Abstract
Glial cell line-derived neurotrophic factor (GDNF) protects dopamine (DA) neurons from 6-hydroxydopamine (6-OHDA) toxicity. We have now explored this protection over 8 weeks following toxin administration. Infusion of Fluoro-Gold (FG) into striatum was followed 1 week later by GDNF (9 μg) or its vehicle. Six hours later, animals received 6-OHDA (4 μg) into the same site. 6-OHDA caused a loss of cells in the substantia nigra that expressed both FG and tyrosine hydroxylase (TH) and striatal terminals expressing TH, the high affinity dopamine transporter (DAT), and the vesicular monoamine transporter 2 (VMAT2) as assessed 2-8 weeks later. Loss of FG+ cells, and striatal DA was completely blocked by GDNF by 2 weeks. In contrast, GDNF only slightly attenuated the loss of TH, DAT, or VMAT2 in striatum at 2 wks, but had restored these markers by 4-8 weeks. Thus, GDNF prevents DA cell death and loss of striatal DA content, but several weeks are required to fully restore the dopaminergic phenotype. These results provide insight into the mechanism of GDNF protection of DA neurons, and may help avoid incorrect interpretations of temporary phenotypic changes.
Keywords: Neuroprotection, oxidative stress, Parkinson's disease, striatum, substantia nigra, glial cell line derived neurotrophic factor
1. Introduction
Among the cells lost in Parkinson's disease (PD) are the dopamine (DA) neurons projecting from the substantia nigra (SN) to the striatum. The loss of these neurons is believed to be responsible for many of the motor deficits associated with the disease. Current pharmacotherapy for PD can alleviate many symptoms of the disorder but does not appear to significantly attenuate the neurodegenerative process. However, neurotrophic factors are a promising avenue for neuroprotective therapies. Much of the evidence for this comes from studies of glial cell line-derived neurotrophic factor (GDNF), a member of the TGFβ family member that is highly expressed in the striatum (Stromberg et al., 1993) as well as other regions of the brain. First, GDNF is a potent survival factor for cultured dopaminergic cells (Lin et al., 1993; Kramer et al, 1999; Gong et al., 1999; Schatz et al., 1999; Ugarte et al., 2003; Ding et al., 2004), and GDNF or a viral vector containing the GDNF gene can protect animals from the behavioral and neuropathological effects of 6-OHDA (Hoffer et al., 1994; Bowenkamp et al., 1995; 1996; Kearns & Gash, 1995; Choi-Lundberg et al., 1998; Garbayo et al., 2009). Second, injury to the brain can increase GDNF (Naveilhan et al., 1997; Liberatore et al., 1997; Sakurai, et al., 1999; Wei et al., 2000; Smith et al., 2003; Cheng et al., 2008). Third, age-related loss of tyrosine hydroxylase (TH) expression in the SN is accelerated in a heterozygous mouse model containing only one copy of the GDNF gene (Boger et al., 2006). Fourth, the loss of DA neurons in patients with PD is accompanied by a reduction of GDNF as compared to age-matched controls (Siegel and Chauhan 2000), suggesting that reduced trophic support may be a causal factor in the genesis of the disease (Appel 1981).
Studies have been somewhat equivocal regarding the efficacy of exogenous GDNF in the treatment of PD. Some groups have reported improvements in clinical symptoms and neuropathology (Gill et al., 2003; Patel et al., 2005; Slevin et al., 2006), whereas others have shown no clinical improvement (Nutt et al., 2003; Lang et al., 2006) (see Sherer et al., 2006 for review of the issues). Despite this controversy, we believe GDNF and its family members to be prime candidates as a therapeutic treatment against degeneration of the nigrostriatal DA system in PD, and that a full understanding of the neuroprotective effects of GDNF will be useful in the development of additional therapies for the disease. Moreover, a better understanding of the changes produced by GDNF on DA neurons should also shed light on the best ligands to use in quantifying the impact of treatment via imaging approaches such as SPECT and PET.
In this report we explore the effects of GDNF in a 6-OHDA rat model of the DA deficiency in PD. We examine several phenotypic markers of the nigrostriatal system over an 8-wk period to gain further insight into the nature of GDNF-induced protection of DA neurons against oxidative stress.
2. Results
2.1 Distribution of exogenous GDNF after intrastriatal administration
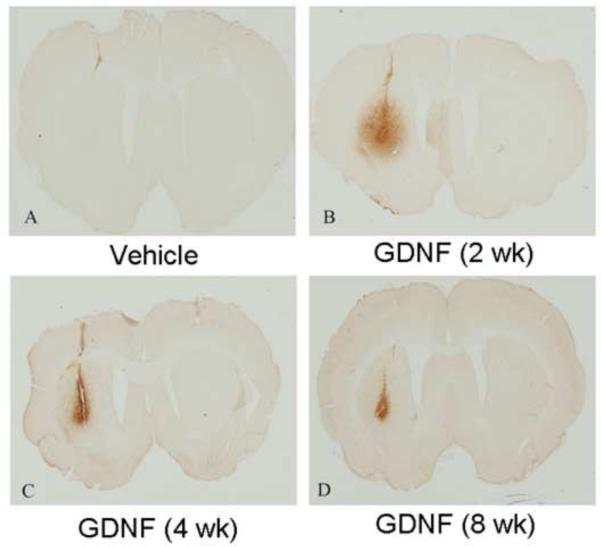
No GDNF immunoreactivity was observed in animals treated with 6-OHDA alone or with the 6-OHDA vehicle at any time point examined. Two weeks after infusion of GDNF alone or with 6-OHDA, a large spread of GDNF immunoreactivity beyond the needle track was observed in striatum. However, by 4 and 8 wks, GDNF was largely confined to the needle track (Fig. 1). No GDNF was observed in the SN of any animals at any time point (data not shown).
Figure 1.
Photomicrographs of GDNF-immunoreactivity in the striatum. Vehicle animals (a) showed no GDNF immunoreactivity in the striatum, while GDNF immunoreactivity was present in the striatum of GDNF (2 wk) (b), GDNF (4 wk) (c), and GDNF (8 wk) (d) treated animals.
2.2 Effect of GDNF on the 6-OHDA-induced loss of TH, DAT, and VMAT2 immunoreactivity in the striatum
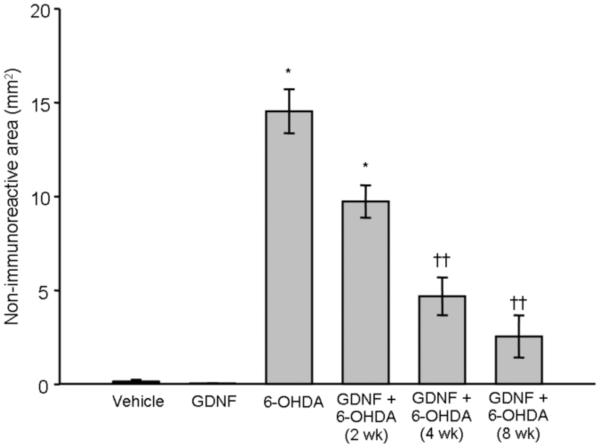
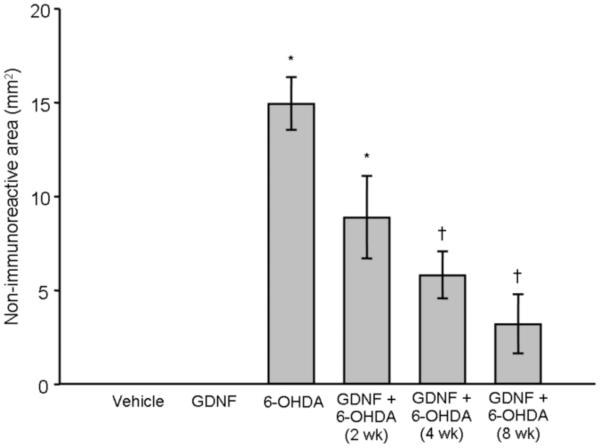
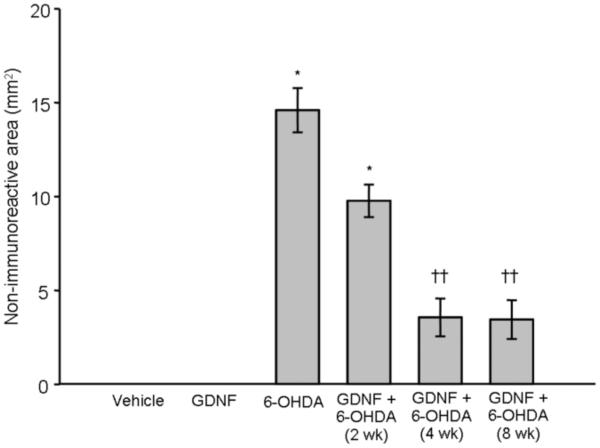
Treatment with vehicle or GDNF alone had no effect on TH, DAT, or VMAT2 immunoreactivity in striatum at any time point examined. In contrast, a one-way ANOVA revealed that animals treated with 6-OHDA alone (n = 25) displayed a significant loss of these phenotypic markers at each time point examined (p < 0.001 vs. vehicle and GDNF alone; Fig 2) [Overall group effect: TH: F(6,55) = 27.96; p < 0.0001; Fig 2a; DAT: F(6,55) = 22.29; p < 0.0001; Fig 2b; VMAT2: F(6,55) = 38.65; p < 0.0001; Fig 2c]. A significant reduction in lesion size was observed with TH, DAT, and VMAT2 at 4 and 8 wks in animals that received GDNF prior to 6-OHDA compared to animals treated with 6-OHDA alone, showing an 84, 80, and 76% reduction in the loss of immunoreactivity (p < 0.01) at 8 wks post-infusion, respectively.
Figure 2.
Effects of GDNF on phenotypic markers of DA terminals in striatum. Significant loss of TH (panel A), DAT (panel B), and VMAT2 (panel C), was observed in the striatum after 6-OHDA (* p < 0.01 vs. vehicle and GDNF). Animals given 6-OHDA with GDNF displayed significant loss of these markers after 2 wks when compared to animals that just received vehicle or GDNF (* p < 0.01). However, this 6-OHDA-induced loss was greatly attenuated by GDNF at 4 and 8 wks (†p < 0.01; ††p < 0.001 vs. 6-OHDA alone) and was not statistically different from animals treated with just vehicle or GDNF. All values are expressed as average lesion area in mm2 ± SEM.
2.3 Effect of GDNF on the 6-OHDA-induced loss of TH positive neurons
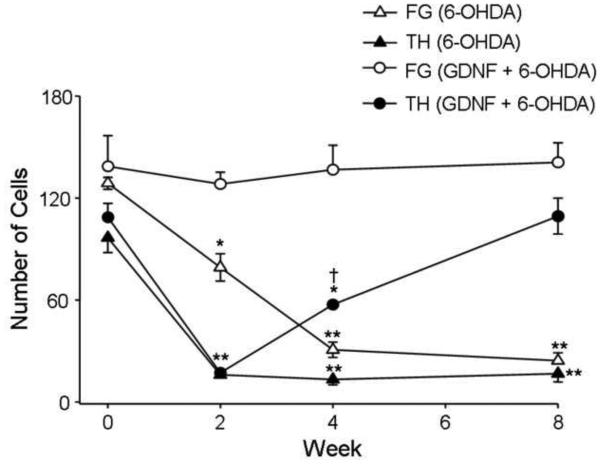
6-OHDA caused a significant loss in TH+ cells in the SN at 2 wks post-6-OHDA infusion that persisted for at least 8 wks (p < 0.001) [Overall group effect: F(7,60) = 15.14; p < 0.001]. GDNF alone had no significant effect on the number of TH+ cells at any time point (p > 0.05), and GDNF infusion prior to 6-OHDA did not prevent the loss of TH+ cells in the SN at 2 wks. However, by 8 wks there was no longer any significant difference between the number of TH+ cells in the SN of animals treated with GDNF plus 6-OHDA and vehicle-treated animals sacrificed at 2, 4 and 8 wks depicted as time 0 (p < 0.001; Fig 3 and supplemental figure).
Figure 3.
Effects of GDNF on loss of TH+ and FG+ cells in the SN after 6-OHDA. Since no significant effect of vehicle treatment was observed at any time, time 0 represents the mean values for all vehicle treated animals sacrificed at 2, 4, and 8 wks. There was a significant loss of TH+ cells in the SN at 2, 4 and 8 wks post infusion in animals treated with only 6-OHDA when compared to vehicle treated animals depicted as time 0 (**p < 0.001). Infusion of GDNF did not prevent a 6-OHDA-induced loss of TH+ cells when assessed at 2 wks compared to vehicle treated animals (**p < 0.001). However, by 4 wks the number of TH+ cells in the animals given both GDNF and 6-OHDA was significantly higher than that at 2 wks (†p < 0.05), although it was still significantly lower than vehicle (*p < 0.05). By 8 wk, no significant loss of TH+ cells was observed in 6-OHDA-treated animals given GDNF when compared to vehicle treated animals. No significant loss of FG+ cells in the SN was observed at any time in animals given 6-OHDA and GDNF. All values are expressed average number of cells ± S.E.M.
Since we had injected FG into the striatum 1 wk prior to administering 6-OHDA into the same region, we also were able to determine the number of FG+ cells in the SN, the great majority of which were co-labeled with TH in control animals (Fig 3 and supplemental figure). A 57% loss of FG+ cells was observed at 2 wks in animals given 6-OHDA alone (p < 0.001), which was even greater at 4 wks (78%; p < 0.05) and 8 wks (83%; p < 0.001), indicating that 6-OHDA caused a gradual but profound loss of the DA cells in the SN. In contrast, GDNF given 6 hr prior to 6-OHDA completely blocked the loss of FG+ cells at each time point examined (p < 0.001, Fig 3 and supplemental figure) [Overall group effect: FG: (F(7,60) = 18.74; p < 0.001) Co-labeled: (F(7,60) = 41.81; p < 0.001)]. GDNF pretreatment failed to block the loss of co-labeling by FG and TH at 2 wks (p < 0.001). However, by 8 wk there was no longer any significant loss of co-labeled cells in the GDNF pretreated animals (Fig 3 and supplemental figure). GDNF alone had no effect on the number of co-labeled cells. In vehicle treated animals, ~56% of TH+ SN cells were co-labeled for TH and FG (data not shown).
2.4 Effect of GDNF on the 6-OHDA-induced loss of DA content in the striatum
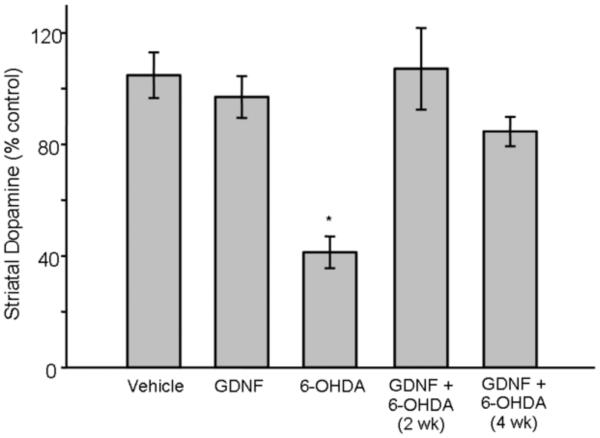
Since TH immunoreactivity was only gradually restored by GDNF pretreatment at early timepoints, we surmised that dopaminergic function also would return gradually. To begin to assess the functional impact of GDNF on the response to 6-OHDA, we measured striatal DA content. Contrary to our prediction, whereas 6-OHDA infused into the striatum produced a significant 60% depletion of DA content in the striatum 2-4 wks post-6-OHDA infusion (p < 0.01) [Overall group effect: (F(4,24) = 13.89; p < 0.0001], prior treatment with GDNF completely blocked this loss at these same time points (Fig 4).
Figure 4.
Effects of GDNF on DA content in the striatum after 6-OHDA. 6-OHDA-treated animals displayed a significant decrease in DA content (* p < 0.01). An injection of GDNF 6 hr prior to 6-OHDA infusion into the same location prevented this loss of DA content as assessed 2 and 4 wks later. Vehicle and GDNF animals showed no significant loss of DA content in the striatum. All values are expressed as a percent of loss of DA content compared to the contralateral side ± SEM.
3. Discussion
3.1 Protection of DA cells versus restoration of the DA phenotype
Our study confirms previous observations that GDNF can protect DA neurons from 6-OHDA in the adult rat (see Introduction), and extends those findings to indicate that protection occurs by several steps, including stabilization of SN cells and maintenance of DA stores, followed by restoration of phenotypic markers of DA neurons. MPTP and 6-OHDA have been reported to cause loss of the TH phenotype without loss of DA neurons in mice and rats (Sauer and Oertel, 1994; Jackson-Lewis et al., 1995; Bowenkamp et al., 1996; Ara et al., 1998; Rosenblad et al., 2003), and infusion of GDNF into the SN has been shown to block loss of SN cells produced by intra-nigral 6-OHDA or axotomy without affecting the initial loss of TH (Bowenkamp et al., 1996; Lu and Hagg, 1997). Thus, loss of the dopaminergic phenotype does not necessarily reflect the loss of the cell. In order to distinguish between these two events, we infused FG into the striatum prior to infusion of 6-OHDA, thereby retrogradely labeling SN neurons that would later be exposed to the toxin. After 6-OHDA treatment, a significant loss of FG+ cells was observed within 2 wks, and cell loss progressed over the next 6 wks as previously reported (Sauer and Oertel, 1994). 6-OHDA also caused a loss of TH+ cells in SN. However, the loss of TH was maximal at 2 wks, the earliest time studied. Similar results were observed when we examined cells co-labeled for TH and FG.
Collectively, these results and those of others (e.g., Sauer and Oertel, 1994; Bowenkamp et al., 1996; Lu and Hagg, 1997) indicate that some of the initial loss of TH immunoreactivity results from downregulation of TH gene expression rather than cell loss. This is an important distinction since it indicates that a window of opportunity is likely to exist during which effects of oxidative stress might be reversible. Indeed, GDNF-induced protection of DA neurons has been observed with intervals as long as 4-5 wks between treatments with neurotoxins and GDNF (Hoffer et al., 1994; Bowenkamp et al., 1996; Aoi et al., 2001; Kirik et al., 2001; Yang et al., 2009).
We administered GDNF 6 hrs prior to 6-OHDA. This treatment ultimately protected against the toxin, but full protection took several weeks. Whereas GDNF blocked the 6-OHDA-induced loss of FG+ cells at all times examined, the trophic factor failed to block the loss of TH+ cells in SN at 2 wks. Instead, TH immunoreactivity in SN – as well as phenotypic markers for DA terminals in striatum – was gradually restored by GDNF over an 8 wk period.
The inability of GDNF to protect cells from the initial 6-OHDA-induced decrease in phenotypic markers indicates that the trophic factor does not initially block the full impact of oxidative stress caused by 6-OHDA. Instead, GDNF may cause a temporary shift in DA neurons from normal maintenance to a mode in which the cell has prioritized repair or regenerative processes. Perhaps only after neuronal survival has been assured, can the full DA phenotype be restored. Studies of gene expression will be required to test this hypothesis.
Our observations contrast with the report that GDNF downregulates TH protein in the striatum and SN many months after a large increase in striatal GDNF produced by the intrastriatal delivery of the GDNF gene using a viral vector and GDNF protein injection in the rat (Rosenblad et al., 2003, Salvatore et al., 2004) since we were unable to detect any significant effects of intrastriatal GDNF given alone on total TH protein or striatal DA content. This discrepancy may reflect differences in the times of measurement and/or the levels of GDNF that were achieved in the nigrostriatal pathway.
3.2 Impact of GDNF on the 6-OHDA-induced loss of DA stores
In order to begin to determine the functional effects of GDNF, we examined striatal DA content. 6-OHDA produced a persistent loss of the neurotransmitter as would be expected from neuronal degeneration. To our surprise, however, no loss of DA was seen at either time point in animals pretreated with GDNF. We had previously observed differential regulation of TH activity and total TH protein in noradrenergic neurons compromised by 6-OHDA (Acheson et al., 1980; Acheson and Zigmond, 1981; see also Reis et al., 1975). In those studies, the ratio of TH activity to norepinephrine was increased while total TH protein was reduced. We and others have also reported an increase in the ratio of DOPAC to DA after the 6-OHDA-induced loss of striatal DA (e.g., Zigmond et al., 1984, Hefti et al., 1985), as well as the maintenance of extracellular DA despite apparent DA neurons loss (e.g., Abercrombie et al.,1990; Robinson et al., 1994). Such phenomenon suggest that mechanisms exist that allow catecholamine neurons to compensate for partial damage. The maintenance of DA content in the face of reduced total TH protein in our study may be an example of such a compensatory mechanism, and could be mediated by the activation of pre-existing TH molecules. Support for this hypothesis comes from several reports that GDNF can phosphorylate TH and increase its activity both in vitro (Kobori et al. 2004) and in vivo (Salvatore et al. 2004). A GDNF-induced increase in DA content (Hudson et al., 1995; Beck et al., 1996; Martin et al., 1996, Rosenblad et al., 2003) and in the stimulus-evoked release of DA (Herbert et al., 1996; Herber & Gerhardt, 1997; Hoffman et al., 1997) has also been reported.
3.3 Duration of action of exogenous GDNF
We observed that GDNF immunoreactivity was detectable for at least 8 wks following administration. GDNF staining was maximal at 2 wks post-infusion, the earliest time point examined, and was largely confined to the needle track at 4 and 8 wks. Of course, we do not know if the GDNF detected by immunohistochemistry at 2-8 wks was biologically active. Thus, in addition to its initial effects in protecting DA neurons against 6-OHDA toxicity, the protein may play a long-term role in the dynamic changes in TH, DAT, and VMAT2, or simply set in motion pro-survival intracellular signaling cascades whose effects are manifest over the subsequent several weeks. We also cannot be certain that all of the GDNF we detected was exogenous, since we and others have shown that endogenous GDNF can be increased in response to injury caused by 6-OHDA (e.g., Smith et al., 2003). However, it is unlikely that such injury-induced increases in endogenous GDNF played a role in our studies, since no such effects were observed in our 6-OHDA-treated animals examined 2-8 wks later (see also Stromberg et al., 1993).
As in our study, Lu and Hagg (1997) demonstrated an initial downregulation of TH after GDNF and 6-OHDA that gradually recovered, as well as the protection of DA cells as measured by a retrograde tracer. However, the protection they observed disappeared if GDNF infusion was terminated, which contrasts with our findings. This might reflect the difference in our experimental paradigms. Lu and Hagg (1997) infused 6-OHDA into the medial forebrain bundle, producing a much larger, more rapid loss of nigrostriatal DA neurons than in our striatal infusion model. In addition, the previous investigators infused GDNF chronically and directly into the SN, whereas in our studies a single bolus of GDNF was infused in the striatum. These may be crucial differences as it has been suggested that GDNF infused in the SN is ineffective at protecting axons and axon terminals from 6-OHDA (Kirik et al., 2004). On the other hand, Bowenkamp and coworkers (Bowenkamp et al., 1996), who delivered 6-OHDA into the medial forebrain bundle followed by a single injection of GDNF into the SN 2 wks later, observed long-lasting restoration of DA neurons, as did we.
3.4 Mechanism of GDNF-induced neuroprotection
Our current hypothesis is that GDNF protects from the loss of DA neurons, but that there is an initial downregulation of the DA phenotype, which is only gradually restored. It remains possible that recovery of striatal markers at later timepoints is a result of sprouting. Indeed, GDNF has been shown to produce axonal sprouting in the striatum after a 6-OHDA lesion (Rosenblad et al., 1998; 1999). However, in a preliminary experiment we found no change of growth associated protein-43, a well established marker of sprouting, in either the striatum or SN of animals treated similarly with GDNF and 6-OHDA. Assuming, then, that GDNF acts to rescue DA neurons from an initial impairment induced by 6-OHDA, a critical question for future research will be the mechanism of that rescue. One factor likely to be involved is GDNF-induced activation of several survival cascades, including those involving Ras/ERK and PI3K/Akt, both of which have been shown by several labs including our own to be associated with GDNF-induced protection (Ugarte et al., 2003; Vallegas et al., 2006; Du et al., 2008; Lindgren et al., 2008). This, in turn may protect mitochondria and/or enhance antioxidant capacity. For example, GDNF has been shown to increase several antioxidant defense enzymes, including copper zinc superoxide dismutase, glutathione peroxidase and catalase, as well as glutathione (Chao and Lee, 1999) and to decrease 6-OHDA induced oxidative stress (Smith and Cass, 2007).
In conclusion, these data indicate that GDNF protected against the 6-OHDA-induced loss of DA neurons. This appeared to occur in several steps. Initially, there was an immediate protection of DA cell as assessed by FG but a loss of several phenotypic markers – TH, VMAT2, and DAT. At the same time there was maintenance of striatal DA stores at normal levels. Protection of DA neurons and striatal DA stores was followed by the gradual restoration of the phenotypic markers over 4-8 weeks. These results confirm previous reports that GDNF can protect DA neurons against oxidative stress, but that this process is more complex than might have previously been realized. Finally, they suggest that studies of GDNF-induced protection in both experimental models and in patients must take into consideration the specific markers used and the interval between trophic factor delivery and analysis of its effects.
4. Experimental Procedures
4.1 Animals
Male Sprague Dawley rats (Hilltop Lab Animals, Scottdale, PA) weighing 250-350 grams were used in these experiments. All animals were housed two per cage and maintained on 12 h light/dark cycle with food (Purina Lab Chow; Purina Labs, St. Louis, MO) and water available ad libitum. All procedures were in strict accordance with the guidelines for the NIH Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
4.2 Surgical Procedures
Seven days prior to GDNF and/or 6-OHDA infusion, animals were anesthetized with isoflurane (1-2% in 100% O2; Halocarbon, River Edge, NJ) and Fluoro-gold (FG) (Fluorochrome, Denver, CO; 2% in sterile saline; 0.2 μl at 0.05 μl/min) was infused into the right striatum (+0.7 mm anterior, −3.1 mm lateral of bregma, and 6.0 mm ventral to dura) according to the atlas of Paxinos and Watson (1982). One week after FG infusion, animals were again deeply anesthetized with isoflurane and 9 μg/3 μl GDNF (Amgen, Thousand Oaks, CA) or 3 μl of its vehicle (citrate buffer, pH 7.2) was infused into the right striatum at 0.5 μl/min at the same coordinates as FG. Six hours following GDNF administration animals were again deeply anesthetized with isoflurane and 6-OHDA (4 μg/0.75 μl at 0.5 μl/min; Regis, Morton Grove, IL) or vehicle (0.02% ascorbate in 0.9% sterile saline; Sigma, St. Louis, MO) was infused into the right striatum at the same coordinates as above.
4.3 Histological Analysis
At 2, 4, or 8 wks after 6-OHDA administration, animals were deeply anesthetized with Equithesin and sacrificed via transcardial perfusion using ice cold saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.6. Following 48 hr cryoprotection in 30% sucrose, brains were sectioned at 60 μm on a cryostat at −20°C. Coronal slices were collected in a one-in-six series. Every sixth section cut from the SN was labeled for TH and every sixth section from the striatum was labeled for TH, vesicular monoamine transporter 2 (VMAT2), DA transporter (DAT), and GDNF.
Immunohistochemistry
All sections were rinsed 3 times in 10 mM phosphate buffered saline (PBS), pH 7.6, prior to and between each incubation. PBS with 0.3% triton-X 100 was used as the diluent for all treatments unless specified otherwise. Sections were pretreated for 15 min with 1% H2O2 in PBS followed by blocking for 1 hr with 10% normal donkey serum. Primary antibody incubations occurred on a rotator overnight at 4°C using a 1:1000 dilution of mouse anti-TH antibody (Chemicon Inc, Temecula, CA, Cat. #MAB318); a 1:250 dilution of goat anti-VMAT2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, Cat.#SC7721); a 1:500 dilution of rat anti-DAT antibody (Chemicon Inc, Temecula, CA, Cat. #MAB369); or a 1:1000 dilution of a goat anti-GDNF antibody (R&D systems, Minneapolis, MN, Cat. #AF-212-NA) with 1% of the appropriate normal serum. This was followed by incubation for 1 hr at room temperature in a 1:200 dilution of the appropriate biotinylated secondary antibody (Jackson ImmunoResearch, West Grove, PA). Tissue was then treated with avidin biotin peroxidase complex (ABC-Elite, Vector Laboratories, Burlingame, CA) and subsequently with 0.02% 3,3-diaminobenzadine as the chromogen. Sections were mounted on gelatin coated slides and dehydrated in ascending concentrations of ethanol before being rinsed in xylenes and coverslipped with Permount mounting medium (Fisher Scientific, Pittsburgh, PA).
Double immunofluorescent labeling in SN
To visualize FG and TH in the same neurons, immunofluorescent labeling was utilized. Sections were treated with 10% normal goat serum for 1 hr and then incubated overnight at 4°C in a 1:1000 dilution of mouse anti-TH antibody. Sections were subsequently labeled with Alexafluor 488 (goat anti-mouse; Molecular Probes, Invitrogen, CA) at a concentration of 1:500 in PBS for 2 hrs at room temperature, washed, mounted, coverslipped in DPX mounting medium (Fisher Scientific, Pittsburgh, PA), and viewed under epifluorescent illumination on a Nikon Inverted Eclipse TE microscope (Nikon Inc., Melville, NY)
4.4 Image Analysis
Lesion area in striatum
Quantification of lesion area was performed in the striatum using MetaMorph software (Molecular Devices Corp., Downington, PA). For quantification of the area devoid of immunoreactivity, the entire coronal section was visualized using a Nikon Supercool scanner (Nikon Inc., Melville, NY). Images were then pseudocolored in MetaMorph, the lesion area on the three sections exhibiting the largest loss was circumscribed in blind fashion, and the area within this region calculated as mm2 by MetaMorph software. The average of these three sections was used as a representation of lesion area for a given animal.
Cell counts in SN
For cell counts in the SN, one of every six sections from each animal was randomly selected by Microsoft Excel for staining. We then counted all FG+, TH+, and co-labeled cells in three sections of the SN at 360 μm intervals.
4.5 HPLC Analysis
Animals were sacrificed via decapitation and a 2 mm section of striatum was dissected centered on the needle track made by prior injections. Dissected striata were assayed using minor modifications of previous method (Smith et al. 2003). Striatal tissue was suspended in 0.1 N HClO2, homogenized and centrifuged at 16,000 × g for 20 min at 4°C, and the supernatant was removed. Tissue samples were assayed for DA by injecting a 10 μl aliquot of the sample onto a reverse phase column (2.0 × 150 mm, ESA Inc., Chelmsford, MA). The mobile phase consisted of 50 mM H2NaPO4, 0.72 mM sodium octyl sulfate, 0.075 mM Na2EDTA and 16% methanol (v/v), pH 2.7. The mobile phase was pumped through the system at 0.3 ml/min using an ESA 580 pump (ESA Inc., Chelmsford, MA). Analyses were performed using an ESA Coulochem Model 4100A detector, an ESA Model 5010 conditioning cell, and an ESA Model 5014B microdialysis cell (ESA, Inc., Chelmsford, MA). The settings for detection were E1=−75mV, E2=+220mV, and guard cell=+350mV. The limits of detection for DA were in the low femtomole range.
4.6 Statistical Analysis
Data were analyzed with a two-way ANOVA, using Bonferonni-corrected multiple comparisons for post hoc analysis. There was no significant difference between the lesion area in striatum of animals treated only with 6-OHDA at the 2 (n = 9), 4 (n = 8), and 8 wk time points (n = 6); therefore these groups were pooled for further analysis.
Supplementary Material
Photomicrographs of TH+ cells in the SN. Vehicle animals (A), (B) and (C) showed no loss of TH+(A) FG+ (B) or co-labeled cells (C), whereas 6-OHDA treated animals showed significant loss of TH+, FG+ and co-labeled cells at 8 wks post-6-OHDA (D-F). Animals administered GDNF prior to 6-OHDA showed a loss of TH+ cells at 2 wks post-6-OHDA (G) but no significant loss at 4 and 8 wks post-6-OHDA (J, M). In contrast, animals administered GDNF prior to 6-OHDA showed no detectable loss of FG+ cells at 2, 4 and 8 wks post-6-OHDA (H, K, N. Such animals did show a significant loss of co-labeled cells at 2 and 4 wks post-6-OHDA (L, O) but no significant loss at 8 wks post-6-OHDA (O).
Acknowledgments
This research was submitted by ADC in partial fulfillment of the requirements for a degree of Doctor of Philosophy at the University of Pittsburgh (2006) and supported in part by grants from the USPHS (NS19608, NS45698 and R01TW008040), the Michael J. Fox Foundation and the U.S. Army (DAMD17-03-0479). ADC was supported as a predoctoral trainee on USPHS grants (NS047831). ADS was supported by a Career Development Award from the USPHS (NS45698). GDNF was a gift of Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Acheson AL, Zigmond MJ, Stricker EM. Compensatory increase in tyrosine hydroxylase activity in rat brain after intraventricular injections of 6-hydroxydopamine. Science. 1980;207:537–40. doi: 10.1126/science.6101509. [DOI] [PubMed] [Google Scholar]
- Acheson AL, Zigmond MJ. Short and long term changes in tyrosine hydroxylase activity in rat brain after subtotal destruction of central noradrenergic neurons. J. Neurosci. 1981;1:493–504. doi: 10.1523/JNEUROSCI.01-05-00493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi M, Date I, Tomita S, Ohmoto T. Single administration of GDNF into the striatum induced protection and repair of the nigrostriatal dopaminergic system in the intrastriatal 6-hydroxydopamine injection model of hemiparkinsonism. Restor. Neurol. Neurosci. 2001;17:31–38. [PubMed] [Google Scholar]
- Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann. Neurol. 1981;10:499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc. Natl. Acad. Sci. 1998;95:7659–63. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Irwin I, Valverde J, Brennan TJ, Langston JW, Hefti F. GDNF induces a dystonia-like state in neonatal rats and stimulates dopamine and serotonin synthesis. Neuron. 1996;16:665–73. doi: 10.1016/s0896-6273(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Zaman V, Smith AC, Hoffer BJ, Tomac AC, Granholm AC. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp. Neurol. 2006;202:336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J. Comp. Neurol. 1995;355:479–89. doi: 10.1002/cne.903550402. [DOI] [PubMed] [Google Scholar]
- Bowenkamp KE, David D, Lapchak PL, Henry MA, Granholm AC, Hoffer BJ, Mahalik TJ. 6-hydroxydopamine induces the loss of the dopaminergic phenotype in substantia nigra neurons of the rat. A possible mechanism for restoration of the nigrostriatal circuit mediated by glial cell line-derived neurotrophic factor. Exp. Brain Res. 1996;111:1–7. doi: 10.1007/BF00229549. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Di Liberto V, Caniglia G, Mudo G. Time-course of GDNF and its receptor expression after brain injury in the rat. Neurosci. Lett. 2008;439:24–9. doi: 10.1016/j.neulet.2008.04.089. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, Chiang YL, Qian J, Bardwaj L, Bohn MC. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Ding YM, Jaumotte JD, Signore AP, Zigmond MJ. Effects of 6-hydroxydopamine on primary cultures of substantia nigra: specific damage to dopamine neurons and the impact of glial cell line-derived neurotrophic factor. J. Neurochem. 2004;89:776–87. doi: 10.1111/j.1471-4159.2004.02415.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Li X, Yang D, Zhang X, Chen S, Huang K, Le W. Multiple molecular pathways are involved in the neuroprotection of GDNF against proteasome inhibitor induced dopamine neuron degeneration in vivo. Exp Biol Med (Maywood) 2008;233:881–90. doi: 10.3181/0712-RM-329. [DOI] [PubMed] [Google Scholar]
- Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS, Blanco-Prieto MJ. Effective GDNF brain delivery using microspheres--a promising strategy for Parkinson's disease. J. Control Release. 2009;135:119–26. doi: 10.1016/j.jconrel.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9:589–95. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Gong L, Wyatt RJ, Baker I, Masserano JM. Brain-derived and glial cell line-derived neurotrophic factors protect a catecholaminergic cell line from dopamine-induced cell death. Neurosci. Lett. 1999;263:153–6. doi: 10.1016/s0304-3940(99)00148-2. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J. Pharmacol. Exp. Ther. 1997;282:760–8. [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J. Pharmacol. Exp. Ther. 1996;279:1181–90. [PubMed] [Google Scholar]
- Hefti F, Enz A, Melamed E. Partial lesions of the nigrostriatal pathway in the rat. Acceleration of transmitter synthesis and release of surviving dopaminergic neurones by drugs. Neuropharmacology. 1985;24:19–23. doi: 10.1016/0028-3908(85)90090-5. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994;182:107–11. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, van Horne CG, Eken S, Hoffer BJ, Gerhardt GA. In vivo microdialysis studies on somatodendritic dopamine release in the rat substantia nigra: effects of unilateral 6-OHDA lesions and GDNF. Exp. Neurol. 1997;147:130–41. doi: 10.1006/exnr.1997.6571. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F, Hoffer B. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res. Bull. 1995;36:425–32. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 1995;672:104–11. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 2004;7:105–10. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Rosenblad C, Bjorklund A. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson's disease. Eur. J. Neurosci. 2001;13:1589–99. doi: 10.1046/j.0953-816x.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- Kobori N, Waymire JC, Haycock JW, Clifton GL, Dash PK. Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J. Biol. Chem. 2004;279:2182–91. doi: 10.1074/jbc.M310734200. [DOI] [PubMed] [Google Scholar]
- Kramer BC, Goldman AD, Mytilineou C. Glial cell line derived neurotrophic factor promotes the recovery of dopamine neurons damaged by 6-hydroxydopamine in vitro. Brain Res. 1999;851:221–7. doi: 10.1016/s0006-8993(99)02191-5. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006;59:459–66. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Wong JY, Porritt MJ, Donnan GA, Howells DW. Expression of glial cell line-derived neurotrophic factor (GDNF) mRNA following mechanical injury to mouse striatum. Neuroreport. 1997;8:3097–101. doi: 10.1097/00001756-199709290-00018. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–2. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Leak RK, Carlson KM, Smith AD, Zigmond MJ. Activation of extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson's disease. J Neurosci Res. 2008;86:2039–49. doi: 10.1002/jnr.21641. [DOI] [PubMed] [Google Scholar]
- Lu X, Hagg T. Glial cell line-derived neurotrophic factor prevents death, but not reductions in tyrosine hydroxylase, of injured nigrostriatal neurons in adult rats. J. Comp. Neurol. 1997;388:484–94. [PubMed] [Google Scholar]
- Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–56. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997;9:1450–60. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF, ICV GDNF Study Group Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann. Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. Academic Press; San Diego: 1982. [Google Scholar]
- Reis DJ, Giliad G, Joh J. Dynamic changes in activity and amounts of tyrosine hydroxylase in the dopaminergic nigrostriatal system in response to axonal damage. Trans. Am. Neurol. Assoc. 1975;100:229–31. [PubMed] [Google Scholar]
- Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J. Neurosci. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur. J. Neurosci. 2003;17:260–70. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle. Eur. J. Neurosci. 1999;11:1554–66. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Björklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson's disease. Neuroscience. 1998;82:129–37. doi: 10.1016/s0306-4522(97)00269-8. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Hayashi T, Abe K, Yaginuma G, Meguro T, Itoyama Y, Tabayashi K. Induction of glial cell line-derived neurotrophic factor and c-ret porto-oncogene-like immunoreactivity in rabbit spinal cord after transient ischemia. Neurosci. Lett. 1999;276:123–6. doi: 10.1016/s0304-3940(99)00804-6. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J. Neurochem. 2004;90:245–54. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schatz DS, Kaufmann WA, Saria A, Humpel C. Dopamine neurons in a simple GDNF-treated meso-striatal organotypic co-culture model. Exp. Brain. Res. 1999;127:270–8. doi: 10.1007/s002210050796. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson's disease. Mov. Disord. 2006;21:136–41. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain. Res. Brain Res. Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB. Unilateral intraputaminal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year each of treatment and withdrawal. Neurosurg. Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.5.2. [DOI] [PubMed] [Google Scholar]
- Smith AD, Antion M, Zigmond MJ, Austin MC. Effect of 6-hydroxydopamine on striatal GDNF and nigral GFRalpha1 and RET mRNAs in the adult rat. Brain Res. Mol. Brain Res. 2003;117:129–138. doi: 10.1016/s0169-328x(03)00289-4. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp. Neurol. 1993;124:401–12. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Ugarte SD, Lin E, Klann E, Zigmond MJ, Perez RG. Effects of GDNF on 6-OHDA-induced death in a dopaminergic cell line: Modulation by inhibitors of PI3 kinase and MEK. J. Neurosci. Res. 2003;73:105–112. doi: 10.1002/jnr.10632. [DOI] [PubMed] [Google Scholar]
- Villegas SN, Njaine B, Linden R, Carri NG. Glial-derived neurotrophic factor (GDNF) prevents ethanol (EtOH) induced B92 glial cell death by both PI3K/AKT and MEK/ERK signaling pathways. Brain Res Bull. 2006;71:116–26. doi: 10.1016/j.brainresbull.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Wei G, Wu G, Cao X. Dynamic expression of glial cell line-derived neurotrophic factor after cerebral ischemia. Neuroreport. 2000;11:1177–83. doi: 10.1097/00001756-200004270-00007. [DOI] [PubMed] [Google Scholar]
- Yang X, Mertens B, Lehtonen E, Vercammen L, Bockstael O, Chtarto A, Levivier M, Brotchi J, Michotte Y, Baekelandt V, Sarre S, Tenenbaum L. Reversible neurochemical changes mediated by delayed intrastriatal glial cell line-derived neurotrophic factor gene delivery in a partial Parkinson's disease rat model. J. Gene Med. 2009;11:899–912. doi: 10.1002/jgm.1377. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch. Neurology. 1984;41:856–61. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Photomicrographs of TH+ cells in the SN. Vehicle animals (A), (B) and (C) showed no loss of TH+(A) FG+ (B) or co-labeled cells (C), whereas 6-OHDA treated animals showed significant loss of TH+, FG+ and co-labeled cells at 8 wks post-6-OHDA (D-F). Animals administered GDNF prior to 6-OHDA showed a loss of TH+ cells at 2 wks post-6-OHDA (G) but no significant loss at 4 and 8 wks post-6-OHDA (J, M). In contrast, animals administered GDNF prior to 6-OHDA showed no detectable loss of FG+ cells at 2, 4 and 8 wks post-6-OHDA (H, K, N. Such animals did show a significant loss of co-labeled cells at 2 and 4 wks post-6-OHDA (L, O) but no significant loss at 8 wks post-6-OHDA (O).