescence ratios at 340/380 nm. (B) Amplitudes of Ca2+ transients (systolic - diastolic values) for all three genotypes. (C) Time to 50% recovery of the Ca2+ transient (TRC 50%) for all three genotypes. For all three genotypes, n = 6 mice (4 females and 2 males) with 9–16 cells for each mouse. *p < 0.03 vs WT; †p < 0.05 vs TG.
4. Discussion
Inhibition of Cl-/HCO3− exchange has been proposed as being potentially cardioprotective in hypertrophy, I/R injury, and arrhythmias [2,8–10]; however, given the expression of at least four different Cl−/HCO3− exchangers in heart and the absence of isoform-specific inhibitors, the role of individual isoforms is unclear. To begin addressing this issue, we assessed the effects of AE3 ablation on cardiac function and hypertrophy in a mouse model of familial hypertrophic cardiomyopathy [15]. As discussed below, the results indicate that the absence of AE3, rather than providing cardioprotection, leads to more rapid decompensation.
Previous studies indicated that AE3-null mice were healthy [14,25] and had normal cardiovascular function [14], although they had a modest reduction in heart weight/body weight ratios [14], consistent with the possibility that inhibition of AE3 might reduce hypertrophy. The rationale for a cardioprotective effect is that AE3-mediated Cl−/HCO3− exchange has the potential to operate in concert with the NHE1 Na+/H+ exchanger [8,26]. Increased NHE1 activity is known to cause hypertrophy [27] and inhibition of NHE1 is anti-hypertrophic [13], so loss of Cl−/HCO3− exchange that is coupled to NHE1 might be expected to reduce hypertrophy. However, the degree of hypertrophy was the same in TM180 and TM180/AE3 double mutants, and TM180/AE3 mutants progressed much more rapidly to heart failure and death.
As in other models of cardiac hypertrophy [28], NHE1 levels were increased in both TM180 and TM180/AE3 mutant hearts, but the loss of AE3 had no effect on the levels of NHE1. Increased Cl−/HCO3− exchange has been observed in hypertrophied hearts of spontaneously hypertensive rats [12,29], which also exhibited increased NHE1 activity [29], and it was suggested that increased expression of AE3fl, the full-length variant of AE3, might provide a balancing Cl−/HCO3− exchange activity that might facilitate increased NHE1 activity [8]. However, mouse ventricle contains almost no AE3fl mRNA [3]. Furthermore, immunoblot analysis showed high levels of AE3c in both WT and hypertrophied TM180 hearts but only trace levels of AE3fl protein, and no change in expression of either AE3 variant was observed in TM180 hearts. These data suggest that AE3fl does not play a major role in cardiac muscle of either WT or TM180 mice and that the enhanced rate of decompensation in TM180/AE3 double relative to TM180 single mutants is due to the loss of AE3c. Unlike mouse heart, however, ventricle tissue from failing human heart expressed similar levels of mRNA for both AE3fl and AE3c [2]. Thus, there are major species differences in expression of the two AE3 variants and it is possible that coupling between NHE1 and AE3fl occurs in human heart.
Although studies using an inhibitory antibody suggest that AE3 balances the alkalinizing activity of NHE1 [30], the current results provide no evidence that expression of NHE1 and AE3 are coordinately regulated in mouse heart. When isolated myocytes are analyzed in Hepes buffer or in the presence of inhibitors of Cl−/HCO3− exchange, NHE1-mediated Na+/H+ exchange leads to an increase in pHi [27,29]. However, this change in pHi does not occur if cells are maintained in the absence of inhibitors or in buffer containing CO2/HCO3−, thus indicating that NHE1 activity is balanced by Cl−/HCO3− exchange [29]. Although AE3 remains a candidate to mediate this activity, one or more of the other Cl−/HCO3− exchangers could also be involved. AE2 is a reasonable candidate, as functional coupling of NHE1 and AE2 on basolateral membranes of mouse colonic epithelium has been shown to support cAMP-stimulated anion secretion [31], both NHE1 and AE2 are sensitive to pHi [32,33] and can function in concert to regulate cell volume [34,35], and AE2 mRNA was modestly upregulated in AE3-null hearts. On the other hand, AE2 is expressed at relatively low levels in heart [4], so the degree to which it might compensate for loss of AE3 is uncertain. NHE1 and AE1 are both present in intercalated discs [36,37], consistent with possible coupling between these transporters in this location, and the very high levels of Slc26a6 in heart [4] make it a good candidate to balance at least some of the activity of NHE1. However, AE1 and Slc26a6 mRNA expression was reduced in TM180 and TM180/AE3 hearts, while NHE1 expression was increased, suggesting that their expression is not coordinately regulated with NHE1 during development of cardiac hypertrophy.
In vivo force-frequency relationships were positive in WT mice, but negative in both mutants, consistent with the negative FFR typically observed in human heart failure [38]. There was little indication of a difference in FFR between single and double mutants as pacing frequency was increased to 500 bpm; however, TM180/AE3 double mutants had more arrhythmic events at the higher pacing frequencies and exhibited greater failure in achieving the desired heart rate as pacing frequencies were increased beyond 500 bpm. In addition, maximal heart rates following β-adrenergic stimulation were substantially lower in TM180/AE3 mutants than in TM180 and WT mice. These data suggest that the loss of AE3 leads to a general reduction in the ability of TM180 mutants to achieve or maintain elevated heart rates, although it should be noted that loss of AE3 alone does not cause a significant reduction in heart rate of anesthetized mice under either basal or stimulated conditions [14].
Basal contractility was normal in 2.5-month-old single and double mutants and basal relaxation, as indicated by −dP/dt, was similarly reduced in both mutants, but TM180/AE3 double mutants exhibited a severe reduction in β-adrenergic responses. The data for WT and TM180 mice are consistent with a previous study using the isolated work-performing heart [15]. In that study, hearts from 2.5-month-old TM180 mice exhibited normal +dP/dt under basal conditions but had sharply reduced absolute values of −dP/dt compared to WT hearts, consistent with a relaxation defect caused by the increased Ca2+-sensitivity of the TM180 contractile apparatus [15], and β-adrenergic responses were reduced to about the same degree as in the current study. When isolated hearts from 4.5-month-old TM180 mice were analyzed, β-adrenergic stimulation of both contraction and relaxation were lost [15], as seen in the current study with 2.5-month-old TM180/AE3 mutants. For both single and double mutants, loss of β-adrenergic stimulation occurs at about the age that a significant incidence of death from heart failure begins to occur. Previous studies showed that loss of AE3 alone or in combination with loss of NKCC1 does not affect responses to dobutamine [14]. These and the current observations suggest that the loss of β-adrenergic responses in young TM180/AE3 mice is due primarily to the TM180 mutation rather than to the loss of AE3 per se, but with more rapid decompensation and heart failure occurring in TM180 mice that also lack AE3.
Phosphatase activity is elevated in end-stage heart failure in humans [39], and cardiac-specific over-expression of PP1 leads to impaired contractility and dilated cardiomyopathy [23]. In AE3-null mice, which appeared outwardly normal, expression of PP1 was elevated and methylation of PP2A was altered [14], supporting the possibility that alterations in these phosphatases, both of which can affect β-adrenergic responses [23,40], might contribute to the more rapid development of heart failure in TM180/AE3 double mutants. However, both single and double mutants exhibited increases in PP1, non-methylated PP2A, and both total and non-methylated PP2A in the myofibrillar fraction, indicating that differences in phosphatase levels are not responsible for the worsening heart failure in TM180/AE3 mice. Expression of CamKII, which is often upregulated and activated in heart failure [41] and mediates some of the effects of β-adrenergic signaling [42], was also increased. CamKII levels were slightly higher in TM180/AE3 hearts, which may contribute to the more rapid decompensation.
Phosphorylation of PLN on Ser16, a major mechanism by which β-adrenergic signaling stimulates cardiac performance, was sharply increased in both mutants under basal conditions. The increased phosphorylation of PLN likely contributes to maintenance of cardiac performance in mutant mice under basal conditions and is consistent with increased catecholamine stimulation that occurs in heart failure. Failing hearts of muscle-LIM-protein (MLP)-null mice exhibited a similar increase in PLN phosphorylation [43], which the investigators noted would lead to a reduced reserve capacity for enhanced Ca2+ handling in response to increased frequency or adrenergic stimulation. Analysis of hearts from surgically instrumented mice revealed a 2.8-fold increase in phosphorylation of Ser16 in WT hearts in response to β-adrenergic stimulation. Under maximally stimulated conditions, however, similar levels of Ser16 phosphorylation were observed in all three genotypes. Given the large differences in Ser16 phosphorylaton between WT and mutant hearts under basal conditions (2.2-fold and 2.7-fold higher in TM180 and in TM180/AE3 hearts, respectively), it is clear that the mutants have a reduced reserve capacity for PLN phosphorylation on Ser16, as suggested for MLP-null mice [43].
Phosphorylation of PLN on Thr17 in WT hearts was near maximum levels under basal conditions (with heart rates at ~350 bpm), and little additional phosphorylation occurred in response to dobutamine (with heart rates at ~560 bpm). Thr17 phosphorylation in TM180 hearts after β-adrenergic stimulation was the same as in WT hearts and levels in TM180/AE3 hearts were slightly reduced. Previous studies have shown that Thr17 phosphorylation responds to increased frequency, but not to β-adrenergic stimulation, whereas the opposite is true for phosphorylation of Ser16 [44]. Thus, the modest reduction in phosphorylation of Thr17 in TM180/AE3 hearts relative to WT or TM180 hearts, which might be due in part to the reduced heart rate, is unlikely to contribute to the impaired β-adrenergic response.
Ca2+-handling was impaired in isolated myocytes from both mutants, with reduced Ca2+ transients and prolonged decay times; however, it was more severely affected in TM180/AE3 myocytes. These alterations may be due in part to increased myofibrillar Ca2+ sensitivity resulting from the TM180 mutation, as the sensitized myofilaments can reduce the amplitude by providing increased Ca2+ buffering power and reduce the decay rate by slowing the dissociation of Ca2+ [45]. Expression of the L-type Ca2+ channel was higher in both mutants, which might be expected to increase, rather than decrease, the amplitude of the Ca2+ transient. However, one of the most striking effects on Ca2+ handling proteins was a reduction in levels of the ryanodine receptor, consistent with the reduced Ca2+ transient amplitudes. Expression of SERCA2a in heart is generally decreased in both human heart failure and in animal models of heart failure [46] and is often considered a major contributing factor to reductions in Ca2+ transients [46,47]. However, SERCA2a levels were not significantly reduced in either TM180 or TM180/AE3 hearts. Although the greater deficit in Ca2+ handling in TM180/AE3 mutants undoubtedly contributes to the phenotype, impaired Ca2+ handling occurs commonly in heart failure [48] and the changes in Ca2+ handling proteins were similar in single and double mutants.
A major unanswered question is the mechanism by which the loss of AE3 in the TM180 model leads to more rapid decompensation and heart failure. Although TM180/AE3 mice exhibit greater impairments of β-adrenergic responses and Ca2+ handling than TM180 mice, these changes are likely due to their more rapid progression to heart failure rather than a direct consequence of the loss of AE3. A deficit in AE3-mediated Cl−/HCO3− exchange would cause a reduction in both HCO3− efflux and Cl− uptake, but the actual effect on cellular ion homeostasis would depend on its interactions with other transporters. For example, several investigators have suggested that AE3 operates in concert with NHE1 to mediate pHi-neutral Na+ uptake [8,26], and other Na+-coupled acid extruders could also serve in this capacity. In addition, there are clear compensatory interactions between AE3 and the NKCC1 [14], consistent with functions in Na+-loading and perhaps Cl−-loading. Given the abundance of Na+-loading mechanisms [49] and the additional Cl−/HCO3− exchangers in cardiac muscle, if AE3 does contribute to this activity, then it is likely to regulate Na+ in subsarcolemmal microdomains rather than in the bulk cytosol. There is evidence that Na+ in subsarcolemmal regions is regulated independently of cytosolic Na+ and that it affects Na+/Ca2+ exchange and contractility [50,51]. Although speculative, changes in Na+ levels (or in pH or Cl−) in subsarcolemmal domains regulated by AE3 might also have the potential to initiate signaling events. For example, salt inducible kinase 1 (Sik1) is expressed in myocytes [52], is involved in Na+ sensing [53], and participates in signaling mechanisms involving PP2A [54], which is affected by the loss of AE3 [14].
In conclusion, loss of AE3 caused no lessening of hypertrophy in the TM180 heart but led to more rapid decompensation and heart failure. This suggests that AE3 might not be a useful target for inhibitory drug therapy, at least in cardiomyopathies that are similar to the TM180 model. Although the data do not rule out the possibility that inhibition of AE3 might be useful in other conditions, the absence of protective effects of AE3 ablation on I/R injury in a Langendorff heart model [14] and the negative effects of AE3 ablation on cardiac performance in mice lacking the NKCC1 Na+-K+-2Cl− cotransporter [14] also argue against a therapeutic value of AE3 inhibition. Finally, the reduced ability of TM180/AE3 mutants to achieve or maintain higher heart rates during pacing suggests that it also might not be an appropriate target for anti-arrhythmic therapy. Rather, the data indicate that AE3-mediated Cl−/HCO3− exchange activity, which appears to be dispensable in a healthy heart [14], contributes to better preservation of cardiac function during heart failure.
Supplementary Material
Acknowledgments
This study was supported by NIH grants HL061974 to GES, HL081680 to DFW, and DK043495 to SLP. We thank Maureen Bender for excellent animal husbandry. Assistance of Dr. Sudarsan Rajan is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Linn SC, Kudrycki KE, Shull GE. The predicted translation product of a cardiac AE3 mRNA contains an N terminus distinct from that of the brain AE3 Cl−/HCO3− exchanger. Cloning of a cardiac AE3 cDNA, organization of the AE3 gene, and identification of an alternative transcription initiation site. J Biol Chem. 1992;267:7927–35. [PubMed] [Google Scholar]
- 2.Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ Res. 1994;75:603–14. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]
- 3.Linn SC, Askew GR, Menon AG, Shull GE. Conservation of an AE3 Cl−/HCO3− exchanger cardiac-specific exon and promoter region and AE3 mRNA expression patterns in murine and human hearts. Circ Res. 1995;76:584–91. doi: 10.1161/01.res.76.4.584. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR. Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J Physiol. 2004;561:721–34. doi: 10.1113/jphysiol.2004.077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niederer SA, Swietach P, Wilson DA, Smith NP, Vaughan-Jones RD. Measuring and modeling chloride-hydroxyl exchange in the guinea-pig ventricular myocyte. Biophys J. 2008;94:2385–403. doi: 10.1529/biophysj.107.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–31. doi: 10.1016/j.yjmcc.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Camilión de Hurtado MC, Alvarez BV, Pérez NG, Ennis IL, Cingolani HE. Angiotensin II activates Na+-independent Cl−-HCO3− exchange in ventricular myocardium. Circ Res. 1998;82:473–81. doi: 10.1161/01.res.82.4.473. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez BV, Fujinaga J, Casey JR. Molecular basis for angiotensin II-induced increase of chloride/bicarbonate exchange in the myocardium. Circ Res. 2001;89:1246–53. doi: 10.1161/hh2401.101907. [DOI] [PubMed] [Google Scholar]
- 9.Farias F, Morgan P, Chiappe de Cingolani G, Camilión de Hurtado MC. Involvement of the Na+-independent Cl−/HCO3− exchange (AE) isoform in the compensation of myocardial Na+/H+ isoform 1 hyperactivity in spontaneously hypertensive rats. Can J Physiol Pharmacol. 2005;83:397–404. doi: 10.1139/y05-025. [DOI] [PubMed] [Google Scholar]
- 10.Lai ZF, Nishi K. Intracellular chloride activity increases in guinea pig ventricular muscle during simulated ischemia. Am J Physiol Heart Circ Physiol. 1998;275:H1613–9. doi: 10.1152/ajpheart.1998.275.5.H1613. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki H, Otani H, Mishima K, Imamura H, Inagaki C. Involvement of anion exchange in the hypoxia/reoxygenation-induced changes in pHi and [Ca2+]i in cardiac myocyte. Eur J Pharmacol. 2001;411:35–43. doi: 10.1016/s0014-2999(00)00893-1. [DOI] [PubMed] [Google Scholar]
- 12.Chiappe de Cingolani G, Morgan P, Mundiña-Weilenmann C, Casey J, Fujinaga J, Camilión de Hurtado M, Cingolani H. Hyperactivity and altered mRNA isoform expression of the Cl−/HCO3− anion-exchanger in the hypertrophied myocardium. Cardiovasc Res. 2001;51:71–9. doi: 10.1016/s0008-6363(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 13.Karmazyn M, Sawyer M, Fliegel L. The Na+/H+ exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:323–35. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 14.Prasad V, Bodi I, Meyer JW, Wang Y, Ashraf M, Engle SJ, Doetschman T, Sisco K, Nieman ML, Miller ML, Lorenz JN, Shull GE. Impaired cardiac contractility in mice lacking both the AE3 Cl−/HCO3− exchanger and the NKCC1 Na+-K+-2Cl− cotransporter: effects on Ca2+ handling and protein phosphatases. J Biol Chem. 2008;283:31303–14. doi: 10.1074/jbc.M803706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro RJ, Wieczorek DF. A familial hypertrophic cardiomyopathy α-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–28. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- 16.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE. α-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–12. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz JN, Robbins J. Measurement of intraventricular pressure and cardiac performance in the intact closed-chest anesthetized mouse. Am J Physiol Heart Circ Physiol. 1997;272:H1137–46. doi: 10.1152/ajpheart.1997.272.3.H1137. [DOI] [PubMed] [Google Scholar]
- 18.Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999;274:2556–62. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- 19.D’Angelo D, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., 2nd Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA. 1997;94:8121–6. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connell TD, Ni YG, Lin K-M, Han H, Yan Z. Isolation and culture of adult mouse cardiac myocytes for signaling studies. AFCS Research Reports [online] 2003;1(5) http://www.signaling-gateway.org/reports/v1/CM0005/CM0005.htm.
- 21.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–77. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 22.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–40. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol. 2002;22:4124–35. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to β-agonists. J Biol Chem. 2000;275:38938–43. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 25.Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl−/HCO3− exchanger AE3 display a reduced seizure threshold. Mol Cell Biol. 2006;26:182–91. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cingolani HE, Ennis IL. Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation. 2007;115:1090–100. doi: 10.1161/CIRCULATIONAHA.106.626929. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura TY, Iwata Y, Arai Y, Komamura K, Wakabayashi S. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res. 2008;103:891–9. doi: 10.1161/CIRCRESAHA.108.175141. [DOI] [PubMed] [Google Scholar]
- 28.Karmazyn M, Kilic A, Javadov S. The role of NHE-1 in myocardial hypertrophy and remodelling. J Mol Cell Cardiol. 2008;44:647–53. doi: 10.1016/j.yjmcc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Pérez NG, Alvarez BV, Camilión de Hurtado MC, Cingolani HE. pHi regulation in myocardium of the spontaneously hypertensive rat. Compensated enhanced activity of the Na+-H+ exchanger. Circ Res. 1995;77:1192–200. doi: 10.1161/01.res.77.6.1192. [DOI] [PubMed] [Google Scholar]
- 30.Chiappe de Cingolani GE, Ennis IL, Morgan PE, Alvarez BV, Casey JR, Camilión de Hurtado MC. Involvement of AE3 isoform of Na+-independent Cl−/HCO3− exchanger in myocardial pHi recovery from intracellular alkalization. Life Sci. 2006;78:3018–26. doi: 10.1016/j.lfs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Gawenis LR, Bradford EM, Alper SL, Prasad V, Shull GE. AE2 Cl−/HCO3− exchanger is required for normal cAMP-stimulated anion secretion in murine proximal colon. Am J Physiol Gastrointest Liver Physiol. 2010;298:G493–503. doi: 10.1152/ajpgi.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakabayashi S, Bertrand B, Shigekawa M, Fafournoux P, Pouysségur J. Growth factor activation and “H+-sensing” of the Na+/H+ exchanger isoform 1 (NHE1). Evidence for an additional mechanism not requiring direct phosphorylation. J Biol Chem. 1994;269:5583–8. [PubMed] [Google Scholar]
- 33.Stewart AK, Kurschat CE, Vaughan-Jones RD, Alper SL. Putative re-entrant loop 1 of AE2 transmembrane domain has a major role in acute regulation of anion exchange by pH. J Biol Chem. 2009;284:6126–39. doi: 10.1074/jbc.M802051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol. 2006;187:159–67. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang L, Chernova MN, Alper SL. Secondary regulatory volume increase conferred on Xenopus oocytes by expression of AE2 anion exchanger. Am J Physiol. 1997;272:C191–202. doi: 10.1152/ajpcell.1997.272.1.C191. [DOI] [PubMed] [Google Scholar]
- 36.Petrecca K, Atanasiu R, Grinstein S, Orlowski J, Shrier A. Subcellular localization of the Na+/H+ exchanger NHE1 in rat myocardium. Am J Physiol Heart Circ Physiol. 1999;276:H709–17. doi: 10.1152/ajpheart.1999.276.2.H709. [DOI] [PubMed] [Google Scholar]
- 37.Moura Lima PR, Salles TS, Costa FF, Saad ST. α-cardiac actin (ACTC) binds to the band 3 (AE1) cardiac isoform. J Cell Biochem. 2003;89:1215–21. doi: 10.1002/jcb.10561. [DOI] [PubMed] [Google Scholar]
- 38.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–50. doi: 10.1161/01.cir.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 39.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–72. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 40.De Arcangelis V, Soto D, Xiang Y. Phosphodiesterase 4 and phosphatase 2A differentially regulate cAMP/protein kinase a signaling for cardiac myocyte contraction under stimulation of β1 adrenergic receptor. Mol Pharmacol. 2008;74:1453–62. doi: 10.1124/mol.108.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson ME. CaMKII and a failing strategy for growth in heart. J Clin Invest. 2009;119:1082–85. doi: 10.1172/JCI39262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm M, Brown JH. β-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48:322–30. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antoons G, Vangheluwe P, Volders PG, Bito V, Holemans P, Ceci M, Wuytack F, Caroni P, Mubagwa K, Sipido KR. Increased phospholamban phosphorylation limits the force-frequency response in the MLP−/− mouse with heart failure. J Mol Cell Cardiol. 2006;40:350–60. doi: 10.1016/j.yjmcc.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–6. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 45.Huke S, Knollmann BC. Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J Mol Cell Cardiol. 2010;48:824–33. doi: 10.1016/j.yjmcc.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arai M, Matsui H, Periasamy M. Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure. Circ Res. 1994;74:555–64. doi: 10.1161/01.res.74.4.555. [DOI] [PubMed] [Google Scholar]
- 47.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology. 2006;21:380–7. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 48.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 49.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 50.Verdonck F, Mubagwa K, Sipido KR. [Na+] in the subsarcolemmal 'fuzzy' space and modulation of [Ca2+]i and contraction in cardiac myocytes. Cell Calcium. 2004;35:603–12. doi: 10.1016/j.ceca.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+/K+-ATPase α2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res. 2007;75:109–17. doi: 10.1016/j.cardiores.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 52.Romito A, Lonardo E, Roma G, Minchiotti G, Ballabio A, Cobellis G. Lack of sik1 in mouse embryonic stem cells impairs cardiomyogenesis by down-regulating the cyclin-dependent kinase inhibitor p57kip2. PLoS One. 2010;5:e9029. doi: 10.1371/journal.pone.0009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaitovich A, Bertorello AM. Intracellular sodium sensing: SIK1 network, hormone action and high blood pressure. Biochim Biophys Acta. 2010 Mar 27; doi: 10.1016/j.bbadis.2010.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 54.Sjöström M, Stenström K, Eneling K, Zwiller J, Katz AI, Takemori H, Bertorello AM. SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc Natl Acad Sci U S A. 2007;104:16922–7. doi: 10.1073/pnas.0706838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.