Abstract
Currently, it is unclear what model of timing best describes temporal processing across millisecond and second timescales in tasks with different response requirements. In the present set of experiments, we assessed whether the popular dedicated scalar model of timing accounts for performance across a restricted timescale surrounding the 1 second duration for different tasks. The first two experiments evaluate whether temporal variability scales proportionally with the timed duration within temporal reproduction. The third experiment compares timing across millisecond and second timescales using temporal reproduction and discrimination tasks designed with parallel structures. The data exhibit violations of the assumptions of a single scalar timekeeper across millisecond and second timescales within temporal reproduction; these violations are less apparent for temporal discrimination. The finding of differences across tasks suggests that task demands influence the mechanisms that are engaged for keeping time.
Keywords: Time, Time perception, Time estimation, Prospective timing, Scalar timing PsycINFO classification: 2340
1. Introduction
Timing is fundamental to many motor and cognitive processes. Temporally coordinated movements are required to perform actions, like swinging a golf club. Timing is also required for conditioned learning and the ability to represent sequential relationships between stimuli. Though many timescales are relevant for human behavior (Buhusi & Meck, 2005; Buonomano & Karmarkar, 2002), debate exists about the nature of the mechanism(s) for timing on the order of milliseconds and seconds.
One core contention is whether timing in this range occurs via a dedicated mechanism or is simply an emergent (intrinsic) product of neural activity during a particular task (Ivry & Schlerf, 2008). Some research implicates motor system specialization for timing milliseconds (Lewis & Miall, 2003b, 2003c), because millisecond-level precision of muscle responses is needed to produce appropriate movements (Mauk & Buonomano, 2004). Intrinsic models of timing, such as state-dependent networks, may be especially suited for timing at this scale, while a dedicated process, such as a clock-counter model, may operate for seconds-length timing (Ivry & Schlerf, 2008; Mauk & Buonomano, 2004). However, a state-dependent network may only operate feasibly over a restricted timescale up to a few hundred milliseconds (Spencer, Karmarkar & Ivry, 2009). Proponents of a dedicated clock, likewise, disagree about the mechanisms involved, such as whether a pacemaker-accumulator device or a series of oscillators is responsible for timing. This leaves open the question of precisely what kind of mechanism times durations of a few hundred milliseconds and longer. If different timers operate for different timescales, where is the transition between timers? Moreover, do tasks with different response requirements depend on the same internal timing mechanism(s) (Ivry & Hazeltine, 1995; Keele, Pokorny, Corcos, & Ivry, 1985; Lewis & Miall, 2003b, Merchant, Zarco, & Prado, 2008)? Translating durations into motor programs for reproduction (motor timing), for instance, is rather different than simply comparing two durations represented in memory (perceptual timing).
1.1. Dedicated Clock - Scalar Timing Theory
Perhaps the most popular dedicated model of timing is the information processing instantiation of scalar expectancy theory (SET) (Gibbon, Church, & Meck, 1984). SET can explain timing performance regularities, such as the superimposition of normalized response rate distributions, in both humans and animals (Allan, 1998; Church, 2003; Gibbon, 1991; Grondin, 2001). Components of SET include a pacemaker that generates pulses at regular intervals and an attentionally-mediated switch (Fortin, 2003; Grondin & Rammsayer, 2003; Meck, 1984; Meck & Benson, 2002). The switch closes at the onset of a relevant stimulus, allowing pulses to flow to an accumulator. At stimulus termination the switch opens and the representation of the accumulated pulses is transferred to working memory and, eventually, long-term memory. When a judgment must be made, individuals use a ratio rule to compare the representation of the duration currently in working memory with one pulled from long-term memory (Allan, 1998; Church, 1984, 2003; Gibbon, Church, & Meck, 1984).
In SET, scalar variance from memory and decision processes is thought to overwhelm all other sources of variability (Allan, 1998; Gibbon et al., 1984; Grondin, 2001). Thus, the relationship between timing variability and the target duration should follow Weber’s law--standard deviation increases proportionally with increasing target duration. Specifically, the coefficient of variation (CV), or standard deviation divided by the mean target interval, should be constant across durations.
SET can account for human timing performance across a variety of tasks, including analogues to those used in the animal literature, (Rakitin et al., 1998; Wearden, 1991a, 1991b, 1992; Wearden, Edwards, Fakhri, & Percival, 1998; Wearden, Rogers, & Thomas, 1997), ones not requiring long-term memory access (Wearden & Bray, 2001), and tasks specially-developed for human research (e.g. temporal production, reproduction, and continuation tapping) (Ivry & Hazeltine, 1995; Keele et al., 1985; Wearden & McShane, 1988). Nevertheless, it remains unclear whether a single scalar mechanism accounts for human timing across milliseconds and seconds in both perceptual and motor tasks. This question remains unanswered by studies that test only a few durations within either the milliseconds or the seconds range and others that confound task and timescale—typically, motor tasks examine milliseconds-length durations while perceptual tasks evaluate longer durations (Allan, 1998; Gibbon, Malapani, Dale, & Gallistel, 1997). Many within-subjects comparisons across timescales usually test a single duration in each (Droit-Volet, 2002; Lavoie & Grondin, 2004; Rammsayer, 1999; Rammsayer & Lima, 1991), whereas similar comparisons across tasks have tested durations of one second and shorter (Ivry & Hazeltine, 1995; Keele et al., 1985; Merchant, Zarco, & Prado, 2008). A few widely spaced durations in a single task are insufficient to accurately characterize the mechanics of timing. Instead, a larger duration set across timescales in multiple tasks must be used to evaluate potential transitions indicative of different timing mechanisms or other critical features of an internal clock (Collyer, Broadbent, & Church, 1992; Crystal, 1999, 2001, 2003; Crystal, Church & Broadbent,1997; Rammsayer, 1999).
1.2. Transitions and nonlinearities across timescales
Researchers show little consensus about where proposed functional transitions on the temporal scale occur. Michon (1985) argued that 500 ms delineates automatic (< 500 ms) versus cognitively-mediated (> 500 ms) temporal processes, while Karmarkar and Buonomano (2007) identified this duration as the transition between a state-dependent (< 500 ms) and a scalar timer (> 500 ms). Others posit that 2–3 seconds marks the upper bound of the “psychological present” in which successively-presented stimuli are still perceived as part of the same group (Lavoie & Grondin, 2004; see Pöppel, 2004 for a review). Finally, several neuroimaging experiments implicate a shift between motor and cognitive timing systems in the region of 1 second (Lewis & Miall, 2003a, 2003b, 2003c, 2006). Pharmacological studies and behavioral studies manipulating cognitive load and controlled attention further implicate executive processes in seconds-length timing (Brown, 1997; Fortin, 2003; Fortin & Breton, 1995; Fortin & Rousseau, 1998; Rammsayer, 1992, 1997, 1999, 2006); their involvement in timing milliseconds-length durations is less clear (Grondin & Rammsayer, 2003; Macar, Grondin & Casini, 1994; Rammsayer & Lima, 1991; Rammsayer & Ulrich, 2005).
More general departures from scalar variability have been observed across a wide range of durations. For example, a review by Gibbon and colleagues (1997) evaluated the CV data from a multitude of human and animal studies and identified patterns of increasing CV for durations up to 100 ms, stable CVs from 100 ms to 1500 ms, and increasing CVs for durations 1500 ms and longer. However, these patterns were derived from visual observation of between-subject patterns, with few studies including tests of durations spanning multiple timescales within the same participants. Some animal studies have found durations that are timed with greater precision than their neighbors (Bizo, Chu, Sanabria, & Killeen, 2006; Crystal 1999, 2001, 2003; Crystal et al., 1997). Regions of maximal sensitivity have similarly been found in humans at points ranging from 272 ms to 800 ms (Collyer, Broadbent, & Church, 1994; Drake & Botte, 1993; Fetterman & Killeen, 1990; Friberg & Sundberg, 1995; Grondin, 1992). In a recent series of temporal discrimination experiments, Grondin (2010) consistently found a smaller CV for 200 ms versus 1000 ms, regardless of the number or range of comparison intervals tested. Interestingly, Lewis & Miall (2009) discovered a steady logarithmic decrease in CV as durations increased from 68 ms to 16.7 minutes (equally-spaced on a logarithmic scale) in an impressive temporal reproduction experiment. They also found greater precision in a discrimination task for a 3 second duration compared to a 600 ms duration. Despite such clear violations of scalar timing, Lewis & Miall (2009) found little evidence of breakpoints between timing mechanisms. Even though they examined a broad swath of durations spanning multiple timescales for temporal reproduction, Lewis & Miall (2009) did not specifically select their durations to focus on any specific possible breakpoint previously identified in the literature, nor did they conduct within-subject comparisons of performance on both temporal reproduction and discrimination. Moreover, in the reproduction task both encoding and reproduction of durations occurred in the presence of distraction to prevent counting. In the present study, we investigate whether a breakpoint occurs in the region around one second where a possible transition between motor and cognitive timing systems might exist. We used no target durations longer than 2 seconds, both to avoid another proposed transition point and to ensure that our durations would be difficult to support with a counting strategy (Grondin, Oullet & Roussel, 2004).
1.3. Task differences in timing
In temporal reproduction, individuals encode a duration and transform it into a motor program to produce the duration via movement. For temporal discrimination, individuals merely compare two or more abstract representations of durations in memory and generate a response to indicate whether or not they match. These different response requirements are presumed to render the tasks more reliant on motor versus perceptual processes for timing, respectively. Some studies point to common mechanism(s) across such tasks for timing in the milliseconds range (400ms: Keele et al., 1985), especially when the response requirements are closely matched (325 ms to 550 ms: Ivry & Hazeltine, 1995). More recent work has revealed hints of cross-task relationships accompanied by task-specific differences in timing variability (Merchant et al., 2008) involving durations of 1 second and less (350 ms to 1000 ms). This latter finding suggests that a distributed network of brain regions might be differentially engaged to time in different task contexts in the sub-seconds range. Indeed, both patient and neuroimaging studies show that certain brain regions (e.g. striatum, supplementary motor area, etc.) may be involved at different times in different types of timing tasks, depending on task constraints and timescales (Coull, 2004; Harrington, Haaland & Hermanowitz, 1998; Harrington, Lee, Boyd, Rapcsak, & Knight, 2004; Ivry & Keele, 1989; Lewis & Miall, 2003b, 2003c; Macar, Anton, Bonnet, & Vidal, 2004; Macar, Coull, & Vidal, 2006; Macar et al., 2002). Thus, the number of different timers, their role across tasks, and their neural implementation remain unclear.
Mounting evidence from behavioral, neuroimaging, and pharmacological studies indicates that a dedicated scalar timer may not adequately explain behaviors across motor and perceptual timing tasks requiring judgments of durations spanning milliseconds and seconds. Therefore, the current experiments systematically examine durations spanning the possible one second transition point to test the feasibility of a unitary scalar timer across this range. Of particular interest is what the interaction between task type and timescale will reveal about the properties of the mechanism(s) responsible for timing. The first two experiments examine whether a common scalar mechanism times sub- and supra-second durations within temporal reproduction. The third experiment investigates these timescales in similarly structured reproduction (motor) and discrimination (perceptual) tasks using a within-subjects design.
If a single scalar mechanism controls timing in this range in task conditions where individuals time implicitly, without counting, constant CVs should be observed across all tested durations. Moreover, Weber plots of performance across short and long durations should reveal positive linear functions with equivalent slopes. Equivalent slopes across timing tasks would, likewise, suggest the operation of a common timer (Ivry & Hazeltine, 1995). Given that the tasks used in the current experiments have different response demands, this latter claim is predicated on the assumption that motor preparation differences do not invoke changes in duration-dependent variance.
2. Experiment 1
We tested whether a single dedicated scalar mechanism accounts for the reproduction of durations several hundred milliseconds to just below two seconds in length. This range should be difficult to support via counting (Grondin, Oullet & Roussel, 2004) and it circumvents durations shorter than 200 ms which have shown violations of scalar variance (Crystal, 1999; Fetterman & Killeen, 1992; Mauk & Buonomano, 2004).
2.1. Methods
2.1.1. Participants
Seventeen (7 females, Age = 19.35 ± 1.06 years) participants completed the experiment for course credit. All participants were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971) and reported no hearing problems. They gave informed consent as approved by the University of Michigan Institutional Review Board and completed a health and activity level questionnaire.
2.1.2. Procedure
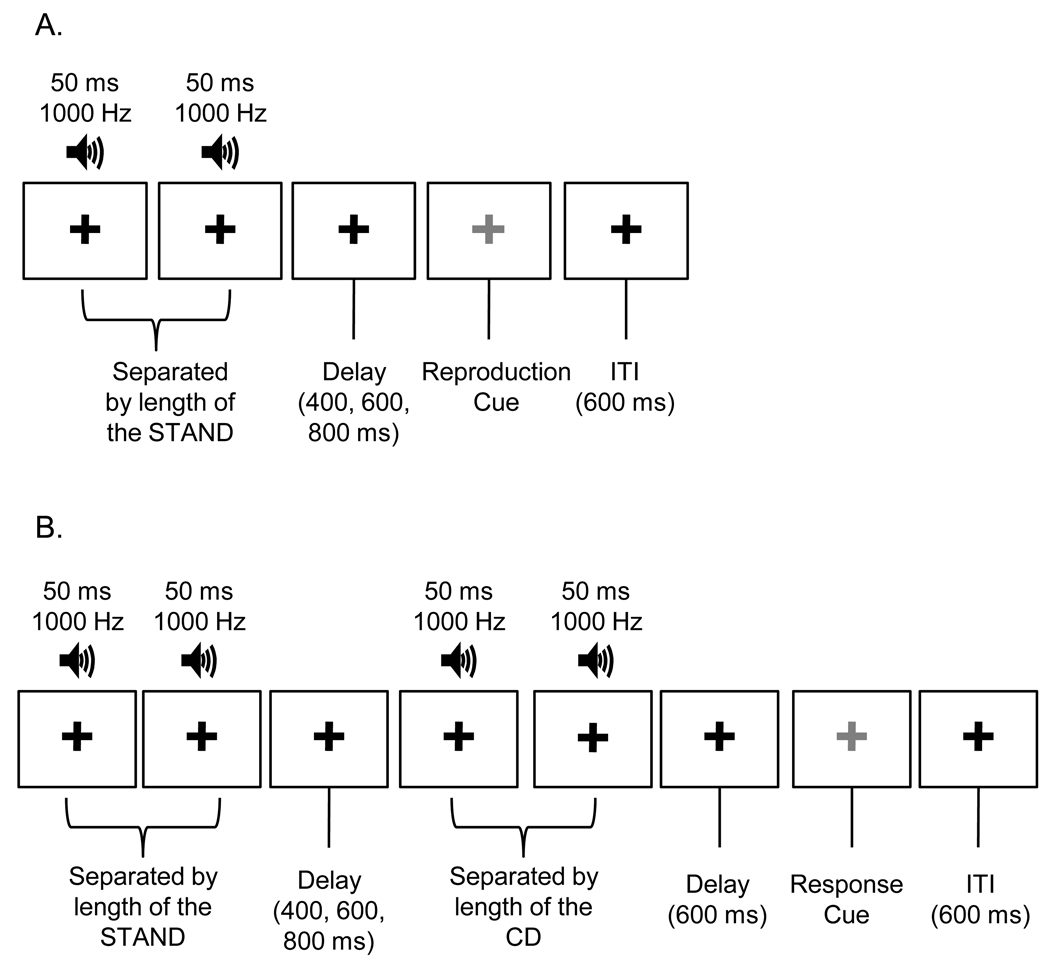
The temporal reproduction task was implemented using E-Prime software on a Dell Optiplex GX150 computer. Tones were presented binaurally via Koss UR-29 headphones. Participants reproduced five standard durations (300 ms, 650 ms, 1000 ms, 1350 ms, and 1700 ms). Instructions included demonstration trials with two durations (475 ms and 1175 ms) not used during the test phase to ensure participants were acclimated to the task. For each standard, there were 2 blocks of 5 runs each. Each run included 12 reproduction trials. In all of the experiments we conducted participants were given a short break to rest their hands and eyes after each run. In the current experiment, one full block was completed for each standard before any second blocks were presented. Within each block set, presentation order of the standards was randomized. At the start of each trial, participants focused on a black fixation cross in the center of the computer screen. Momentarily, they heard a pair of 50 ms 1000 Hz tones separated by an empty interval the length of the specified standard duration. After a variable delay (400, 600, or 800 ms) the fixation cross turned green to cue participants to reproduce the standard with two right index finger taps on the space bar. Figure 1, panel A illustrates a single trial. Participants were not told the length of the durations and were instructed not to count or produce any movements beyond those required to reproduce the standard interval. In this and the subsequent experiments, an experimenter remained in the room to monitor participants’ compliance with instructions.
Figure 1.
Trial schematic for A) temporal reproduction and B) temporal discrimination. STAND = Standard Duration, CD = Comparison Duration. Note that the grey fixation cross was green when presented to participants.
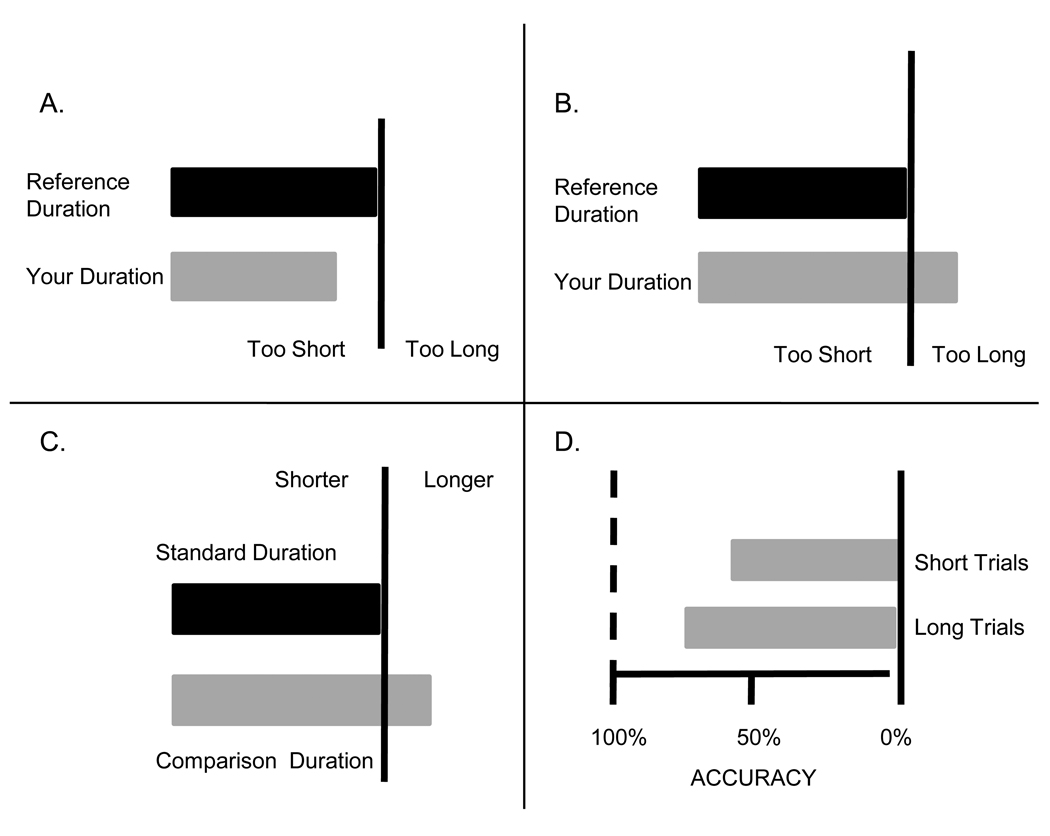
During the first run of each block, participants received trial-by-trial visual feedback consisting of a horizontal black bar representing the length of the standard duration and another bar representing the length of their reproduced duration (see figure 2 panels A and B). The length of the reproduction bar indicated whether their reproduction was shorter (panel A) or longer (panel B) than the standard without giving numerical information. For the last four runs of each block, feedback was given only at the end of the run--the mean reproduction across the entire run was reflected in the reproduction bar. At the end of the study, an exit survey assessed participants’ strategy use and compliance with instructions not to move or count. One individual reported counting and was excluded from further analyses.
Figure 2.
Feedback screens for the reproduction (Panels A and B) and discrimination (Panels C and D) tasks. In both panels A and B, the black bar represents the reference, or standard duration and the grey bar indicates the length of the participant’s reproduced duration. The vertical line is the cutoff between a reproduction that is too short or too long. Panel A shows a reproduction that was too short. Panel B shows one that was too long. Panel C shows the type of feedback given at the end of a single practice trial during the discrimination task. The black bar represents the standard duration and the grey bar represents the length of the comparison duration that was just presented on the trial. The vertical line is the cutoff between a value that is shorter than the standard and one that is longer. This example shows feedback after a longer comparison was presented. Panel D shows the type of feedback given at the end of each test run for discrimination. Feedback about the overall percentage of correct classifications of each comparison duration type (short or long) over the run are shown in the feedback. The closer the grey bars are to 100%, the better the classification of comparison durations in relation to the standard. Note that the grey bars in all of the feedback screens were actually red when presented to participants.
2.1.3. Data analysis
The first run in each block was considered practice and was excluded from analyses. For the test runs, we excluded trials where individuals responded prior to the cue. Within participants we also eliminated reproductions falling more than 2.5 standard deviations outside of the individual’s overall mean. For each test run we then calculated median latency to first tap (the time for individuals to begin their reproduction), accuracy index (ratio of mean reproduced duration to standard duration) and coefficient of variation (CV) (the standard deviation of reproductions divided by the mean reproduced value). CV is a measure of temporal sensitivity where lower values indicate a better ability to discriminate or reproduce a particular duration with consistency (Gibbon et al., 1997). The accuracy index allows for normalized comparisons across standard durations. A value of one represents perfect accuracy; values greater than one and less than one indicate reproductions that are longer and shorter than the standard, respectively (Baudouin, Vanneste, Pouthas, & Isingrini, 2006). We examined latency to first tap to determine whether people show an increase in the time needed to access and translate memory representations into motor responses with increasing standard duration length.
In addition, Weber slope analysis (Ivry & Hazeltine, 1995; Spencer & Zelaznik, 2003) was used to distinguish duration-dependent, or “clock” variance from variance due to peripheral factors, such as motor variability. These sources of variability are indistinguishable using the CV. Following the generalized form of Weber’s law, variance is plotted on the ordinate with reproduced duration squared on the abscissa. The slope of the linear regression function fit to these data represents duration-dependent (“clock”) variance while the intercept represents peripheral variance.
To determine whether clock variance for short and long durations was equivalent, we first examined where each individual showed the most pronounced break (last point of the first fitted line) in performance. This was achieved by finding the best-fitting independent bi-linear regression function to the data, indicated by the lowest sum of squared error. Lewis & Miall (2009) used a similar bi-segmental fitting procedure with logarithmic regression to search for breakpoints in CV data. We searched for breaks in the present manner instead of using an arbitrary breakpoint of one second, because we expected individual differences in the location of any transition between timing systems. For each portion of the bilinear fit, we calculated the Weber fraction (square root of the regression slope) as an estimate of the rate of change in variability with changes in duration. We then compared these fractions to assess whether the mechanisms for timing across the break are equivalent.
Repeated measures (RM) analysis of variance (ANOVA) with standard duration (5) and run (8) as within-subjects variables was used to examine accuracy index, CV, and first tap latency performance. In this and all subsequent experiments the Huynh-Feldt correction was applied when sphericity was violated, and main effects of duration and run were further examined for linear or quadratic trends. Main effects were also explored using post-hoc t-tests. All post-hoc tests and correlations were assessed with Bonferroni-corrected α = .05, two-tailed, unless otherwise noted.
2.2. Results
2.2.1. Accuracy index
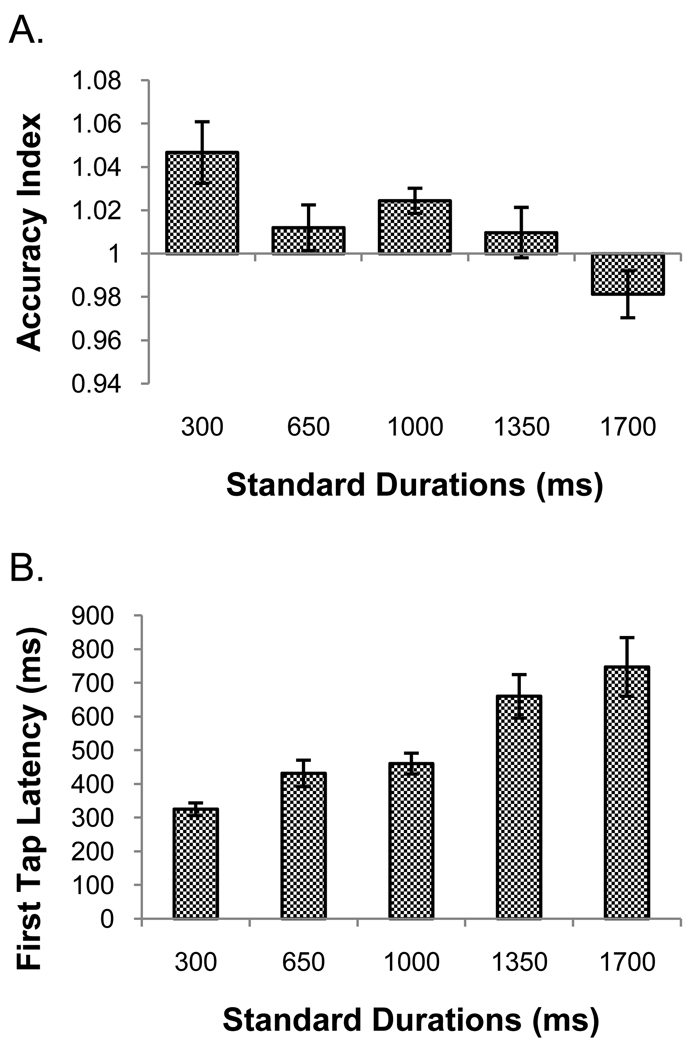
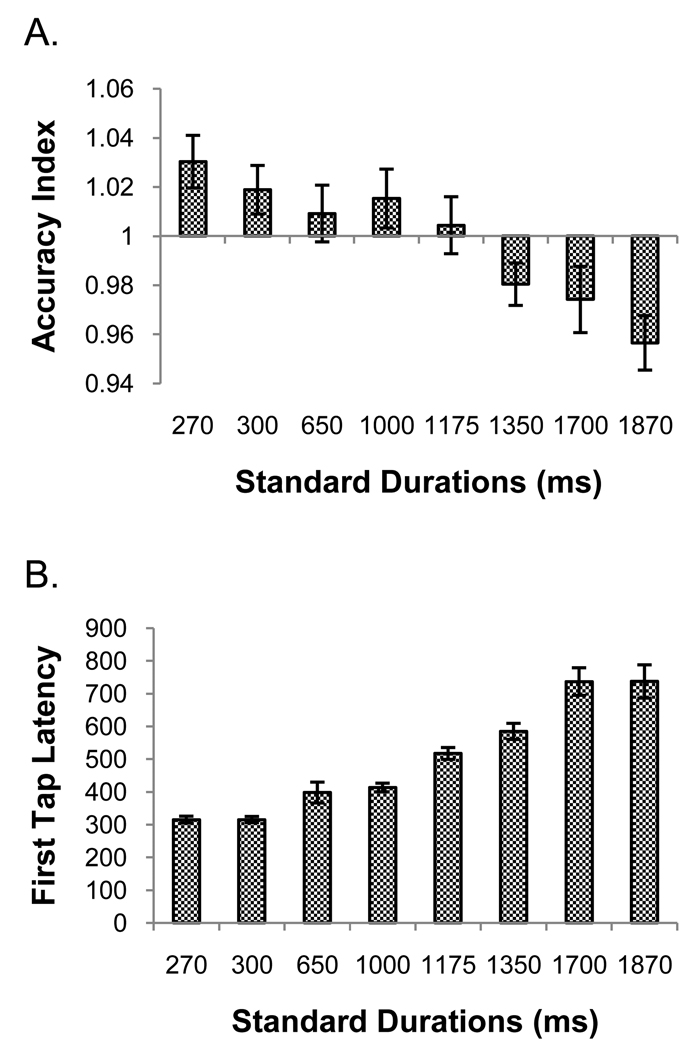
One participant’s data was excluded because her accuracy index score for the 1000 ms duration fell outside of the group mean ± 2.5 standard deviations. A RM ANOVA revealed no significant interaction, but main effects of run, F(7, 98) = 2.73, p = .013, ηp2 = .16 and duration, F(4, 56) = 5.82, p = .002., ηp2 = .29. No significant linear or quadratic trends were found for the run effect. The duration effect was explained by a significant linear trend, F(1, 14) = 9.19, p = .009, ηp2 = .40, of decreasing accuracy index with increasing length of the standard durations. Figure 3 panel A shows each duration’s accuracy index collapsed across runs. Participants tended to overshoot their reproductions for the shortest durations and undershoot for the longest duration. However, the range of the values on the ordinate is greatly compressed, indicating that, on the whole, reproductions were fairly accurate.1
Figure 3.
Panel A shows the accuracy index data from temporal reproduction in experiment 1. The abscissa crosses the ordinate at the point which represents perfect accuracy. Values greater than 1 indicate over-reproductions, while values less than 1 indicate under-reproductions. Panel B shows the latency to first tap data for reproduction from experiment 1. Error bars are mean ± 1 standard error.
2.2.2. Measures of Sensitivity: CV
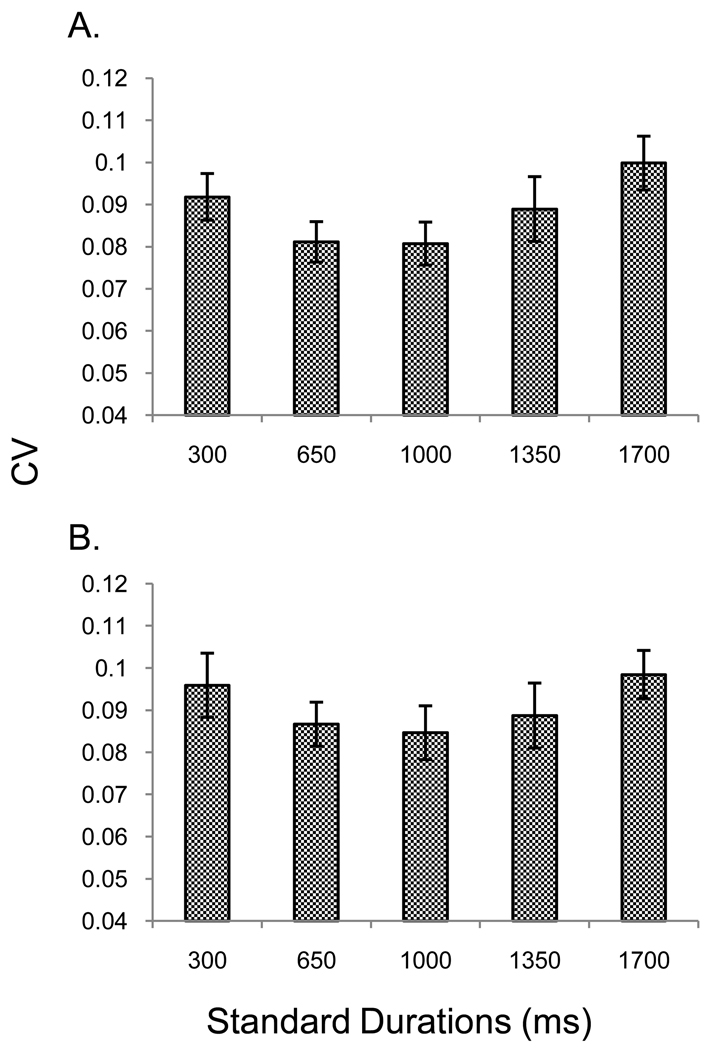
A RM ANOVA on CV revealed no interaction, but main effects of both duration, F(2.56, 38.40) = 3.14, p = .043, ηp2 = .17 and run, F(5.02, 75.23) = 2.46, p = .041, ηp2 = .14. Neither a linear nor a quadratic trend was found for the run effect. We did find a quadratic trend for duration, F(1, 15) = 17.99, p = .001, ηp2 = .55, with the minimum value of the function, M = .08, at both the 650 ms and 1000 ms standards.2 Figure 4 panel A shows the mean CV collapsed across runs. Post-hoc pairwise comparisons revealed a significant difference between CVs for 650 and 1700 ms (p = .049).
Figure 4.
Panel A shows the CV data from the reproduction task of experiment 1 collapsed across all participants and runs. Panel B shows the CV data for the first set of blocks only. Error bars represent the mean ± 1 standard error.
We performed correlations between CVs across standard durations; a single timer would result in significant positive relationships between all CVs, because the timing mechanism should contribute to performance in similar ways for all durations. Specifically, an individual’s performance at one duration should enable you to predict that individual’s performance at other durations. Significant correlations were found between 650 ms and 1000 ms (r = .66) and 1350 ms and 1700 ms (r = .86), with corrected α = .05, one-tailed. There was also a trend towards a positive relationship between 300 ms and 1000 ms, r = .61, p = .060, one-tailed. This hints at a relationship within short durations and long durations but not across these ranges.
It is possible that we elicited the quadratic pattern for CV due to repetition of the full set of durations. Durations in the middle of the set could have benefited in two ways. First, neighboring durations may have overlapped in their distribution of representations of the value to be reproduced, leading to greater sensitivity for mid-point durations. Second, knowledge of the entire duration set may have led to use of an anchoring approach, whereby the endpoint durations were reproduced by simply recreating durations subjectively judged “short” and “long”, while a more discriminative strategy was used to reproduce the harder to discriminate midpoint durations. To address this issue, we examined CV data from only the first presentation of the durations (shown in figure 4 panel B). RM ANOVAS revealed no main effect of duration (p = .375) but the quadratic contrast remained significant, F(1, 15) = 6.93, p = .019, ηp2 = .32; the minimum (M = .09) occurred at 1000 ms.3 This approach must be viewed with caution, however, since it eliminated half of the observations in the experiment.
2.2.3. Measures of Sensitivity: Weber Fraction
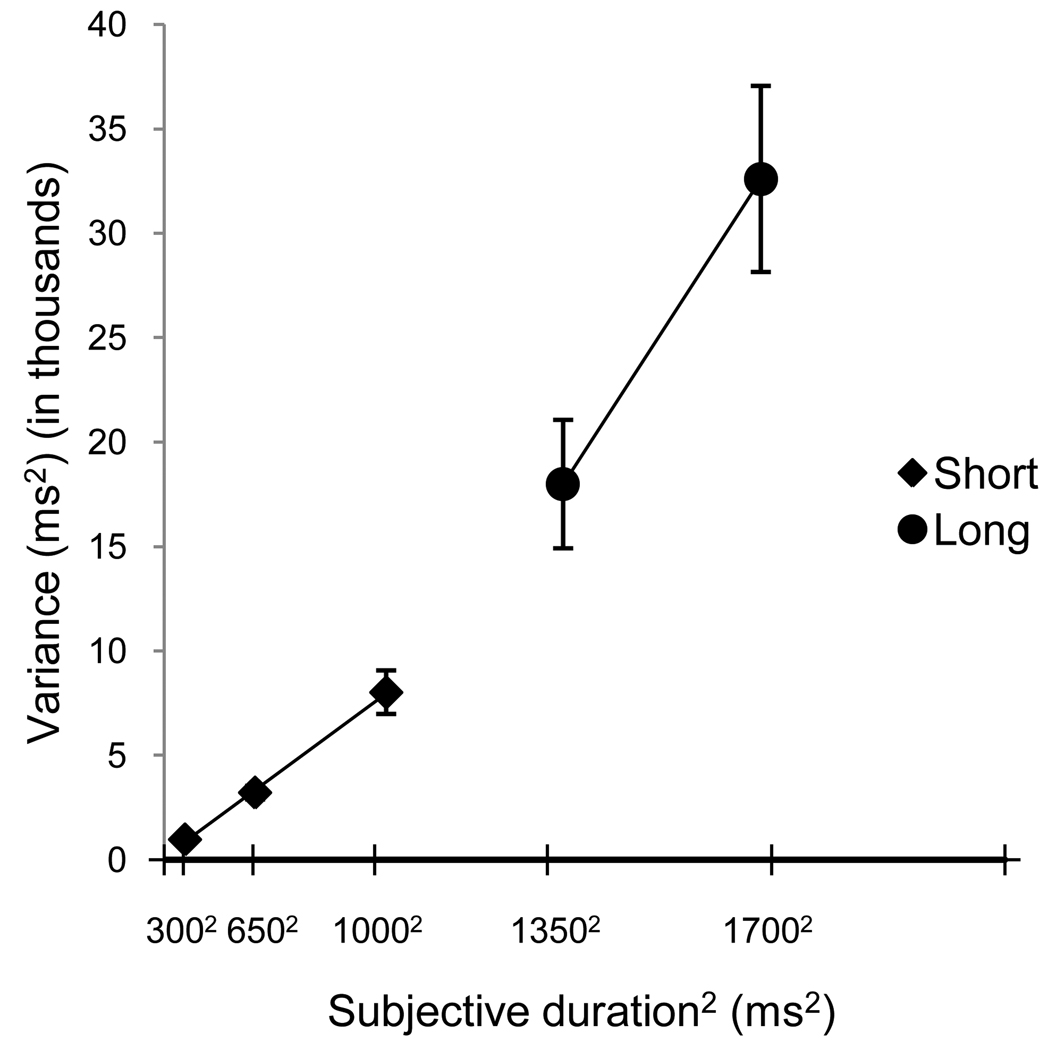
The mean breakpoint was 956 ± 120 ms. Figure 5 shows the bi-linear Weber plots fit to the averaged data across participants using the mean breakpoint. There was a significant difference, t (15) = −2.95, p = .010 between the short (M = .08 ± .02) and long (M = .12 ± .04) Weber fractions.
Figure 5.
The bi-linear Weber plots from experiment 1's temporal reproduction task fit to the averaged data across participants using the mean breakpoint. Error bars are ±1 standard error.
2.2.4. Latency to first tap
One participant whose mean latency for the 300 ms standard fell outside a range of 2.5 standard deviations around the group mean was excluded from the following analyses. A RM ANOVA showed no interaction but main effects of both run, F(2.78, 38.91) = 4.84, p = .007, ηp2 = .26, and duration, F(1.42, 19.89) = 26.16, p < .001, ηp2 = .65. The run effect showed a linear trend, F(1, 14) = 7.25, p = .018, ηp2 = .34, with tap latency decreasing over time with practice. A linear trend, F(1, 14) = 31.10, p < .001, ηp2 = .69, also explained the duration effect. As seen in figure 3 panel B, tap latency increased as the duration to be timed increased.4
2.3. Discussion
The purpose of this experiment was to determine whether the scalar property holds across a restricted set of durations spanning one second in temporal reproduction. Though individuals tended to over-reproduce short durations and under-reproduce long durations, they were generally accurate. This pattern, known as Vierordt’s Law, has been found in other studies of temporal reproduction (Wearden, 2003). Of critical note, non-constant CVs as well as different Weber fractions across the breakpoint argue against a single scalar timer. The fact that the quadratic pattern of CVs was found even for the first duration set presentation supports this finding, since individuals did not have knowledge of the entire set of standards to benefit performance for the midpoint (1000 ms). The Weber slope breakpoint was close to one second, strengthening the argument that one second marks a transition between timing systems (Lewis and Miall, 2003b; 2003c; 2006). This idea is buttressed by correlations between CVs for durations within the short set and within the long set with no significant correlations across duration sets (all p > .05, Bonferroni-corrected).
Interestingly, the latency to make the first reproduction tap increased as the standard duration increased, despite the fact that individuals experienced many trials over which to memorize each standard. It is possible that individuals rehearsed the standard on every trial before reproducing it. However, latencies were not always equal to the standard duration values, especially for longer durations. Another possibility is that the processes involved in accessing the representation of a standard duration in working memory and translating it into a motor program with the appropriate temporal properties are time sensitive. In particular, motor preparatory processes may take longer for the reproduction of longer durations.
We note that the quadratic pattern for the CVs could have resulted from use of a different strategy for reproducing the shortest and longest standards than for reproducing the intermediate standards, especially in the second block set. Instead of creating an accurate reproduction using their internal “clock” for the endpoint standards individuals may have reproduced values they categorically deemed “short” and “long”. Intermediate values would need to be timed with the more accurate “clock” since they would not lend as easily to simple categorizations. Although these issues should not have contributed to the quadratic pattern of CVs in the first duration block set, those data are tenuous, given the small number of observations that comprised them.
It is important to confirm that the quadratic pattern will hold with more experimental observations and when identification of the endpoint durations of the standard duration set is more difficult. Thus, the subsequent experiments include additional durations objectively close to the endpoints used in this experiment to force active encoding of each standard rather than use of a categorization or anchoring strategy. Individuals show greater sensitivity when they must compare hard-to-discriminate durations (Ferrara, Lejeune, & Wearden, 1997; Penney, Allan, Meck, & Gibbon, 1998), therefore, inclusion of additional values close to the endpoint standards may foster more accurate representations of these durations. An additional duration was added to the middle of the set to increase resolution around the putative breakpoint. Each standard was presented in only one block in subsequent experiments.
3. Experiment 2
This experiment examined whether a common scalar clock controls timing across durations spanning one second when the endpoints of the standard duration set are difficult to discriminate. If so, similar patterns of sensitivity (as measured by CV and Weber fractions) should be evident across all durations.
3.1. Method
3.1.1. Participants
Twenty-nine (16 females, Age = 20.41 ± 1.72 years) right-handed students with no reported hearing problems participated for course credit. Four individuals were excluded from analyses because they reported using either a counting or movement-encoding (i.e. tapping their foot) strategy during the task.
3.1.2. Procedure
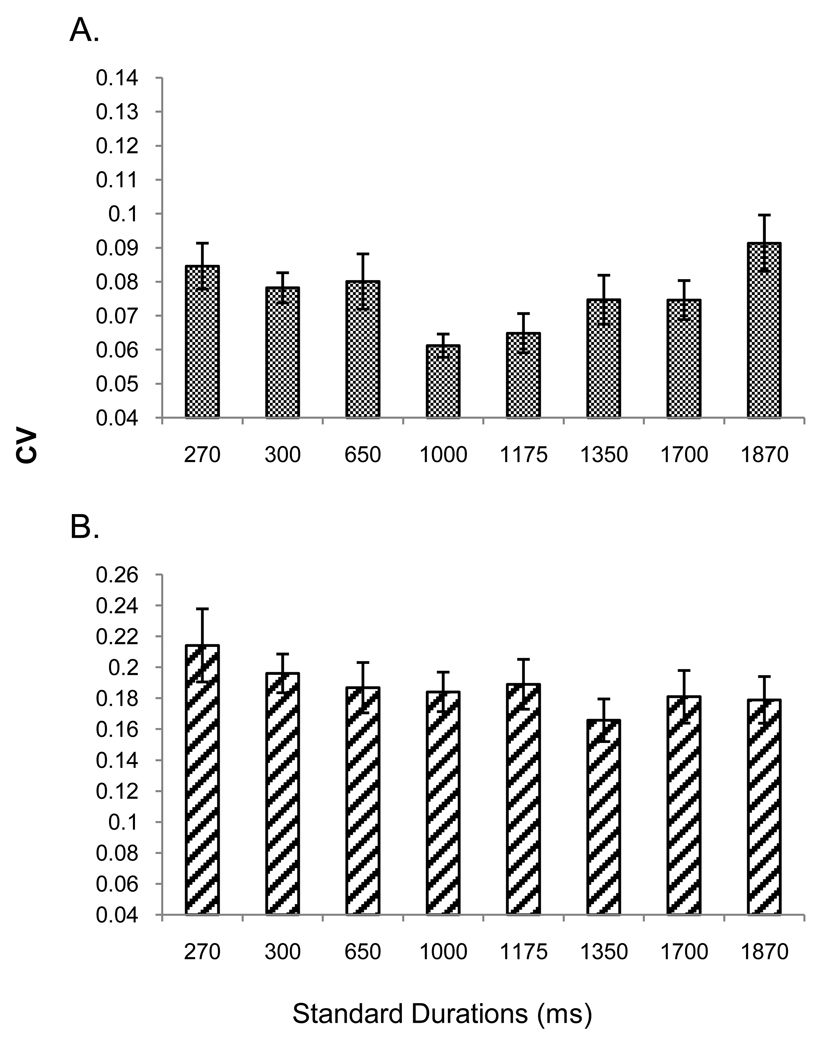
The software and hardware were the same as in experiment 1. Participants performed temporal reproduction with eight standard durations: 270, 300, 650, 1000, 1175, 1350, 1700, and 1870 ms. New endpoints were created by subtracting (for the shortest duration) or adding (for the longest duration) 10% of the value of the previous endpoints. Research suggests that a ten percent difference marks the threshold at which humans begin to be able to discriminate different durations in the milliseconds and seconds ranges (Mauk & Buonomano, 2004). The experiment involved one 3-hour testing session. Each standard was presented within a single block of 7 runs of 12 trials each. As with experiment 1 the first run was practice, included trial-by-trial feedback (see figure 2 panels A and B), and was excluded from further analyses. Feedback was given at the end of each of the remaining 6 runs. Participants were instructed not to count or move and after each duration block, they were asked to stand and stretch to combat fatigue; they were given a longer 5-minute break after completion of the first 4 duration blocks. Trial structure was identical to experiment 1 (see figure 1 panel A).
3.1.3. Data analysis
The trimming procedures, dependent measures and analyses were the same as experiment 1. RM ANOVAs with duration (8) and run (6) as within-subjects factors were used to assess accuracy index, CV, and tap latency. Planned comparisons evaluated whether the minimum CV differed from the endpoint duration CVs.
3.2. Results
3.2.1. Accuracy Index
Two participants’ data were excluded because their accuracy index score for at least one duration fell outside of the group mean ± 2.5 standard deviations. Similar to experiment 1, a RM ANOVA with the remaining participants revealed main effects of run, F(5, 35) = 2.36, p = .044, ηp2 = .10, and duration, F(7, 154) = 6.21, p < .001, ηp2 = .22, but a non-significant trend for a run by duration interaction (p = .08). The duration effect followed a linear trend, F(1, 22) = 26.13, p < .001, ηp2 = .54; individuals over-reproduced durations of 1175 ms or less and under-reproduced durations longer than 1175 ms (see figure 6 panel A). Additionally, 650 ms and 1175 ms were reproduced with the greatest accuracy.5
Figure 6.
Panel A shows the accuracy index data from the reproduction task of experiment 2. The abscissa crosses the ordinate at the point which represents perfect accuracy. Values greater than 1 indicate over-reproductions, while values less than 1 indicate under-reproductions. Panel B shows the latency to first tap data for reproduction from experiment 2. Error bars are mean ± 1 standard error.
3.2.2. Measures of Sensitivity: CV
A RM ANOVA revealed a main effect of duration, F(4.39, 105.33) = 3.52, p = .008, ηp2 = .13 and a significant run by duration interaction, F(25.31, 607.31) = 2.11, p = .001, ηp2 = .08, but no effect of run. A quadratic trend explained the duration effect, F(1, 24) = 7.44, p = .012, ηp2 =.24.6 The minimum of this function (M = .08) was found at the 1000 ms duration, as seen in figure 7. Comparisons between this point and the two endpoint durations revealed significant differences (1000 ms versus 270 ms, t(24) = − 3.07, p = .005; 1000 ms versus 1870 ms, t(24) = −3.64, p = .001). There were also differences between 1000 ms and 650 ms, t(24) = −3.79, p = .001, as well as 1000 ms and 1700 ms, t(24) = −.3.32, p = .003, all p-values one-tailed, Bonferroni-corrected.
Figure 7.
CV data from the reproduction task of experiment 2. Error bars represent the mean ± 1 standard error.
Correlations between CVs are shown at the top of Table 1. Of interest are many highly significant correlations between the long durations. There were only a few correlations between the short durations or between durations that cross one second.
Table 1.
Reproduction CV Correlations across standard durations for experiments 2 and 3.
| 270 | 300 | 650 | 1000 | 1175 | 1350 | 1700 | 1870 | |
|---|---|---|---|---|---|---|---|---|
| Experiment 2 | ||||||||
| 270 | ||||||||
| 300 | .51 | |||||||
| 650 | .41 | .54* | ||||||
| 1000 | .18 | .05 | .64** | |||||
| 1175 | .22 | .13 | .56* | .71** | ||||
| 1350 | .18 | .20 | .57** | .77** | .84** | |||
| 1700 | .14 | .03 | .49 | .74** | .88** | .75** | ||
| 1870 | .16 | .18 | .54* | .73** | .73** | .81** | .72** | |
| Experiment 3 | ||||||||
| 270 | ||||||||
| 300 | .58 | |||||||
| 650 | .71** | .58 | ||||||
| 1000 | .57 | .51 | .49 | |||||
| 1175 | .63 | .67 | .58 | .85** | ||||
| 1350 | .70* | .84** | .73** | .73** | .89** | |||
| 1700 | .78** | .58 | .54 | .81** | .84** | .80** | ||
| 1870 | .53 | .89** | .55 | .50 | .64 | .78** | .58 | |
p < .10, one-tailed
p < .05, one-tailed
3.2.3. Measures of Sensitivity: Weber Fraction
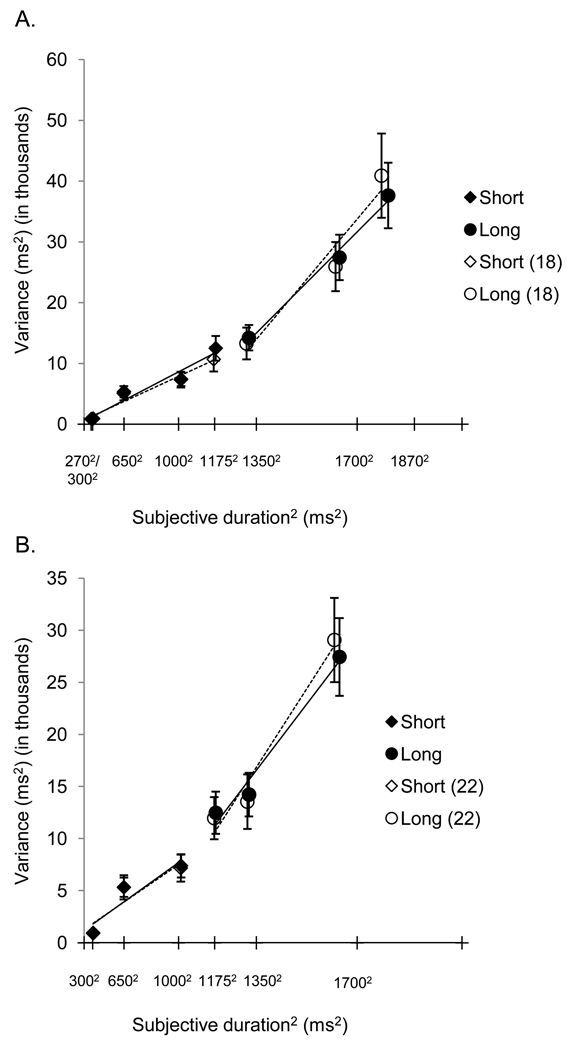
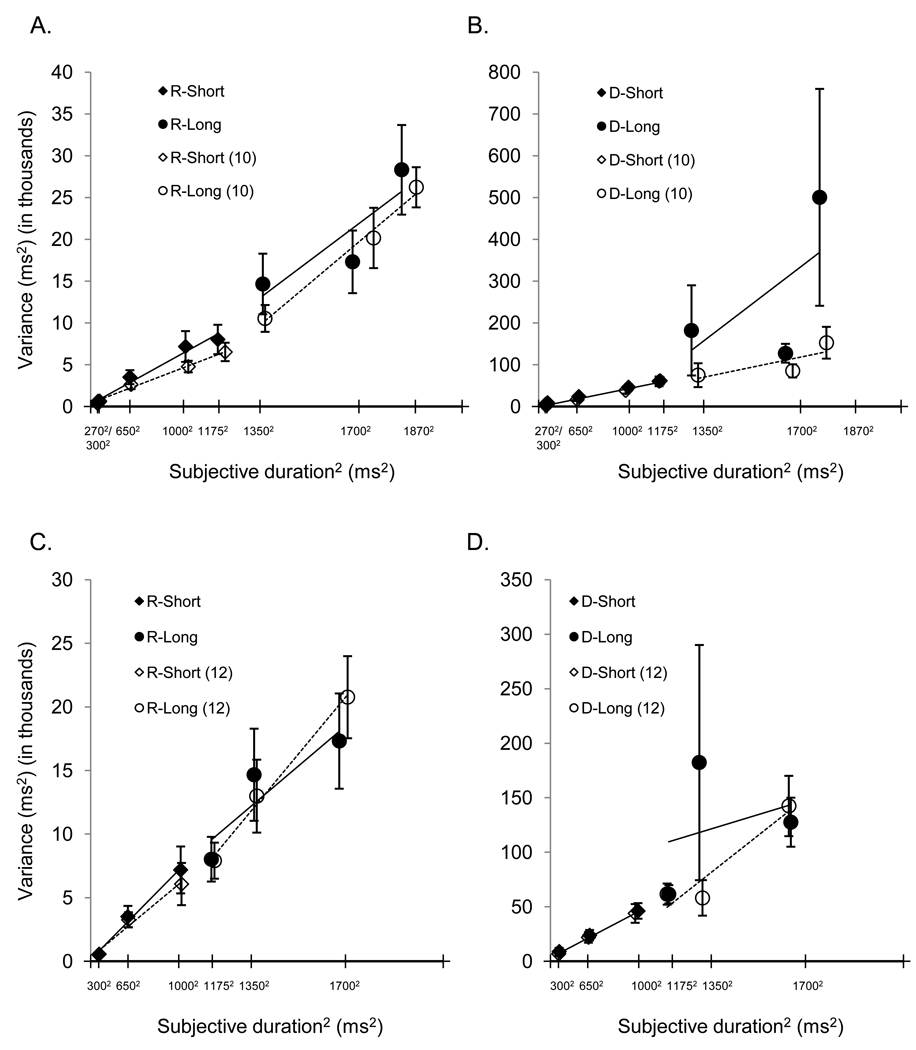
The mean breakpoint was 1252 ± 160 ms. One problem with calculating Weber fractions in this study was that the portion of the bilinear function that spanned the long duration set had a negative slope for some individuals. This violates the assumptions of scalar expectancy theory; therefore, the seven individuals who showed this pattern were eliminated from further Weber fraction analysis. Interestingly, no participant had a negative slope across the short duration set. Mean Weber fractions across the remaining participants were M = .08 ± .03 across short durations and M = .18 ± .13 across long durations; the long duration set slope was steeper than that for the short duration set. A paired t-test confirmed that these Weber fractions were significantly different, t(17) = −3.31, p = .004. Figure 8 panel A shows the average Weber function and bilinear fits calculated across all participants and across only the 18 individuals with positive slopes using the average breakpoint across all participants.
Figure 8.
Panel A shows the average Weber function and bilinear fits calculated across all participants and across only the 18 individuals with positive slopes using the average breakpoint across all participants for experiment 2's reproduction task. Panel B shows the average Weber function and bilinear fits for the truncated data from all participants and only the 22 individuals with positive slopes using the average breakpoint. In both panels, solid lines indicate the data for all participants, while dashed lines indicate data for only the individuals with positive Weber slopes. Error bars are represent the mean ±1 standard error.
One explanation for negative slopes is that having two closely spaced durations at each end of the duration set may have led to the predicted increased sensitivity for these durations. However, a consequence of this for the two longest durations is that in some cases their variability was so similar that the best fitting line across long durations was fit strictly to these two data points. Several of the resulting slopes were close to zero or negative. This could have led to an overestimate of the breakpoint via bilinear fits. Thus, our attempt to eliminate use of a categorization strategy for the endpoint durations may have complicated the Weber slope analysis. Therefore, we calculated Weber breakpoints and slopes from truncated data, with values from the two endpoints (270 and 1870 ms) removed. This analysis was on par with that conducted in experiment 1, but without the concern that the endpoint durations were reproduced using a categorization strategy. Only 3 participants had negative slopes across the long duration set. The breakpoint (1021 ± 204 ms) was similar to the value obtained in experiment 1. A paired t-test revealed a trend towards a significant difference between short and long Weber fractions, t(21) = −1.79, p = .088 (short: M = .09 ± .05; long: M = .13 ± .09).7 Figure 8 panel B shows the average Weber function and bilinear fits across all participants and across only the 22 individuals with positive slopes using the average breakpoint across all participants.
3.2.4. Latency to first tap
Two participants whose latencies fell 2.5 standard deviations outside of the group mean were excluded. For the remaining participants we found an effect of duration, F(2.35, 51.64) = 49.50, p < .001, ηp2 = .69, explained by a linear trend, F(1, 22) = 92.24, p < .001, ηp2 = .81.8 Figure 6 panel B shows the tap latency collapsed across runs; latency again increased with increases in length of the standard durations.
3.3. Discussion
The findings of experiment 2 largely replicated those from experiment 1. In particular, the Vierordt pattern of slight over-reproduction for short durations and under-reproduction for long durations held for the accuracy index as did the linear increase in tap latency across increasing standard durations. Duration also significantly affected CVs. Even with inclusion of endpoint standards designed to prevent use of a categorization strategy, a quadratic function explained the effect of duration on CV, with the minimum value, again, at the 1000 ms standard. This demonstrates a violation of the scalar property. The estimated breakpoint using all durations (M = 1252) was larger than the estimate from experiment 1 (M = 956), but the significant difference between short and long Weber fractions was replicated. One difficulty with the present experiment was negative long duration Weber slopes for some participants, which may have resulted from including endpoint durations that were difficult to discriminate from their neighbors to prevent use of a categorization strategy. Weber fractions from a truncated data set still hinted at a timescale difference for temporal reproduction, as well as a breakpoint (M = 1021) similar to that seen in experiment 1.
Correlations amongst CV values suggested that individuals engaged similar mechanisms to time longer durations. While experiment 1 suggested a common mechanism for timing short durations, as well, the current findings do not replicate this. It is possible that the durations tested within the milliseconds range actually spanned several mechanistically distinct timescales (Fetterman & Killeen, 1992; Michon, 1985). If this were the case, we would expect great variability in breakpoints, with some located close to this other transition near the shortest durations in the set. However, we found no evidence for the latter. Indeed, given the size of some of the Pearson correlation coefficients between short durations, more power could potentially reveal evidence for a common timer within this range.
Despite minor inconsistencies with the findings from the first experiment and problems fully investigating Weber fractions for the long duration sets, there is clear evidence for non-scalar timing across milliseconds and seconds in temporal reproduction. The next experiment addresses whether a similar pattern of non-scalar timing will be found in a perceptual timing task that does not require translation of durations into motor responses.
4. Experiment 3
This within-subjects experiment examined whether the patterns of variability in timing behavior in temporal reproduction would extend to a perceptual timing task with a parallel task structure. In particular, will temporal discrimination show the same non-linearity found for temporal reproduction in experiments 1 and 2? We also wanted to investigate whether individuals show inter-task CV correlations, suggesting that similar processes might be engaged for timing in both motor and perceptual tasks. If a common timing mechanism is engaged across tasks we should find also find similar Weber fractions across timescales for the two tasks. Additionally, we were interested in whether the latency to respond in temporal discrimination would increase with increasing standard duration length. Increases in latency would suggest that simply accessing a memory representation of a longer standard takes more time even in cases where recoding the duration into the motor response is not required.
4.1. Method
4.1.1. Participants
Nineteen right-handed (14 females, Age = 21 ± 3.21 years) participants with no reported hearing difficulties completed both temporal reproduction and discrimination and were paid for their participation. They completed a health history and activity level questionnaire on the first testing day and exit surveys at the end of each testing session to determine whether they used counting or movement-encoding strategies. No participant reported use of these strategies.
4.1.2. Procedure
The same 8 standard durations from experiment 2 (270, 300, 650, 1000, 1175, 1350, 1700, and 1870 ms) were used in the current experiment. The software and hardware used were also the same. Testing was completed over eight separate sessions on different days. Each individual timing task was completed in four days. All sessions for one task were completed before any of the sessions were completed for the other task; task order was counterbalanced (10 participants completed reproduction first). Each standard duration was presented within a single block of 21 runs of 18 trials each. Two standard duration blocks were completed each day. Order of blocks was pseudo-randomized so that participants were never presented with adjacent durations (e.g. 270 ms and 300 ms) from the set on the same day. The first run of each block served as practice, included trial by trial feedback, and was excluded from further analysis. Feedback was given at the end of the run for all remaining runs (see figure 2, panels A and B for temporal reproduction and panels C and D for temporal discrimination). Participants were told not to count or move during the tasks and were asked to stand and stretch to combat fatigue in the middle of each duration block; they were given a 5-minute break after completion of the first duration block each day.
The individual trial structure for each task can be seen in figure 1(panel A shows temporal reproduction, panel B shows temporal discrimination). For temporal discrimination, participants made judgments about whether comparison durations were shorter or longer than the standard duration, typical of a two-alternative forced choice procedure (Grondin & Rammsayer, 2003; Morgan, Watamaniuk, & McKee, 2000). At the start of the task, participants focused on a fixation cross and then heard two 50 ms 1000 Hz tones separated by an empty interval the length of the standard duration. After a variable delay of 400, 600, or 800 ms, they heard another pair of tones separated by the relevant comparison duration. Then the fixation turned green, cueing participants to respond either “shorter” or “longer” with a button press on the keyboard. The six comparison durations for each standard were ± 40%, ± 15%, and ± 6% of the length of the standard. Percentages were based on reports of similar comparison values used in prior studies (Grondin, 2005; Grondin et al., 2004; Grondin, Roussel, Gamache, Roy, & Ouellet, 2005) and on pilot data we collected.9 Each comparison was presented 3 times per run, totaling 60 repetitions. Cumulative responses to the comparison durations were used to construct 6-point psychometric functions; probability of responding “long” was plotted on the ordinate and comparison duration values were plotted on the abscissa.
4.1.3. Data analysis
Dependent variables (accuracy index, CV, Weber fractions, and latency to first tap) for reproduction were calculated as in experiments 1 and 2. For temporal discrimination the two critical dependent variables included the point of subjective equality (PSE) and the standard deviation. PSE is the duration which is equivalently judged as either shorter or longer than the standard duration. The standard deviation is derived from the inverse of the slope of the psychometric function at the PSE. The probit transform, which assumes a cumulative normal distribution for the form of the psychometric function, was used to determine the threshold and slope parameters from which the PSE and standard deviation were derived (see Treutwein, 1995).10 The PSE and standard deviation were used to calculate accuracy index, CV and Weber fractions for each participant. The mean across runs of the median latency to respond was also investigated. Calculation of the dependent variables for temporal discrimination was dependent on estimates derived from cumulative responses; therefore, we were unable to assess the effect of run for this task and for comparisons with temporal reproduction.
RM ANOVAs with duration (8) and task (2) as within-subjects factors were used to assess CV, accuracy index, and tap latency. For analysis of Weber fractions, the RM ANOVAs included duration set (2) and task (2) as within-subjects factors. When significant task by duration interactions were found, we conducted follow-up RM ANOVAS within each task to clarify the pattern of simple effects. In addition, we investigated within and between task correlations on CVs to determine whether, within individuals, temporal sensitivity was related across durations and tasks. These correlations were examined using Bonferroni-corrected α = .05, two-tailed.
4.2. Results
4.2.1. Accuracy index
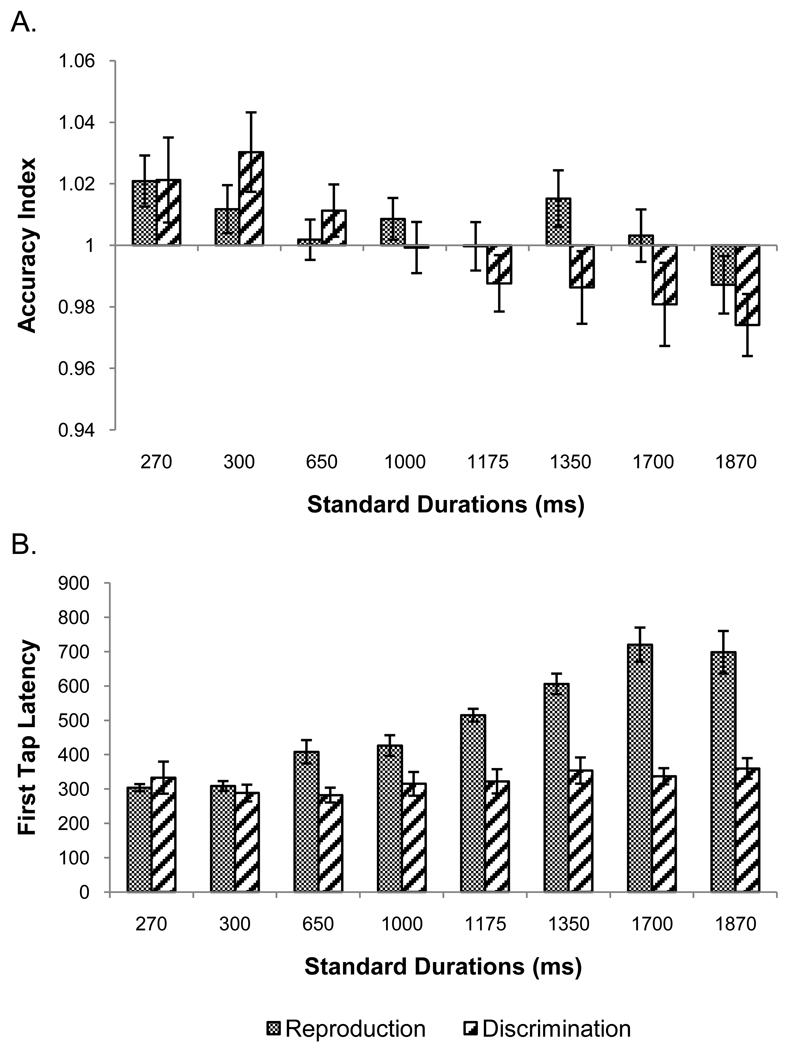
Three participants were excluded because they showed a group-level outlier accuracy index value for at least one duration in one of the timing tasks. There was no duration by task interaction (p = .211) and no effect of task (p = .317), but a significant effect of duration, F(7, 105) = 5.76, p < .001, ηp2 = .28. A linear contrast explained this effect, F(1, 15) = 17.93, p = .001, ηp2 = .54. Figure 9 panel A shows similar patterns of accurate responding across durations in the two tasks.11
Figure 9.
Panel A shows the accuracy index data from the reproduction and discrimination tasks of experiment 3. The abscissa crosses the ordinate at the point which represents perfect accuracy. Values greater than 1 indicate over-reproductions, while values less than 1 indicate under-reproductions. Panel B shows the latency to first tap data for the two tasks from experiment 3. Error bars are mean ± 1 standard error.
4.2.2. Measures of Sensitivity: CV
Data from 5 participants were excluded from the following analysis because at least one of their data points in a task was a group outlier. There was no significant duration by task interaction (p = .171). However, there were main effects of task, F(1, 13) = 77.62, p < .001, ηp2 = .86, and duration, F(7, 91) = 2.56, p = .019 ηp2 = .17. A quadratic trend explained the pattern of data across durations, F(1, 13) = 13.38, p = .003 ηp2 = .51, with minimum values (M = .12) located at the 1000 ms and 1350 ms standards.12,13 Figure 10 panels A and B show that these minimums were driven by the reproduction and discrimination tasks, respectively. Clearly, the effect of task is due to larger CVs for temporal discrimination.
Figure 10.
CV data from experiment 3 for A) reproduction and B) discrimination. Error bars represent the mean ± 1 standard error.
4.2.3. Measures of Sensitivity: Weber Fractions
The mean breakpoint for temporal reproduction was M = 1258 ± 179 and M = 1276 ± 89 for discrimination. Figure 11 panels A and B show the average Weber function and bilinear fits for the two tasks calculated across all participants and across only the participants with positive slopes using the average breakpoint for each task.
Figure 11.
Panel A shows the average Weber function and bilinear fits calculated for the reproduction task across all participants and across only the 10 individuals with positive slopes using the average breakpoint across all participants. Panel B shows these same data from the discrimination task. Panel C shows the average Weber function and bilinear fits for the reproduction task truncated data from all participants and only the 12 individuals with positive slopes using the average breakpoint. Panel D shows the same data for the discrimination task. In all four panels, solid lines indicate the data for all participants, while dashed lines indicate data for only the individuals with positive Weber slopes. Error bars are ±1 standard error.
Nine participants had negative slopes across the long duration set for at least one timing task and were excluded from the RM ANOVA. There was no significant task by duration set interaction (p = .260) nor effects of either task (p = .113) or duration set (p = .212). A cross-task comparison of the short duration set Weber fractions from all participants, however, revealed a significant difference, t(18) = −6.12, p < .001.
As with experiment 2, negative Weber slopes may have resulted from our method of eliminating anchor effects. This is supported by the fact that the most common breakpoint for participants in both tasks was 1350 ms, leaving the 1700 and 1870 ms standards as the points constituting the long duration set. As we did in experiment 2, we applied new bilinear fits to truncated versions of each individual’s Weber function for each task. The newly obtained breakpoints were M = 1074 ± 196 for reproduction and M = 1147 ± 120 for discrimination. Figure 11 panels C and D show the average Weber function and bilinear fits for the truncated data calculated across all participants and across participants with only positive slopes for the two tasks using the average breakpoints for each task.
Seven participants with negative long duration set slopes were excluded from analysis. There was neither a significant task by duration set interaction (p = .240) nor an effect of duration set (p = .128). There was, however, a main effect of task, F(1, 11) = 103.96, p < .001, ηp2 = .90, due to larger Weber fractions for discrimination (M = .24) as compared to reproduction (M = .08).14 Five participants showed negative slopes only for the discrimination task. Given that experiments 1 and 2 found a duration set effect for temporal reproduction, we examined this effect when truncated data from these five participants were included; there was no effect (p = .410).
4.2.4. Latency to first tap
Data from two individuals were excluded because their latency for at least one standard duration in either task was a group outlier. We found a significant task by duration interaction, F(5.25, 83.98) = 15.40, p < .001, ηp2 = .49. There were also main effects of task, F(1, 16) = 57.04, p < .001, ηp2 = .78 and duration, F(4.16, 66.57) = 22.43, p < .001, ηp2 = .58. A linear trend explained the duration effect, F(1, 16) = 50.70, p < .001, ηp2 = .76.15 Figure 9 panel B shows the data for each task. The interaction was driven by increasing latencies for reproduction accompanied by relatively stable latencies for discrimination as the standard durations increased.
Simple effects analyses within each task revealed a main effect of duration for temporal reproduction, F(2.99, 47.90) = 31.26, p < .001, ηp2 = .66, with a significant linear trend, F(1, 16) = 68.65, p < .001, ηp2 = .81, as found in experiments 1 and 2. Temporal discrimination showed no effect of duration (p = .263).
4.2.5. CV Correlations
As Table 2 shows, no significant cross-task relationships were found, even where standard durations matched. This suggests that the two tasks rely on different timing mechanisms. There are, however, a few scattered Pearson correlation coefficients that might achieve significance with more statistical power.
Table 2.
Between-task CV correlations for experiment 3.
| Reproduction | 270 | 300 | 650 | 1000 | 1175 | 1350 | 1700 | 1870 |
|---|---|---|---|---|---|---|---|---|
| Discrimination | ||||||||
| 270 | .14 | −.10 | .32 | −.08 | −.09 | −.11 | .03 | .02 |
| 300 | .11 | −.03 | .49 | .19 | .15 | .13 | .17 | .11 |
| 650 | .04 | −.14 | .18 | .08 | .12 | −.03 | .16 | .23 |
| 1000 | .22 | −.11 | .33 | −.10 | −.16 | −.15 | −.05 | .18 |
| 1175 | .10 | −.17 | .22 | −.16 | −.15 | −.18 | −.11 | .04 |
| 1350 | −.01 | .14 | .34 | .02 | .07 | .08 | .03 | .44 |
| 1700 | .50 | .08 | .64 | .17 | .31 | .26 | .27 | .09 |
| 1870 | .26 | −.16 | −.09 | .19 | .34 | −.02 | .29 | −.03 |
p < .10, one-tailed
p < .05, one-tailed
The bottom of Table 1 shows within-task correlations between CVs for temporal reproduction. Significant correlations emerged between durations in the midpoint of the range as well as between midpoint durations and several of the shortest and longest standards. However, almost all of the Pearson correlation coefficients were rather large and would likely reach significance with more participants. This pattern differs somewhat from that found in experiments 1 and 2. In an effort to increase power and clarify the relationship between CVs across durations within temporal reproduction, we performed correlations using the data (except outliers) from all three experiments (see Table 3). Note that the number of participants contributing to each correlation differs, because only 5 of the durations (300, 650, 1000, 1350, and 1700 ms) were used in all 3 experiments. Durations longer than 650 ms correlated with one another, and the two shortest durations correlated with each other. Other than a few correlations between the 650 ms CV and some shorter and longer durations, we did not find significant relationships spanning across timescales. Therefore, temporal reproduction at these timescales may largely rely on different mechanisms. Within-task correlations for discrimination are shown in Table 4. Significant relationships emerged between midpoint durations as well as between these and several of the shortest standards.
Table 3.
Reproduction CV Correlations across standard durations collapsed across all three experiments.
| 270 | 300 | 650 | 1000 | 1175 | 1350 | 1700 | 1870 | |
|---|---|---|---|---|---|---|---|---|
| 270 | ||||||||
| 300 | .51* | |||||||
| 650 | .40 | .39* | ||||||
| 1000 | .12 | .29 | .34 | |||||
| 1175 | .28 | .22 | .55** | .59** | ||||
| 1350 | .22 | .23 | .41** | .60** | .79** | |||
| 1700 | .33 | .21 | .28 | .52** | .74** | .66** | ||
| 1870 | .08 | .15 | .28 | .60** | .54** | .64** | .61** |
p < .05, one-tailed
p < .01, one-tailed
Table 4.
Discrimination CV Correlations across standard durations for experiment 3.
| 270 | 300 | 650 | 1000 | 1175 | 1350 | 1700 | 1870 | |
|---|---|---|---|---|---|---|---|---|
| 270 | ||||||||
| 300 | .65 | |||||||
| 650 | .54 | .69* | ||||||
| 1000 | .76** | .59 | .74** | |||||
| 1175 | .85** | .72** | .69* | .87** | ||||
| 1350 | .64 | .70* | .82** | .78** | .70* | |||
| 1700 | .39 | .42 | .41 | .45 | .44 | .24 | ||
| 1870 | .22 | .14 | .52 | .28 | .39 | .10 | .47 |
p < .10, one-tailed
p < .05, one-tailed
4.3. Discussion
This experiment investigated whether the non-scalar variability that was found for temporal reproduction in experiments 1 and 2 would extend to a temporal discrimination task with a parallel structure. Clear task-related differences were evident for several performance measures. Specifically, CV values, truncated Weber fractions, and the short Weber fraction from the non-truncated data set were larger for discrimination than reproduction. Also, tap latency increased with increasing duration for reproduction, but remained constant for discrimination. Absence of cross-task correlations for CV suggests non-shared timing mechanisms across the two tasks; correlations are expected if timing components are shared, despite task differences in response requirements. The task effect for non-truncated Weber fractions across short durations indicates, in particular, that different processes may be engaged for timing in the milliseconds range. This result challenges prior work supporting a common timing mechanism across tasks in this range (Ivry & Hazeltine, 1995; Keele et al., 1985).
Accuracy was similar across tasks. Consistent with many prior studies of timing (Treisman,1963), subjective estimates were longer than objective values for shorter durations and shorter for longer durations. The fact that individuals’ reproduction and PSE estimates were close to the objective durations fits well with prior studies indicating that humans tend to exhibit a 1:1 relationship between these values (Baudouin et al., 2006; Collyer et al., 1992; Wearden, 1991a; Wearden, 2003; Wearden et al., 1997).
Given the similar patterns of accuracy across tasks, larger CVs for discrimination than reproduction were driven by greater variability. While the inclusion of comparison durations that were ±40% of the standard durations (compared to smaller percentages from prior studies) might have led to these larger variance estimates, the present result is in line with Ivry & Hazeltine’s (1995) finding that perceptual timing leads to greater variance than production or reproduction. Our Weber fractions for both the perceptual and motor tasks, however, were much larger than those obtained by these researchers, whose Weber fractions tended to hover between .02 and .05. This difference might be due to a context-driven difference in timing sensitivity due to the restricted set of millisecond-range durations (325 ms to 550 ms) used in their study, compared to the durations used in the present study (Ferrara et al., 1997; Penney et al., 1998).
With regard to timescale, results indicate no task difference in the manner in which CVs changed across durations--a quadratic fit explained the data. The minimum values for each task did, however, occur at different durations. Data from the full and truncated duration sets indicate that Weber fractions changed in similar ways across the breakpoint for each task. In contrast to the first two experiments, there was no duration set effect on Weber fractions for temporal reproduction. However, several individuals exhibited negative slopes; these were primarily found for the discrimination task, suggesting that very different processing may, indeed, be engaged for the two types of tasks, despite leading to similar patterns of performance across timescale.
The combined reproduction CV data from all three experiments indicated strong correlations between all durations of 1000 ms and longer, suggesting that longer durations engage a common timing mechanism in this task. Correlations for discrimination, though less reliable, point towards significant relationships between most durations of 1350 ms and shorter, suggesting a common mechanism for timing shorter durations in this task. These patterns are interesting, given that 1000 ms and 1350 ms are the locations where minimum CV’s were found for temporal reproduction and discrimination, respectively. These durations may mark important transition points in timing for the two types of tasks. The average Weber function breakpoints from the final two experiments tend to suggest a transition at a somewhat longer duration than 1000 ms for temporal reproduction, however. Future work should include additional longer durations so that 1000 ms is not directly located in the center of the tested duration set to accurately identify the Weber slope and breakpoint estimates.
Overall, our findings implicate different mechanisms for timing across motor and perceptual tasks, even though these mechanisms may lead to relatively similar performance changes across durations. Evidence against a single scalar clock across timescales is also supported by the quadratic pattern of CVs across durations.
5. General Discussion
The current goal was to investigate whether a dedicated scalar timing mechanism consistent with scalar expectancy theory (Gibbon et al., 1984), is ubiquitous across millisecond and second durations for both perceptual and motor tasks. We were particularly interested in the performance of human participants on a number of closely-spaced durations encompassing one second. The overall results cast doubt on the view that a unitary scalar mechanism appropriately describes timing across these tasks and durations. Instead, different timers may operate for perceptual and motor tasks, and there is evidence for departures from scalar variability.
5.1. Timing across tasks
Of the few studies that have directly compared timing performance across motor and perceptual tasks within the same participants, (Ivry & Hazeltine, 1995; Keele et al., 1985, Merchant, Zarco, & Prado, 2008), performance has been assessed within a single timescale (e.g. milliseconds range). Some of these studies found support for a common scalar clock across tasks (Ivry & Hazeltine, 1995, Keele et al., 1985). More recent work using a broader range of durations of 1 second or less found overall patterns of scalar variability, but also revealed that differences in modality, encoding context and task type (e.g. perception or production) influenced estimates of duration-dependent variance (Merchant, Zarco, & Prado, 2008); the authors argued that while some features of timing in different tasks may involve common timing mechanisms, some distributed mechanisms are also differentially engaged, depending on task constraints.
The tasks used in the current set of experiments were designed to equate perceptual and encoding requirements; therefore, we would have expected evidence supporting a common scalar timer. However, we found no significant relationships across tasks in CV measures, and we found task differences in CVs and the Weber slopes from the truncated data and from the non-truncated short duration sets. The absence of significant correlations between CVs for the two timing tasks, despite involvement of the same study participants, is surprising. Though differences in motor preparation and performance may have contributed noise to the CV data, between-task relationships should have emerged if the tasks shared one or more processes for timing. While the small number of participants urges a cautious interpretation of these results we note that our sample size is comparable to that of prior studies using similar analyses (Keele et al., 1985; Merchant, Zarco, & Prado, 2008; Robertson et al., 1999). The significant task effect for short duration set Weber fractions and the absence of cross-task correlations in the milliseconds range are especially noteworthy because they contradict the oft-cited finding of similar timing across tasks for durations in this range (Ivry & Hazeltine, 1995; Keele et al., 1985). Interestingly, while timing sensitivity was poorer for temporal discrimination than reproduction, accuracy was similar, suggesting that the mechanisms responsible for timing in the two tasks lead to similar representations of the standard durations.
Reproduction and discrimination are also clearly distinguished by their different patterns of tap latency. Increased latency across durations for reproduction but not discrimination is consistent with the finding by Vidal, Bonnet, and Macar (1991; 1992) that action duration is coded as part of a motor program and can be processed prior to motor execution. This feature of temporal reproduction also fits with Lewis and Miall’s (2003b; 2003c; 2006) theory that tasks that require replication of a duration via an action may be especially reliant on the motor system; including duration as a dimension of the response may necessitate different encoding and memory processes than those engaged for temporal discrimination. On the other hand, task differences might be due to strategy differences. For example, participants may rehearse the standard duration prior to response or create a latency that is temporally and rhythmically congruous with the standard to support reproduction. Note, however, that latency length did not always match standard duration length in our experiments. Thus, this process of encoding may involve some translation or compression of the duration. Additional work manipulating task encoding, memory, and motor preparation requirements is needed to draw definitive conclusions about the source of task differences on this measure.
We went to great lengths to equate the features of our two timing tasks in experiment 3. Despite our efforts, we acknowledge that the tasks differ in their motor requirements and that contamination from motor processes could have influenced our results. This challenge exists, however, for all studies that compare motor and perceptual timing tasks. Moreover, our efforts to match the features of the reproduction and discrimination tasks in experiment 3 went beyond many prior reports. We also took deliberate steps to prevent participants from using explicit movement-based strategies (e.g. foot tapping), which included monitoring by an experimenter and removal of individuals who reported their use on self-report questionnaires. While we cannot rule out the use of covert or implicit movement-based strategies, our efforts to eliminate their explicit use make our findings of cross-task differences all the more informative.
5.2. Timing across durations
The quadratic, U-shaped pattern of CVs in temporal reproduction from all 3 experiments suggests that timing across millisecond and second durations in this task does not rely on a single scalar clock. This type of pattern has previously been found for both production and categorization timing tasks in pigeons using durations of 500 ms up to 64 s (Bizo et al., 2006) and rats using durations of several seconds in length (Crystal, 2001, 2003). In the present experiments one minimum reproduction CV occurred at 1000 ms--the point where some researchers argue for a transition between timing mechanisms reliant on the motor system and those that engage executive control processes (Lewis & Miall, 2003a, 2003b, 2003c, 2006). The Weber slope data from the first two experiments further implicates different clocks across timescales, with breakpoints from experiment 1 (M = 956 ms) and the truncated data sets for experiments 2 and 3 (M = 1021 ms; M = 1074 ms) occurring near 1000 ms.
While the quadratic CV data provide consistent evidence of nonlinear timing in temporal reproduction, it is important to note that this may not point to the operation of different timers across timescales. Instead, reproduction may involve a single timer that is simply not a pacemaker-accumulator mechanism adhering to the scalar property. Two possible alternatives include a multiple oscillator clock (Church & Broadbent, 1990) and the striatal beat frequency (SBF) model (Matell & Meck, 2004). Both mechanisms could produce scalar variability across most durations along with points of maximal sensitivity at durations that match or are multiplicative values of the component oscillator periods (Church & Broadbent, 1990; Crystal, 1999, 2001, 2003; Matell & Meck, 2004). Our temporal reproduction CVs could be due to such a mechanism with at least one oscillator period close to 1000 ms. SBF could also account for people's increased sensitivity at 1000 ms via well-tuned coincidence detection due to repeated reinforcement from the observation of timepieces and physiological processes, such as heartbeat. In our experiments, however, there was little indication of significant correlations across timescales in temporal reproduction. If a non-scalar clock were wholly responsible for timing, significant correlations across timescales should still emerge. Moreover, the Weber slope analyses in the first two experiments hint at different mechanisms for timing milliseconds versus seconds-length durations. Thus, the quadratic pattern in CVs may implicate two different timers across timescales.
CV data from experiment 3 suggest that, despite task differences in temporal sensitivity, the manner in which sensitivity changed across timescales did not differ by task. However, the CV correlations within temporal discrimination complicate the picture concerning the mechanism(s) that operates across timescales; correlations emerged between the intermediate standard durations along with many large Pearson correlation coefficients between CVs for shorter durations. Correlations were weak for the longer durations. Additional power could yield a clearer pattern, elucidating whether temporal discrimination in the milliseconds and seconds ranges engages different timers.
Findings from the Weber slope analysis were difficult to interpret. Importantly, fitting independent bilinear regressions to the data assumes two separate scalar timers across a break, but these assumptions may not be correct. Indeed, in experiment 3 nearly half of our participants revealed negative Weber slopes across the long duration set (even for truncated data). These negative slopes may have been due to the durations we used to prevent use of a categorization strategy in experiments 2 and 3 coupled with our bi-linear regression technique. This idea is supported by the fact that we did not find negative slopes in experiment 1. Moreover, prior studies using Weber slope analysis have typically involved durations of one second or less, have investigated a single regression line through all data points, and have no reported negative slopes (Ivry & Hazeltine, 1995; Merchant, Zarco & Prado, 2008; Robertson et al., 1999; Spencer & Zelaznik, 2003). It is noteworthy that negative slopes were not ubiquitous in our experiments, occurring for some individuals but not others, and more frequently with temporal discrimination. Therefore, they may reflect strategy differences in response to differing task demands associated with timing of longer durations.
Negative slopes for longer durations could result from attempts to subdivide the standard and comparison durations in such a way that variability for longer durations is reduced. One manner of subdivision, counting, is known to cause departures from scalar timing in this way (Grondin, Meilleur-Wells & Lachance, 1999; Hinton & Rao, 2004; Killeen & Weiss, 1987). We eliminated data from individuals who reported counting in our experiments, but some individuals with negative slopes reported trying to associate the standard and comparison durations with the length of a word or words they repeated silently. Negative slopes could also emerge from difficulty maintaining attentional focus when encoding longer durations. Attention is posited to modulate the output of the pacemaker component of SET (see Zakay & Block, 1996, 1997). Difficulty maintaining attention at encoding, therefore, could ultimately lead to duration representations with missed pulses, reducing their discriminability from their shorter neighbors and violating the expected increase in variance with increasing durations. Future studies need to address whether negative Weber slopes are due to individual differences, such as differences in attention, or strategy differences in timing per se.
5.3. Future directions
An important future goal is to determine with greater certainty whether the quadratic pattern of data for temporal reproduction indicates a shift between different timing mechanisms or, instead, points to a single timer which may not adhere to scalar variance. Our current data point to the prior possibility. However, exploration of other models of timing is warranted. Reproduction performance should be systematically investigated with additional durations, including multiples (e.g. 2000 ms, 3000 ms) of the 1000 ms point of maximal sensitivity. Increases in sensitivity at these harmonic values would be consistent with either a multiple oscillator clock or the SBF model of timing. Moreover, exploring additional durations beyond the range of 2 seconds will help clarify whether the quadratic pattern in CV continues with longer durations or whether another transition between timing mechanisms emerges in the 2–3 second range. Given that the evidence of non-scalar timing was much weaker for temporal discrimination in our study, it is similarly important to examine this task in more detail to identify the features of the timing mechanism(s) engaged for the milliseconds and seconds ranges.
Negative Weber slopes were problematic for investigating duration-dependent sources of variance in our studies. Evaluation of truncated data sets solved the problem, to some degree, but these data are not necessarily equivalent to those obtained from duration sets lacking similar end-point pairs. We confirmed in experiments 2 and 3 that the quadratic pattern in the CV data was likely not due to an anchoring strategy, so future research can confidently use Weber slope analysis incorporating bi-linear regression with endpoint durations that are easier to discriminate from their neighbors. Persisting negative slopes will lend further credence to the presence of some difference in the mechanisms or strategies engaged for timing between short and long durations, especially for temporal discrimination.
The present results call attention to the need for further detailed investigations of motor and perceptual timing across an even larger set of durations spanning tens of milliseconds to several seconds in length to clarify where and how many functional breaks in timing exist. Two or more timescale shifts in this range have been suggested (Buhusi & Meck, 2005; Buonomano & Karmarkar, 2002), with the current study strongly implicating a shift in the region of one second for temporal reproduction.
Acknowledgments
We would like to thank David E. Meyer, Cindy A. Lustig, J. Wayne Aldridge, Warren Meck and Trevor Penney for their thoughtful comments and assistance with this work. We also thank the following undergraduates for their help with data collection and analysis: Holly Borchardt, Pranali Koradia, Jessica Imas, Kirsten Rose, Marisa Terry, Alyse Grossman, Megan Walsh, Amanda Szabo, and Samar Elabed. This work was supported by NIH AG024106 (RDS), AG18286 (PARL), NIA Training Grant AG000030, a National Science Foundation Graduate Research Fellowship, a Rackham Graduate School pre-doctoral fellowship, and an APA dissertation research award (ASB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The pattern of data was unchanged when all participants were included.
We assessed the reliability of the CVs across runs within each duration using Chronbach's alpha in an effort to address concerns that individuals might shift strategies across time. For each duration, 8 items (runs) were included in the analysis, and all alpha levels for each duration fell within the acceptable range (all α > .77, with all durations longer than 300 ms having α > .85).
There is a difference between short and long Weber fractions calculated across the first set of duration blocks, t(13) = −5.13, p < .001. Two participants were excluded from this comparison due to negative Weber fractions across the long duration set.
This pattern of findings held when all participants were included in the analysis.
When all participants were included in the analysis, the main effect of run disappeared and an interaction between run and duration emerged, F(35, 840) = 1.53, p = .027, ηp2 =.06. Otherwise, the pattern of results remained the same.
We assessed the reliability of the CVs across runs within each duration using Chronbach's alpha. For each duration, 6 items (runs) were included and all alpha levels were in the acceptable range (all α > .78; for durations longer than 270 ms, all α > .86).
Evaluating the truncated CV data revealed an effect of duration, F(3.35, 80.45) = 4.30, p = .005, ηp2 = .15, following a quadratic trend, F(1, 24) = 7.57, p = .011, ηp2 = .24.
This pattern of findings did not change when all participants were included.
In our pilot study, the most discriminable comparison durations were ± 25% of the standard. However, for the shortest standards half of our 8 participants could not reliably discriminate these comparisons from the standard at least 75% of the time. Therefore, we made the extreme comparisons easier to discriminate (± 40% of the standard) in an effort to construct more representative psychometric functions.
We transformed the probabilities obtained for each comparison duration into Z-scores using the inverse cumulative normal distribution. We then performed a linear regression across these points and determined the slope (c1 ) and intercept (c0 ) of the function. The threshold was derived using the equation, , while the standard deviation was derived using (Treutwein,1995).
This overall pattern did not change when all participants were included.
With all participants included, the same basic findings emerged. However, the minimum values occurred at 1000 ms, 1175 ms, and 1350 ms (all M = .14).
As with experiments 1 and 2, we assessed the reliability of the CVs across runs within each duration using Chronbach's alpha. For each duration, 20 items (runs) were included in the analysis and all alpha levels were quite high (all α > .91).
An evaluation of the truncated CV data revealed no task by duration interaction (p = .220) and no duration effect (p = .144), but a main effect of task, F(1,13) = 81.80, p < .001, ηp2 = .86. Looking within temporal reproduction we saw that the duration effect was robust, F(5, 65) = 4.38, p = .002, ηp2 = .25, and followed a quadratic trend, F(1, 13) = 23.61, p < .001, ηp2 =.65, while discrimination showed no duration effect (p = .337).
This pattern of findings held when all participants were included.
References
- Allan LG. The influence of the scalar timing model on human timing research. Behavioural Processes. 1998;44:101–117. doi: 10.1016/s0376-6357(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Baudouin A, Vanneste S, Pouthas V, Isingrini M. Age-related changes in duration reproduction: Involvement of working memory processes. Brain and Cognition. 2006;62(1):17–23. doi: 10.1016/j.bandc.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Bizo LA, Chu JYM, Sanabria F, Killeen PR. The failure of Weber's law in time perception and production. Behavioural Processes. 2006;71(2):201–210. doi: 10.1016/j.beproc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Brown SW. Attentional resources in timing: Interference effects in concurrent temporal and nontemporal working memory tasks. Perception & Psychophysics. 1997;59(7):1118–1140. doi: 10.3758/bf03205526. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Karmarkar UR. How Do We Tell Time? 2002;Vol. 8:42–51. doi: 10.1177/107385840200800109. [DOI] [PubMed] [Google Scholar]
- Church RM. Properties of the internal clock. Annals of the New York Academy of Sciences. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- Church RM. A concise introduction to scalar timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; 2003. pp. 3–22. [Google Scholar]
- Church RM, Broadbent HA. Alternative representations of time, number, and rate. Cognition. 1990;37(1):55–81. doi: 10.1016/0010-0277(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Collyer CE, Broadbent HA, Church RM. Categorical time production: Evidence for discrete timing in motor control. Perception & Psychophysics. 1992;51(2):134–144. doi: 10.3758/bf03212238. [DOI] [PubMed] [Google Scholar]
- Collyer CE, Broadbent HA, Church RM. Preferred rates of repetitive tapping and categorical time production. Perception & Psychophysics. 1994;55(4):443–453. doi: 10.3758/bf03205301. [DOI] [PubMed] [Google Scholar]
- Coull JT. fMRI studies of temporal attention: Allocating attention within, or towards, time. Cognitive Brain Research. 2004;21(2):216–226. doi: 10.1016/j.cogbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Systematic Nonlinearities in the Perception of Temporal Intervals. Journal of Experimental Psychology:Animal Behavior Processes. 1999;25(1):3–17. [PubMed] [Google Scholar]
- Crystal JD. Nonlinear time perception. Behavioural Processes. 2001;55:35–49. doi: 10.1016/s0376-6357(01)00167-x. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Nonlinearities in sensitivity to time: Implications for oscillator-based representations of interval and circadian clocks. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; 2003. pp. 61–75. [Google Scholar]
- Crystal JD, Church RM, Broadbent HA. Systematic nonlinearities in the memory representation of time. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23(3):267–282. doi: 10.1037//0097-7403.23.3.267. [DOI] [PubMed] [Google Scholar]
- Drake C, Botte M-C. Tempo sensitivity in auditory sequences: Evidence for a multiple-look model. Perception & Psychophysics. 1993;54(3):277–286. doi: 10.3758/bf03205262. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S. Scalar timing in temporal generalization in children with short and long stimulus durations. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 2002;55(4):1193–1209. doi: 10.1080/02724980244000161. [DOI] [PubMed] [Google Scholar]
- Ferrara A, Lejeune H, Wearden JH. Changing sensitivity to duration in human scalar timing: An experiment, a review, and some possible explanations. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1997;50(3):217–237. [Google Scholar]
- Fetterman JG, Killeen PR. A componential analysis of pacemaker-counter timing systems. Journal of Experimental Psychology: Human Perception and Performance. 1990;16(4):766–780. doi: 10.1037//0096-1523.16.4.766. [DOI] [PubMed] [Google Scholar]
- Fetterman JG, Killeen PR. Time discrimination in Columba livia and Homo sapiens. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18(1):80–94. doi: 10.1037//0097-7403.18.1.80. [DOI] [PubMed] [Google Scholar]
- Fortin C. Attentional time-sharing in interval timing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; 2003. pp. 235–260. [Google Scholar]
- Fortin C, Breton R. Temporal interval production and processing in working memory. Perception & Psychophysics. 1995;57(2):203–215. doi: 10.3758/bf03206507. [DOI] [PubMed] [Google Scholar]
- Fortin C, Rousseau R. Interference from short-term memory processing on encoding and reproducing brief durations. Psychological Research/Psychologische Forschung. 1998;61(4):269–276. doi: 10.1007/s004260050031. [DOI] [PubMed] [Google Scholar]
- Friberg A, Sundberg J. Time discrimination in a monotonic, isochronous sequence. Journal of the Acoustical Society of America. 1995;98(5):2524–2531. [Google Scholar]
- Gibbon J. Origins of Scalar Timing. Learning and Motivation. 1991;22:3–38. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: Advances and challenges. Current Opinion in Neurobiology. 1997;7(2):170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Grondin S. Production of time intervals from segmented and nonsegmented inputs. Perception & Psychophysics. 1992;52(3):345–350. doi: 10.3758/bf03209151. [DOI] [PubMed] [Google Scholar]
- Grondin S. From physical time to the first and second moments of psychological time. Psychological Bulletin. 2001;127(1):22–44. doi: 10.1037/0033-2909.127.1.22. [DOI] [PubMed] [Google Scholar]
- Grondin S. Overloading Temporal Memory. Journal of Experimental Psychology: Human Perception and Performance. 2005;5:869–879. doi: 10.1037/0096-1523.31.5.869. [DOI] [PubMed] [Google Scholar]
- Grondin S. Unequal Weber fractions for the categorization of brief temporal intervals. Attention, Perception & Psychophysics. 2010;72(5):1422–1430. doi: 10.3758/APP.72.5.1422. [DOI] [PubMed] [Google Scholar]
- Grondin S, Meilleur-Wells G, Lachance Re. When to start explicit counting in a time-intervals discrimination task: A critical point in the timing process of humans. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(4):993–1004. [Google Scholar]
- Grondin S, Ouellet B, Roussel M-E. Benefits and Limits of Explicit Counting for Discriminating Temporal Intervals. Canadian Journal of Experimental Psychology. 2004;58(1):1–12. doi: 10.1037/h0087436. [DOI] [PubMed] [Google Scholar]
- Grondin S, Rammsayer TH. Variable foreperiods and temporal discrimination. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 2003;56(4):731–765. doi: 10.1080/02724980244000611. [DOI] [PubMed] [Google Scholar]
- Grondin S, Roussel M-E, Gamache PL, Roy M, Ouellet B. The structure of sensory events and the accuracy of time judgments. Perception. 2005;34(1):45–58. doi: 10.1068/p5369. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowitz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12(1):3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain: A Journal of Neurology. 2004;127(3):561–574. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Rao SM. 'One-thousand one…one-thousand two…': Chronometric counting violates the scalar property in interval timing. Psychonomic Bulletin & Review. 2004;11(1):24–30. doi: 10.3758/bf03206456. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Hazeltine RE. Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(1):3–18. doi: 10.1037//0096-1523.21.1.3. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. Journal of Cognitive Neuroscience. 1989;1(2):136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences. 2008;12(7):273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Telling time in the absence of clocks. Neuron. 2007;53(3):427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele SW, Pokorny RA, Corcos DM, Ivry R. Do perception and motor production share common timing mechanisms: A correlational analysis. Acta Psychologica. 1985;60(2):173–191. doi: 10.1016/0001-6918(85)90054-x. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Weiss NA. Optimal timing and the Weber function. Psychological Review. 1987;94(4):455–468. [PubMed] [Google Scholar]
- Lavoie P, Grondin S. Information processing limitations as revealed by temporal discrimination. Brain and Cognition. 2004;54(3):198–200. doi: 10.1016/j.bandc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia. 2003a;41(12):1583–1592. doi: 10.1016/s0028-3932(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. Current Opinion in Neurobiology. 2003b;13(2):250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. Overview: An image of human neural timing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. CRC Press; 2003c. pp. 515–532. [Google Scholar]
- Lewis PA, Miall RC. Remembering the time: A continuous clock. Trends in Cognitive Sciences. 2006;10(9):401–406. doi: 10.1016/j.tics.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. The precision of temporal judgment. Philosophical Transactions of The Royal Society B. 2009;364:1897–1905. doi: 10.1098/rstb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macar F, Anton J-L, Bonnet M, Vidal F. Timing functions of the supplementary motor area: An event-related fMRI study. Cognitive Brain Research. 2004;21(2):206–215. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Macar F, Coull J, Vidal F. The supplementary motor area in motor and perceptual time processing: fMRI studies. Cognitive Processing. 2006;7(2):89–94. doi: 10.1007/s10339-005-0025-7. [DOI] [PubMed] [Google Scholar]
- Macar F, Grondin S, Casini L. Controlled attention sharing influences time estimation. Memory & Cognition. 1994;22(6):673–686. doi: 10.3758/bf03209252. [DOI] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Experimental Brain Research. 2002;142(4):475–485. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The Neural Basis of Temporal Processing. Annual Review of Neuroscience. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Meck WH. Attentional bias between modalities: Effect on the internal clock, memory, and decision stages used in animal time discrimination. Annals of the New York Academy of Sciences. 1984;423:528–541. doi: 10.1111/j.1749-6632.1984.tb23457.x. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain's internal clock: How frontal-striatal circuitry keeps time and shifts attention. Brain and Cognition. 2002;48(1):195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Merchant H, Zarco W, Prado L. Do we have a common mechanisms to measuring time in the hundreds of milliseconds range? Evidence from multiple-interval timing task. Journal of Neurophysiology. 2008;99:939–949. doi: 10.1152/jn.01225.2007. [DOI] [PubMed] [Google Scholar]
- Michon JA. The compleat time experiencer. In: Jackson JAMJL, editor. Time, mind, and behavior. Berlin: Springer Verlag; 1985. pp. 20–54. [Google Scholar]
- Morgan MJ, Watamaniuk SNJ, McKee SP. The use of an implicit standard for measuring discrimination thresholds. Vision Research. 2000;40(17):2341–2349. doi: 10.1016/s0042-6989(00)00093-6. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;Vol. 9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Penney TB, Allan LG, Meck WH, Gibbon J. Memory mixing in duration bisection. In: Rosenbaum DA, Collyer CE, editors. Timing of behavior: Neural, psychological, and computational perspectives. The MIT Press; 1998. pp. 165–193. [Google Scholar]
- Pöppel E. Lost in time: a historical frame, elementary processing units and the 3-second window. Acta Neurobiologiae Experimentalis. 2004;64:295–301. doi: 10.55782/ane-2004-1514. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24(1):15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Effects of benzodiazepine-induced sedation on temporal processing. Human Psychopharmacology: Clinical and Experimental. 1992;7(5):311–318. [Google Scholar]
- Rammsayer TH. Are there dissociable roles of the mesostriatal and mesolimbocortical dopamine systems on temporal information processing in humans? Neuropsychobiology. 1997;35(1):36–45. doi: 10.1159/000119328. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1999;52(3):273–286. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH. Effects of pharmacologically induced changes in NMDA receptor activity on human timing and sensorimotor performance. Brain Research. 2006;1073:407–416. doi: 10.1016/j.brainres.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH, Lima SD. Duration discrimination of filled and empty auditory intervals: Cognitive and perceptual factors. Perception & Psychophysics. 1991;50(6):565–574. doi: 10.3758/bf03207541. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH, Ulrich R. No evidence for qualitative differences in the processing of short and long temporal intervals. Acta Psychologica. 2005;120(2):141–171. doi: 10.1016/j.actpsy.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Zelaznik HN, Lantero DA, Bojczyk KG, Spencer RM, Doffin JG, et al. Correlations for timing consistency among tapping and drawing tasks: Evidence against a single timing process for motor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25(5):1316–1330. doi: 10.1037//0096-1523.25.5.1316. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Karmarkar U, Ivry RB. Evaluating dedicated and intrinsic models of temporal encoding by varying context. Philosophical Transactions of The Royal Society B. 2009;364:1853–1863. doi: 10.1098/rstb.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC, Zelaznik HN. Weber (slope) analyses of timing variability in tapping and drawing tasks. Journal of Motor Behavior. 2003;35(4):371–381. doi: 10.1080/00222890309603157. [DOI] [PubMed] [Google Scholar]
- Treisman M. Temporal discrimination and the indifference interval: implications for a model of the "internal clock". Psychological Monographs: General and Applied. 1963;77:1–31. doi: 10.1037/h0093864. [DOI] [PubMed] [Google Scholar]
- Treutwein B. Adaptive psychophysical procedures. Vision Research. 1995;35(17):2503–2522. [PubMed] [Google Scholar]
- Vidal F, Bonnet M, Macar F. Programming response duration in a precueing reaction time paradigm. Journal of Motor Behavior. 1991;23(4):226–234. doi: 10.1080/00222895.1991.9942033. [DOI] [PubMed] [Google Scholar]
- Vidal F, Bonnet M, Macar F. Can duration be a relevant dimension of motor programs? In: Macar F, Pouthas V, Friedman WJ, editors. Time, action and cognition: Towards bridging the gap. Kluwer Academic/Plenum Publishers; 1992. pp. 263–273. [Google Scholar]
- Wearden JH. Do humans possess an internal clock with scalar timing properties? Learning and Motivation. 1991a;22(1):59–83. [Google Scholar]
- Wearden JH. Human performance on an analogue of an interval bisection task. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1991b;43(1):59–81. [PubMed] [Google Scholar]
- Wearden JH. Temporal generalization in humans. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18(2):134–144. doi: 10.1037//0097-7403.23.4.502. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Bray S. Scalar timing without reference memory? Episodic temporal generalization and bisection in humans. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 2001;54(4):289–309. doi: 10.1080/02724990042000173. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Edwards H, Fakhri M, Percival A. Why 'sounds are judged longer than lights': Application of a model of the internal clock in humans. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1998;51(2):97–120. doi: 10.1080/713932672. [DOI] [PubMed] [Google Scholar]
- Wearden JH. Applying the scalar timing model to human time psychology: Progress and challenges. In: Helfrich H, editor. Time and mind II: Information processing perspectives. Göttingen: Hogrefe & Huber; 2003. pp. 21–39. [Google Scholar]
- Wearden JH, McShane B. Interval production as an analogue of the peak procedure: Evidence for similarity of human and animal timing processes. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1988;40(4):363–375. [Google Scholar]
- Wearden JH, Rogers P, Thomas R. Temporal bisection in humans with longer stimulus durations. The Quarterly Journal of Experimental Psychology B: Comparative and Physiological Psychology. 1997;50(1):79–94. doi: 10.1080/027249997393655. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Current Directions in Psychological Science. 1997;6:12–16. [Google Scholar]
- Zakay D, Block RA. The role of attention in time estimation processes. In: Pastor MA, Artieda J, editors. Time, internal clocks and movement. North-Holland/Elsevier Science Publishers; 1996. pp. 143–164. [Google Scholar]