Abstract
Background
In Brazil it is mandatory to screen donors for HIV antibodies using two immunoassays (IAs) in parallel. Confirmatory testing is performed only on reactive donors who return for counseling. The goal of this analysis was to determine if concordant IA reactivity accurately predicts infection and can be used for HIV incidence/prevalence analyses.
Methods
We reviewed HIV screening and confirmatory results obtained for 307,407 donations in the first year of the REDS-II study in Brazil (2007), and for 2,304,755 donations collected from 1996 to 2006 in one of the REDS-II sites (Sao Paulo).
Results
In the Sao Paulo site, 11,410 (0.50%) HIV-IA-reactive donations were discarded, but only 2,095 (0.09%) were reactive to both IAs. Western blot was positive on 1,002 (48%) dual-IA-reactive donors who returned for counseling. Only 4 HIV-infected donors were detected who had been missed at screening by one of the IAs; all occurred prior to 2002. The positive predictive value (PPVs) of dual-IA-reactivity varied from 45.8 to 100%, with 80–90% PPVs when using IAs from different manufacturers. If both assays yielded signal-to-cutoff (S/C) values ≥3.0, PPVs ranged from 91–99%, with ~99% sensitivity for true HIV seropositivity.
Conclusion
Parallel testing of all donations has limited efficacy when highly sensitive IAs are used. Reactivity by two sequential IAs is useful for prevalence studies if the assays are from different manufacturers and especially if high S/C values are considered.
Keywords: HIV, Algorithm, Serology, Blood bank screening
INTRODUCTION
Since development of the first HIV immunoassays (IAs) in 1985, donor screening for HIV antibodies has generally been performed with one IA (enzyme immunoassay or chemiluminescent immunoassay ), followed by retesting reactive samples in duplicate with the same IA. In 1998, the Brazilian Ministry of Health, made it mandatory to screen samples from all donated blood units using two parallel IAs.1 The rationale for this policy was related to concern over the quality of HIV assays available in Brazil and the performance of testing laboratories in the country at that time. Parallel testing of blood donors by two assays was a practice that had been employed since the 1970s in Latin America for Chagas disease, because of the presence of low titer T cruzi antibodies in some infected donors and the variable sensitivities of these antibody assays2. Experience with Chagas screening probably influenced the policy makers as they considered the serious consequences of transfusion transmission of HIV, a virus which was spreading rapidly in Brazil at the time.
In Brazil (and many other developing or resource-constrained countries), repeat reactive units that are detected by HIV screening test(s) are discarded, and the donors are notified of “abnormal test results” and asked to return to the collection center to provide a new specimen for retesting. Confirmatory assays (generally a Western blot [WB] in the case of HIV) are only required to be performed for donors who return for counseling, and this testing is done on the follow-up samples. This approach is employed to reduce the cost of performing relatively expensive confirmatory assays on index donation samples from donors who fail to return for notification and counseling, as well as to corroborate the index donation HIV seroreactivity using a second specimen collected weeks later, thus allowing for evolution of seroconversion enabling more accurate confirmation of recently infected cases. One disadvantage of this approach is that confirmatory test data are only available for a subset of HIV seroreactive donors, such that the definition of true positive cases based on index donation test results is incomplete, precluding accurate assessment of HIV prevalence and incidence in the donor population.
As part of the Retrovirus Epidemiology Donors Study-II (REDS-II) program in Brazil3, we decided to analyze this parallel IA testing strategy in terms of efficiency and accuracy, as well as to evaluate whether we could use concordance and or levels of reactivity on both IAs as an accurate predictor of true HIV infection status for prevalence and incidence analyses. In addition to informing Brazilian HIV donor screening policies (potentially guiding revisions to the currently recommended testing algorithm) and enhancing REDS-II data interpretations, our analysis of the results of parallel IA screening in Brazil is relevant to donor screening algorithms in other developing countries. It is also relevant to developed countries such as Australia where two serological assays for HIV, HCV and HTLV are used sequentially (rather than in parallel) to reduce costs and, more important, to avoid indeterminate results by confirmatory Western blot and recombinant immunoblot assays which result in confusing counseling messages leading to donor anxiety and further unnecessary testing 4. HIV testing algorithms based on sequential application of two or more rapid- or laboratory-based immunoassays are also frequently used in diagnostic screening settings, an approach endorsed by WHO and CDC, and hence our data from a large number of donors screened in parallel in Brazil also yields information to assess the accuracy of such algorithms 5–8.
METHODS
Overall study design and setting
Two sets of data were evaluated in this analysis:
Parallel IAs results on 2,304,755 blood donations collected from 1996 to 2006 at Fundação Pró-Sangue/Blood Center in São Paulo (FPS/HSP), and WB results performed on the consequent follow-up samples from reactive donors who returned for counseling.
HIV test data from the first year database of the REDS II international study (2007). This database included a total of 307,425 donations collected by the 3 blood centers participating in the REDS-II program: 1) Fundação Pró-Sangue – Blood Center of São Paulo (FPS/HSP - São Paulo), 2) Fundação Hemominas (Belo Horizonte), and 3) Fundação Hemope (Recife). For this dataset we reviewed the patterns of parallel IAs reactivity on index donations relative to WB results obtained at the time of return visits and the results of detuned HIV-1 antibody assays performed at Blood Systems Research Institute BSRI. For cases where the donors did not return, retained aliquots of serum or plasma from the index donation were tested by WB, and if necessary by PCR to define these donors’ HIV infection status.
The HIV screening kits used by blood centers in Brazil change frequently due to regulatory approval, availability of assays and instruments, and the prices for test kits, which are negotiated by a bid and tender process on an annual basis. For the analysis of the first set of historical data, the assays used were classified into three different groups according to the generation of HIV antibody screening:
Group A: Parallel IA screening was performed with one first generation assay-HEMOBIO ANTI-HIV ( EMBRABIO – São Paulo, Brazil), and one second or third generation assay: Innotest TM HIV-1/HIV-2Ab s.p. (Innogenetics – Ghent, Belgium), ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO -Raritan, NJ, USA) or Vironostika® HIV Uni-Form II (Biomerieux - Boxtel, The Netherlands);
Group B: Parallel IA screening included one second- and one third-generation assay: Vironostika® HIV Uni-Form II plus O (Biomerieux - Boxtel, The Netherlands and [Murex ICE HIV-1.O.2 (Genelabs Diagnótics Pte.Ltda -Singapore- Singapore) or ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, NJ, USA)].
-
Group C: Parallel screening was performed using one third and one fourth generation (HIV antigen/antibody combination) assay:
Vironostika® HIV Uni-Form II Ag-Ab (Biomerieux, Boxtel, The Netherlands) and [ ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, New Jersey) or Abbott Murex HIV 1.2.O ( Abbott Murex -Dartford, UK)]
Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK) and ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, New Jersey)
Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) and Vironostika® HIV Uni-Form II (Biomerieux - Boxtel, The Netherlands)
For the REDS-II 2007 dataset, all centers were using one fourth-generation assay in different combinations as described below:
-
FPS/HSP:
Vironostika® HIV Uni-Form II (Biomerieux - Boxtel, The Netherlands) and [Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) or Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France)]
-
Hemominas:
Abbott Prism HIV O Plus (Wiesbaden, German) and Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK).
Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) and Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France).
-
Hemope:
Abbott Murex HIV 1.2.O ( Dartford, UK) and [Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK) or Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France)].
During all time periods and at all three centers, when a donor tested repeat reactive by either of the parallel screening IAs, the unit was discarded and the sample was sent to Sao Paulo for further testing as follows:
Samples reactive to only one IA were submitted to an in house PCR in pools of 10 based on the technique described by Candotti et al 9.
Samples reactive to both IAs were sent to Blood Systems Research Institute to classify the infection as recent (i.e., incident) or remote using the Standardized Testing Algorithm for Recent HIV Seroconversion (STARHS), which is based on a sensitive/less-sensitive enzyme immunoassay (“detuned” EIA)10. For this study, the Vironostika® HIV-1 Microelisa (bioMérieux Industry, Raleigh, NC, USA) assay for anti-HIV was modified for “detuned” EIA application by increasing the sample dilution from 1:76 to 1:20,000 and reducing the sample incubation time from 100 minutes to 30 minutes while retaining the kit-specified conjugate incubation time of 30 minutes 10. Donors with remotely acquired HIV infectiona will remain positive in this detuned procedure (reflecting high titer and high avidity HIV-specific antibodies, whereas recently infected or false positive donors will give negative assay results. For such non reactive samples, if the donor had returned for confirmation, we used the final Western blot results obtained by the blood banks to classify the donor as HIV infected or not infected. If the donor had not returned for follow-up testing, the sample from the index donation was tested by Western blot, and samples with WB indeterminate or negative results were further tested by individual (non-pooled) PCR testing when enough volume was available.
It is important to note that in Brazil most donor screening laboratories apply “gray zones” to the interpretation of cut-off values. HIV antibody IAs tests are considered “reactive” when the optical density signal-to-cutoff (S/C) ratio is >1.1, “inconclusive” or “gray-zone reactive” when the S/C ratio is >0.9 and <1.1, and “non-reactive when the S/C ratio is <0.9. Indeterminate/gray-zone reactive units are subjected to repeat testing and if repeat reactive they are discarded and donor follow-up notification and testing are triggered in the same way as for “reactive” units.
RESULTS
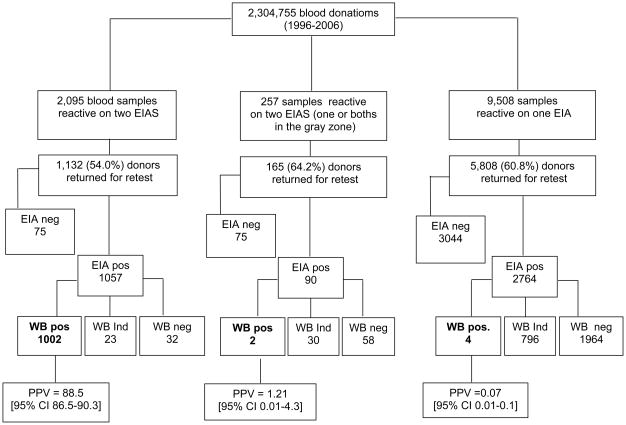
The distributions of screening and confirmatory HIV test results for blood donors at our São Paulo blood center from 1996 to 2006 are summarized in Figure 1. There were 2,304,755 blood donations at FPS/HSP during the study period. A total of 11,860 (0.51%) units were discarded due to repeat reactivity (above the grey-zone S/C cutoff) on one or both HIV screening tests. The rate of discarded units due to HIV seroreactivity decreased significantly over time, from 1.87% in 1996 to 0.26% in 2006. Of the 11,860 HIV seroreactive/discarded units, 2,095 (17.7%) had index donation samples that were reactive (S/C>1.1) on both HIV screening tests, 257 (2.2%) additional donations were reactive by both IAs but at least one test had an S/C ratio that was in the grey zone, and 9,508 (80.2%) units tested reactive on only one IA.
Figure 1.
Distribution of HIV IAs reactivity and Positive Predictive Values of reactivity patterns for 2,304,755 blood donors screened for HIV utilizing a parallel HIV IA algorithm at Fundação Pró-Sangue- Hemocentro de São Paulo from 1996 to 2006.
Figure 1 also presents the positive predictive values (PPV), defined based on the results of follow-up sample IA and WB results, for the seroreactive donations, according to the pattern of index donation reactivity on the parallel screening HIV antibody assays. The PPV was very high for samples that were reactive by both IAs (88.5% [95% CI 86.5–90.3]), low for the indeterminate/gray zone reactive samples (1.21% [95% CI 0.01–4.3], and very low for the single IA reactive samples 0.07% [95% CI 0.01–0.1].
Of the 307,425 units screened by the REDS centers in 2007, 1098 (0.36%) samples were discarded due to HIV IA reactivity; 844(0.27%) were reactive to only one assay, 213 (0.07%) were positive on both IAs, and 41 (0.01%) had a borderline result in one assay with a positive or borderline result in the second assay (Figure 2). Of the 1098 samples, 957 (91.3%) were available for further testing. Of the 213 samples positive by two IAs, 198 (93%) were submitted to detuned EIA testing using the STAHRS algorithm, and 90 (45%) were reactive and considered long standing infection; for 63 of these detuned EIA-reactive donations WB results were available and all were strongly positive. Of the 108 STAHRS negative samples, 33 were WB negative, 16 WB indeterminate and 59 WB positive. PCR was performed in 7 of the indeterminate cases and in 4 of the negative samples and one WB indeterminate sample was positive. So overall we classified 150 samples as confirmed positives (90 long-standing infections, and 60 recent infections [59 WB positive plus one WB-indeterminate sample that tested PCR-positive)
Figure 2.
Summary of HIV 2007 REDS II serological results.
Table 1 presents the PPV for samples that tested reactive by 2 assays in the dataset in more detail, with Panel A summarizing the results for the 1996–2006 FPS/HSP dataset and Panel B the results for the REDS-II 2007 dataset. In general the PPV of dual assay repeat reactivity was high, approaching 90% for most of the combinations of assays. The only exception was when two different IA kits from the same manufacturer were used in parallel (e.g., BioRad third-generation HIV1+2 and BioRad fourth-generation Ag/Ab kits). This was the case in Hemominas, and for part of the period in Hemope during the REDS-II time period.
Table 1.
Results of screening for HIV antibodies, according to parallel screening assays employed, for 2,304,755 donations at Hemocentro de São Paulo (Upper Panel) and at the REDS-II Program in Brazil (Lower Panel).
| Location(Period) | Kits Used* | Blood Donations (N) | Samples reactive on two HIV assays (N) | Available for confirmation (N) | Confirmed positive† (N) | PPV (%) |
|---|---|---|---|---|---|---|
| São Paulo (01/96 – 06/97) | A | 335,123 | 516 | 243 | 218 | 89.71 |
| São Paulo (06/97 – 07/02) | B | 1,196,522 | 1,100 | 565 | 500 | 88.49 |
| São Paulo (07/02 – 12/06) | C | 910,156 | 479 | 324 | 284 | 87.65 |
| Total São Paulo 1996 – 2006 | 2,304,755 | 2,095 | 1,132 | 1,002 | 88.51 | |
| FPS/HSP (2007) | D | 137,654 | 68 | 68 | 68 | 100.00 |
| Hemominas (2007) | E | 69,974 | 69 | 59 | 25 | 45.76 |
| Hemope (2007) | F | 99,797 | 76 | 71 | 55 | 77.46 |
| Toral REDS-II (2007) | 307,425 | 213 | 198 | 150 | 77.08 | |
A: HEMOBIO ANTI-HIV ( EMBRABIO – São Paulo, Brazil), and [Innotest TM HIV-1/HIV-2Ab s.p. (Innogenetics – Ghent, Belgium), ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, NJ, USA) or Vironostika® HIV UniForm II (Biomerieux - Boxtel, The Netherlands)];
B: Vironostika® HIV Uni-Form II plus O (Biomerieux - Boxtel, The Netherlands and [Murex ICE HIV-1.O.2 (Genelabs Diagnótics Pte.Ltda - Singapore- Singapore) or ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, NJ, USA)].
C: Vironostika® HIV Uni-Form II Ag-Ab (Biomerieux, Boxtel, The Netherlands) and [ ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, New Jersey) or Abbott Murex HIV 1.2.O ( Abbott Murex - Dartford, UK)]; Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK) and ORTHO HIV-1/HIV2 Ab Capture ELISA Test System (ORTHO - Raritan, New Jersey); Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) and Vironostika® HIV Uni-Form II (Biomerieux - Boxtel, The Netherlands)
D Vironostika® HIV Uni-Form II (Biomerieux - Boxtel, The Netherlands) and [Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) or Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France)]
E Abbott Prism HIV O Plus (Wiesbaden, German) and Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK); Genscreen® PLUS HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France) and Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France).
F Abbott Murex HIV 1.2.O ( Dartford, UK) and [Murex HIV Ag-Ab combo (Abbott Murex - Dartford, UK) or Genscreen®ULTRA HIV Ag-Ab (Bio-Rad – Marnes-la-Coquette, France)].
Confirmed by Western blot
As shown in Table 2, the PPVs were significantly higher when S/C values on both IAs were higher than 3.0, even for samples that tested reactive by two kits from the same vendor. When considering IA reactive results at the time of return (which excludes samples that were highly reactive at screening due to cross-contamination the PPV was 99% (95%CI-97.9–99.6) for samples that tested reactive with S/C values >3.0 on both IAs (Table 2).
Table 2.
Correlation of sample/cut-off (S/C) ratios of screening IAs and HIV Western blot positivity or inconclusive for dual IA-reactive specimens from Sao Paolo (1996 to 2006) and the three REDS-II blood centers (2007).
| Donors reactive on two screening IAs who returned for counseling and retesting at FPS (1996 – 2006) | Donors reactive on two IA assays at REDS-II Blood centers (2007) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening Assay † | Reactive on two IA assays |
Confirmed positive‡ | Screening PPV§ (%) | Return PPV§ (%) | Screening test | Available for confirmation | Confirmed positive|| | PPV§ (%) | |
| At screening | At return | ||||||||
| One of both IAs <3.0 | 270 | 148 | 14 | 5.18 | 9.45 | 79 | 74 | 1 | 1.35 |
| Both IAs ≥ 3.0 | 1027 | 1000 | 990 | 96.39 | 99.00 | 175 | 163 | 149 | 91.41 |
Signal-to-cutoff optical density ratio ratio
Confirmed by Western blot at retest
PPV = Positive Predictive Value
Confirmed by Western blot on the screening sample
For samples with single IA reactivity only (n=5008), there were 6 cases in which donors tested WB-positive on follow-up. Detailed results for these six cases that were borderline or discordant reactive at the time of donation and tested positive on both IA and Western blot on follow-up specimens are shown in Table 3. Donor 1 was determined to be falsely positive on initial testing of the return sample due to carry-over contamination; the donor was confirmed as seronegative on two additional follow-up samples and hence classified as not infected. Donor 2 was probably falsely positive at the time of the index donation, because this individual had a previous false reactive result to this kit and was seroconverting at the time of return one year after the index donation (WB pattern had only three bands). The four other cases (donors 3–6) were true HIV infections, who were detected as reactive by only one of the two screening assays. Two of these 4 cases were missed by 1st and two by 2nd generation assays. All four cases were probably seroconverters because S/C reactivity of the IAs had increased between the time of return and the index donation sample; however, in one case (missed by a 1st generation IA) a different kit was used on the return sample, so we cannot exclude that the discordant reactivity was due to lack of sensitivity of the assay to a prevalent (non-seroconversion) sample.
Table 3.
Donors reactive to only one or two HIV antibody IA screening tests and then determined to be Western blot positive in the confirmatory sample at retest at Fundação Pró-Sangue – Blood center of São Paulo from 1996 to 2006.
| Donor | Gender | Donation sample | Follow-up sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date (MM/YY§) | IA|| Kit 1 | IA|| Kit 2 | Date | IA|| Kit 1 | IA|| Kit 2 | Western blot | Result | ||||||
| S/C | Result | S/C | Result | (MM/YY§) | S/C | Result | S/C | Result | Reactive Bands | ||||
| 1* | Male | 01/96 | 1.28 | R¶ | 0.02 | NR | 03/98 | 13.76 | R¶ | 2.38 | R | p24, p31, gp41, p51, p66, gp120, gp160 | R |
| 2‡ | Male | 01/97 | 0.0 | NR** | 3.74 | R¶ | 10/98 | 1.35 | R¶ | 4.18 | R | p17, p24, gp160 | R |
| 3† | Male | 06/97 | 0.0 | NR** | 4.19 | R¶ | 07/97 | 10.56 | R¶ | 6.47 | R | p17, p24, p31, gp41, p51, p55, p66, gp120, gp160 | R |
| 4† | Male | 12/96 | 0.94 | I†† | 7.54 | R¶ | 01/97 | 5.0 | R¶ | 13.39 | R | p17, p24, gp120, gp160 | R |
| 5† | Female | 01/99 | 0.0 | NR** | 1.37 | R¶ | 08/99 | 12.41 | R¶ | 10.50 | R | p17, p24, p31, p39, gp41, p51, p55, p66, gp120, gp160 | R |
| 6† | Female | 04/01 | 0.94 | I†† | 11.07 | R¶ | 06/01 | 17.17 | R¶ | 11.76 | R | p17, p24, p31, gp41, p51, p55, p66, gp120, gp160 | R |
False positive WB result due sample contamination
Possible false-positive result on the screening sample
Seroconvertion
MM/YY = Month/Year
IA = Immunoassay
R = Reactive
NR = non-reactive
I = Inconclusive
Of note, all of these true infection cases that were detected by only one of the two parallel IAs occurred prior to April 2001. No cases of confirmed infected donors with discordant reactivity on the two screening IAs performed on the index donation were identified from 2002–2007 among 2,249 such discordant reactive donors for whom follow-up samples were available or whose index samples were tested by WB (for the 2007 REDS-II dataset index donations).
DISCUSSION
This study presents a systematic analysis of results of serological screening of more than two and one-half million blood donations tested in parallel for HIV antibodies by two different screening tests. The decision to add a second assay for HIV screening was made in 1998 by the Brazilian Ministry of Health and until our study, had not been fully evaluated. We sought to determine if this policy is warranted, and also to gain insights into the accuracy of alternative screening based on sequential screening assays, which is practiced both for HIV blood screening and in diagnostic testing settings in a number of countries4,8.
Our data show that this parallel IA strategy detected 4 infected donors who were reactive by only one of the two screening assays and who were determined to be in the early seroconversion stage of infection based on a comparison of index donation and follow-up testing results. The cases were all detected as reactive by the later generation assays employed in the parallel screening algorithm, and two of these cases had borderline non-reactive results (0.94 S/C values) on the less sensitive IAs. Since 2001, no case of HIV infection detected by only a single screening assay has been identified among 1,200,305 screened donors including 825 HIV infected donors with concordant screening assay reactivity. These results suggest that this parallel screening strategy is no longer efficacious in the context of the improved and generally comparable sensitivity of contemporary screening assays. Given absence of any HIV infections among donors who screened discordant reactive since April 2001, consideration should be given to allow screening with a single sensitive IA, employing the second IA as part of a confirmatory algorithm rather than as a parallel screening test.
In most countries, the serodiagnosis of HIV infection, whether in blood donor or clinical diagnostic settings, relies on a sequential algorithm using an initial screening immunoassay (either a rapid point-of-care assay or a lab-based IA) followed by a more specific supplemental test such as a WB or immunofluorescence assay (IFA)11. One problem with this strategy is that contemporary 3rd and 4th generation screening assays are more sensitive than WB or IFA during the early post seroconversion window phase; consequently some acutely infected donors are misclassified as false positives due to lack of sensitivity of the confirmation assay12. Another problem is that a substantial proportion of samples that are falsely reactive in screening assays subsequently yield an indeterminate result by the WB or IFA, which results in additional costs for further testing and/or follow-up testing, and a confusing and often disturbing counseling message4,8,13. Our data support the use of algorithms based on sequential application of screening immunoassays to reduce the number of specimens subjected to more expensive supplemental assays as well as indeterminate notifications4–6.
With respect to donor screening policies in Brazil, we recommend that the current algorithm that requires that a WB be performed on follow-up samples that remain reactive to only one assay should be discontinued. Such a policy change would have reduced cost by decreasing the number of WBs performed during the period of this study by approximately 2,760 WB and would have avoided approximately 800 indeterminate WB results without missing any case of HIV infection.
Using follow-up samples to define HIV serostatus resolves the problem of the lower sensitivity of the WB assay during window phase and also prevents confirming a donor as positive due to sample mix-up or cross-contamination. As shown in Table 2, at FPS/HSP during the period there were 10 donors with index donation samples with S/C levels >3 that were negative on follow up, indicating probable specimen mix-up or contamination. Hence obtaining a new specimen and retesting donors who tested reactive on initial screening is warranted in all settings to avoid misclassification of HIV infection, which can have a devastating impact on an individual and result in legal liability and distrust of the testing laboratory/program.
Figure 3 presents our recommendation for an algorithm for screening and confirmation of HIV at blood banks in Brazil based on our analysis. We recommend that screening be performed with one 4th generation IA due to published results showing that this test is more sensitive during the window phase14–16. Repeat reactive donors would be asked to return to give a follow-up sample which would be tested by the original screening IAs and if reactive, submitted to a second IAs. WB would only be performed if both IAs are reactive, which would reduce the number of WB indeterminate results and the resulting counseling problems. Reentry would be considered if the individual test negative by original IAS screening assays. Discrepant results on IAs would be considered negative for infection, but would not allow for donor reentry since the individual might again test reactive on a screening assay and the unit would need to be discarded. This algorithm implies that optimally sensitive and high quality IAs are used for both primary screening and follow-up sample testing. This algorithm follows recommendations by WHO, and more recently by CDC, for consecutive use of IAs tests for diagnostic HIV screening. The final infectious status is defined after confirmatory assays, since the positive predictive value (PPV) of two screening assays is still not high enough for diagnostic purposes17 For prevalence analyses using donation data only (i.e., when definitive follow-up data are available for only a subset of screening test reactive donors), our analyses demonstrates that reactivity by two screening assays, employed sequentially in a confirmatory algorithm, would have PPV higher than 90% if the assays are selected from different manufacturers. By incorporating higher S/C values into the interpretation, the PPV could be increased to >95% with a minimal loss of sensitivity of ~1%.
Figure 3.
Proposal of an algorithm for HIV screening at Blood Banks in Brazil.
Acknowledgments
This work was supported by NHLBI contract HHSN268200417175C
Abbreviations
- IA
Immunoassays
- S/C
Signal-to-cutoff optical density ratio
- PPV
positive-predictive value
- WB
Western blot
- WHO
World Health Organization
- CDC
Centers for Disease Control
Footnotes
Conflict of interest: none
References
- 1.Brasil. Ministério da Saúde. Secretaria de Vigilância Sanitária. Portaria no488 de 17 de junho de 1998. In: Sanitária SdV., editor. Agência Nacional de Vigilância Sanitária. Diário Oficial da União; jun 18, 1998. pp. 3–4. [Google Scholar]
- 2.Camargo ME, Segura EL, Kagan IG, et al. Three years of collaboration on the standardization of Chagas’ disease serodiagnosis in the Americas: an appraisal. Bull Pan Am Health Organ. 1986;20:233–44. [PubMed] [Google Scholar]
- 3.Carneiro-Proietti AB, Sabino EC, Sampaio D, et al. Demographic profile of blood donors at three major Brazilian blood centers: results from the International REDS-II study, 2007 to 2008. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seed CR, Margaritis AR, Bolton WV, et al. Improved efficiency of national HIV, HCV, and HTLV antibody testing algorithms based on sequential screening immunoassays. Transfusion. 2003;43:226–34. doi: 10.1046/j.1537-2995.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 5.Owen SM, Yang C, Spira T, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol. 2008;46:1588–95. doi: 10.1128/JCM.02196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Groen G, Van Kerckhoven I, Vercauteren G, Piot P. Simplified and less expensive confirmatory HIV testing. Bull World Health Organ. 1991;69:747–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Joint United Nations Programme on HIV/AIDS (UNAIDS)-WHO. Revised recommendations for the selection and use of HIV antibody tests. Wkly Epidemiol Rec. 1997;72:81–7. [PubMed] [Google Scholar]
- 8.Bennett B, Branson B, Delaney K, et al. The association of Public Health Laboratories and the centers for Disease Control and Prevention; 2009. HIV testing algorithms a status report, 2009 [monograph on the internet] Available from: http://www.aphl.org/aphlprograms/infectious/hiv/Documents/StatusReportFINAL.pdf. [Google Scholar]
- 9.Candotti D, Temple J, Owusu-Ofori S, Allain JP. Multiplex real-time quantitative RT-PCR assay for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. J Virol Methods. 2004;118:39–47. doi: 10.1016/j.jviromet.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Rawal BD, Degula A, Lebedeva L, et al. Development of a new less-sensitive enzyme immunoassay for detection of early HIV-1 infection. J Acquir Immune Defic Syndr. 2003;33:349–55. doi: 10.1097/00126334-200307010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Interpretation and use of the western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb Mortal Wkly Rep. 1989;38:1–7. [PubMed] [Google Scholar]
- 12.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–9. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Guan M. Frequency, causes, and new challenges of indeterminate results in Western blot confirmatory testing for antibodies to human immunodeficiency virus. Clin Vaccine Immunol. 2007;14:649–59. doi: 10.1128/CVI.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laperche S. Antigen-antibody combination assays for blood donor screening: weighing the advantages and costs. Transfusion. 2008;48:576–9. doi: 10.1111/j.1537-2995.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 15.Pilcher CD, Christopoulos KA, Golden M. Public health rationale for rapid nucleic acid or p24 antigen tests for HIV. J Infect Dis. 201(Suppl 1):S7–15. doi: 10.1086/650393. [DOI] [PubMed] [Google Scholar]
- 16.Sickinger E, Jonas G, Yem AW, et al. Performance evaluation of the new fully automated human immunodeficiency virus antigen-antibody combination assay designed for blood screening. Transfusion. 2008;48:584–93. doi: 10.1111/j.1537-2995.2007.01583.x. [DOI] [PubMed] [Google Scholar]
- 17.Klarkowski DB, Wazome JM, Lokuge KM, et al. The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm. PLoS One. 2009;4:e4351. doi: 10.1371/journal.pone.0004351. [DOI] [PMC free article] [PubMed] [Google Scholar]