Abstract
Background
Application of annuloplasty rings during mitral valve (MV) repair has been shown to significantly change the mitral annular geometry. Until recently, a comprehensive two-dimensional echocardiographic evaluation of annular geometric changes was difficult owing to its nonplanar orientation. In this study, an analysis of the three-dimensional intraoperative transesophageal echocardiographic evaluation of the MV annulus is presented before and immediately after repair.
Methods
We performed three-dimensional geometric analysis on 75 patients undergoing MV repair during coronary artery bypass graft surgery for mitral regurgitation or myxomatous mitral valve disease. Geometric analysis of the MV was performed before and immediately after valve repair with full rings and annuloplasty bands. The acquired three-dimensional volumetric data were analyzed in the operating room. Specific measurements included annular diameter, leaflet lengths, the nonplanarity angle, and the circularity index. Before and after repair data were compared.
Results
Complete echocardiographic assessment of the MV was feasible in 69 of 75 patients (92%) within 2 to 3 minutes of acquisition. Placement of full rings resulted in an increase in the nonplanarity angle or a less saddle shape of the native mitral annulus (137 ±14 versus 146 ± 14; p = 0.002. By contrast, the nonplanarity angle did not change significantly after placement of partial rings.
Conclusions
Mitral annular nonplanarity can be assessed in the operating room. Application of full annuloplasty rings resulted in the mitral annulus becoming more planar. Partial annuloplasty bands did not significantly change the nonplanarity angle. Neither of the two types of rings restored the native annular planarity.
The normal mitral valve (MV) annulus is a nonplanar, saddle-shaped structure with its highest point in the mid anterior annulus near the aortic valve and a second smaller peak near the mid portion of the posterior annulus [1-11]. The nonplanarity of the annulus is believed to contribute to leaflet stress reduction [12, 13]. Appreciation of the importance of this nonplanarity has led to the introduction of surgical repair techniques and prostheses that either maintain or restore the saddle-shape of mitral annulus [3, 6, 14]. Restoration of nonplanarity after repair can also potentially reduce leaflet stress and thus improve the durability of repair [10].
Owing to its saddle shape and difference in axes and levels of the various portions, the mitral annulus cannot be examined in a single two-dimensional ultrasound plane [7, 15-18]. The analysis of mitral annular nonplanarity has so far been performed using either video fluoroscopy to follow implanted annular markers in animals [4, 5, 19, 20] or magnetic resonance imaging [21, 22] or echocardiography in humans and animals [2, 6, 10, 23-26]. The annulus was then mathematically reconstructed off-line, thus making the information impractical for clinical use in the operating room. In recent years, the introduction of three-dimensional echocardiography has been instrumental in developing a better description and understanding of the shape of the mitral valve annulus [27]. Also, with the availability of rapid three-dimensional reconstruction, the geometric analysis of the MV is generally available with in a few minutes of acquisition [27].
In this study, we describe the feasibility and initial results of performing complex yet rapid analyses of MV annular shape routinely using intraoperative three-dimensional transesophageal echocardiography (TEE). Specifically, we compared the changes in the saddle shape of the native mitral annulus before and after repair with different annuloplasty devices by measurement of the nonplanarity angle (NPA). Our ability to appreciate the effects of annuloplasty rings and bands on the annular nonplanarity can potentially help the clinicians to select and choose the devices best suited for particular clinical situations.
Material and Methods
After Institutional Review Board approval, a total of 75 patients undergoing MV surgery at our institution consented to participate in the study. Six patients were excluded from the study owing to incomplete or unsatisfactory data acquisition. Perioperative TEE examinations were carried out after induction of general anesthesia and endotracheal intubation. All studies were performed on a Sequoia C512 ultrasound system (Siemens, Mountainview, California) utilizing a V5M multiplane TEE transducer. The ultrasound system was also equipped with the TomTec (GmBH, Munich, Germany) “on-line perspective box,” a commercially available computer capable of automatic triggered rotational acquisition of ultrasound images from 0 to 180 degrees in 3- to 5-degree rotations of the scan plane. All images were acquired before start of the surgical procedure during a brief period of apnea and then again after separation from cardiopulmonary bypass after MV repair.
Echocardiographic Imaging
After performing a comprehensive two-dimensional and color-flow Doppler examination [28], a three-dimensional acquisition of MV was performed. A mid-esophageal four-chamber view was obtained with the TEE, the region of interest (mitral valve) was identified, and a 5-degree rotational acquisition was started gated to the electrocardiographic R wave. The images were immediately rendered and reconstructed and analyzed utilizing the mitral valve assessment package software by TomTec (GmBH Munich, Germany) available on the perspective box. The mitral valve geometric reconstruction was performed according to the previously described methodology briefly outlined below [27].
We also carried out similar echocardiographic examination on 8 patients undergoing coronary artery bypass graft surgery with normal ejection fraction, trivial mitral regurgitation, and no other valvular pathology for assessment and calculation of “normal” geometric determinants for comparison.
Mitral Valve Three-Dimensional Reconstruction
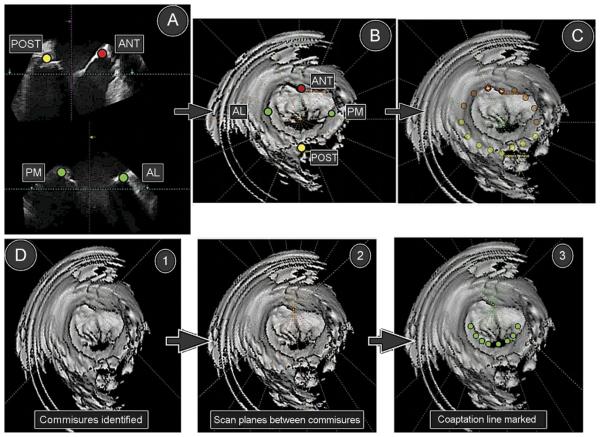
Briefly, the mitral valve geometric reconstruction was performed in a series of sequential steps, as follows. Reference image set-up: identification of the aortic valve in the upper outer quadrant (Fig 1A). Image of interest: an end-systolic frame is identified. Landmark identification: anterior, posterior, anterolateral (AL), and posteromedial (PM) landmarks are identified on the orthogonal views from the reference image set-up, establishing the anteroposterior (AP) and mediolateral dimensions of the mitral annulus (Fig 1A, B). Line of sight adjustment: an en-face view of the mitral valve (maximal annular cross-sectional area). Mitral annulus marking: the mitral annulus is manually marked in a series of 10 rotated two-dimensional slices evenly placed between the already established AP and AL-PM dimensions of the mitral annulus (Fig 1C). Commissural identification (see Fig 1D, 1). Coaptation line identification: in a series of 10 rotated two-dimensional slices, the coaptation line is defined and manually marked (Fig 1D, Figs 2 and 3). Geometric analysis: in the last step, the geometric data based on the three-dimensional reconstruction are generated and analyzed.
Fig 1.
(A) Anterior (ANT), posterior (POST), anterolateral (AL), and posteromedial (PM) landmark identification. (B) Annular markings displayed on the en-face view of the mitral valve. (C) Circumferential annular marking. (D) Identification of the commissures (1), identification of the coaptation line (2), and manually marked coaptation line (3).
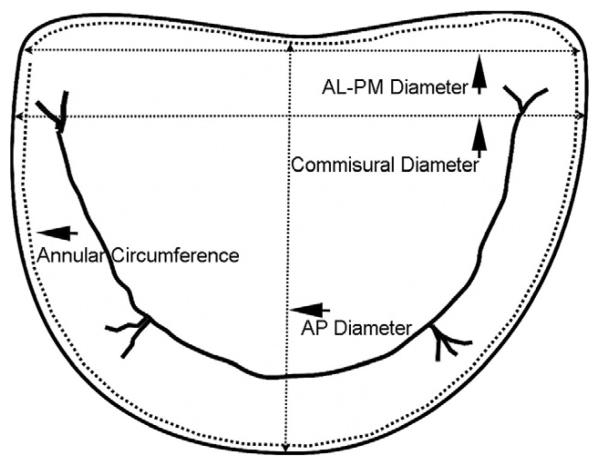
Fig 2.
Identification of the dimensions of the mitral valve automatically measured during geometric analysis. (AL-PM = anterolateralposteromedial; AP = anteroposterior.)
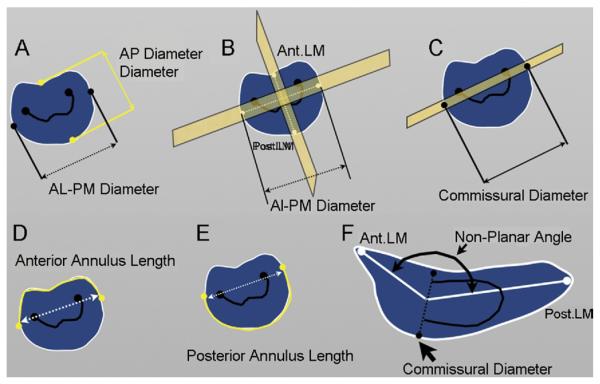
Fig 3.
Geometric measurements during mitral valve analysis. (A) Anteroposterior (AP) diameter is the distance between the anterior and the posterior landmark. (B) Anterolateralposteromedial (AL-AP) diameter is the distance between the anterolateral and posteromedial landmarks. (C) Commissural diameter is the annular diameter at the point of identification of commissures. (D) Anterior annulus length. (E) Posterior annulus length. (F) Non-planarity angle is the angle subtended between the anterior landmark (Ant. LM) and posterior landmark (Post. LM) at the commissural diameter.
Geometric Variables
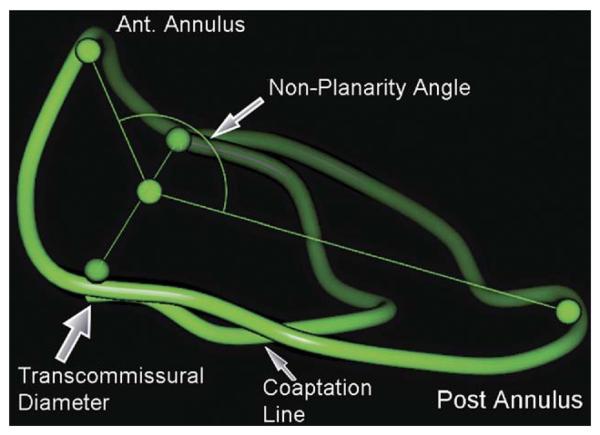
The following geometric variables were measured and generated automatically by the mitral valve assessment package based on the above workflow (Figs 2, 3). Antero-posterior diameter is measured between the anterior and posterior landmarks identified in the third step of the workflow (Figs 2, 3A). The AL-PM diameter is measured between the AL and PM landmarks of the third step of the workflow (Figs 2, 3B). Commissural diameter is the diameter of the mitral annulus at the level of the two commissures (Figs 2, 3C). For annular circumference, see Figure 2. Anterior annulus length is the curve length between the endpoints of the commissural diameter through the anterior part of the annulus ring (Fig 3D). Posterior annulus length is the curve length between the endpoints of the commissural diameter through the posterior part of the annulus ring (Fig 3F). Nonplanarity angle is a three-dimensional measure of the nonplanarity of the mitral annulus (Figs 3F, 4); this is the angle subtended at the middle point of the commissural diameter, and the anterior and posterior landmarks, namely, the annular high points (Fig 4); the more nonplanar the annulus, the smaller the NPA. Circularity index is a derived ratio of AP diameter and AL-PM diameter and is automatically generated.
Fig 4.
Measurement of nonplanarity angle by the mitral valve assessment package. (Ant = anterior; Post = posterior.)
Mitral Valve Geometric Assessment After Repair
The next three-dimensional data sets were also acquired as electrocardiography-triggered three-dimensional volume sets after separation from cardiopulmonary bypass and were analyzed in the same fashion. Before and after repair, geometric measurements were then compared. All geometric reconstruction and analyses were performed by an experienced echocardiographer (F.M.) after acquisition. The R-wave- gated three-dimensional acquisition of MV data sets is completed in 30 to 40 s for a patient with a heart rate of 70 beats per minute, and the reconstruction and geometric analysis and data in less than 2 minutes.
Statistical Methods
Only patients with complete data sets (before and after cardiopulmonary bypass) were included and analyzed in the study. The MV geometric data were checked for normality with skewness and kurtosis. These normally distributed data were analyzed by a paired t test for before and after repair comparisons and a Student t test for comparison of means between groups. Any p value less than 0.05 was considered significant. The software SPSS 15.0 for Windows (SPSS, Chicago, IL) was used for analyses.
Results
Seventy-five consecutive patients scheduled to undergo mitral valve repair/replacement were enrolled in the study. We were able to acquire complete three-dimensional data sets (before and after repair) in 69 patients. In 6 patients, we were unable to acquire complete data sets owing to partial annulus drop. In total, there were 29 ischemic MV repairs performed for ischemic mitral regurgitation along with coronary artery bypass graft surgery and 40 for myxomatous MV disease. The ischemic MVs had a larger baseline NPA (138.06 degrees) as compared with the myxomatous MVs (132.88 degrees) and the normal patients (127 degrees), but this difference did not reach statistical significance (p = 0.07; Table 1). Before and after repair MV geometry data (circularity index and NPA) for all patients who had MV surgery with complete annuloplasty rings and partial bands for ischemic and myxomatous MV diseases is shown in Table 2. Patients whose MV repair included complete annuloplasty rings (all types) had significantly (p = 0.002) flatter native annuli after repair than before (NPA before and after repair was 137 ± 14 degrees and 146 ± 14 degrees, respectively; p = 0.002). Patients with MV repair using partial annuloplasty rings did not have any significant difference between before and after NPA (before repair 133 ± 16 degrees versus after repair 137 ± 16 degrees; p = 0.184).
Table 1.
Baseline Characteristics of Nonplanarity Angle and Circularity Index for Normal, Myxomatous, and Ischemic Mitral Valves
| Ischemic Mitral Valves (n = 29) Mean (SD) |
Nonischemic Mitral Valves (n = 40) Mean (SD) |
Normal Mitral Valves (n = 8) Mean (SD) |
|
|---|---|---|---|
| Before nonplanarity angle (degrees) | 138 (15) | 133 (15) | 127 (5) |
| p = 0.07 | |||
| Before circularity index | 0.90 (0.1) | 0.89 (0.1) | 0.90 (0.1) |
| NS | |||
NS = not significant.
Table 2.
Comparison of Nonplanarity Angle and Circularity Index Between Full Rings (Ischemic/Myxomatous) and Partial Bands (Ischemic/Myxomatous)
| Before Repair |
After Repair |
p Value |
|
|---|---|---|---|
| Full rings | |||
| All rings: CE-Physio (Edwards) and CG-Future (Medtronic), n = 29 | |||
| Nonplanarity angle (degrees) | 137 ± 14 | 146 ± 14 | 0.002a |
| Circularity index (AP/AL-PM diameter) | 0.93 ± 0.1 | 0.86 ± 0.1 | 0.007a |
| Partial bands | |||
| All bands: Cosgrove Bands (Edwards) and Duran Bands (Medtronic), n = 40 | |||
| Circularity index (AP/AL-PM diameter) | 0.87 ± 0.1 | 0.88 ± 0.1 | 0.371a |
Statistically significant.
AL = anterolateral; AP = anteroposterior; CE = Carpentier-Edwards; CG = Collvin-Galloway; PM = posteromedial.
The data were then analyzed based on the type of the annuloplasty device for ischemic and myxomatous MV disease. A total of 24 Carpentier-Edwards Physio-Rings (CE Rings [Edwards Lifesciences, Irwine, CA]) were used, 12 for ischemic and 12 for myxomatous mitral valves (Table 3). There were 38 Cosgrove-Edwards annuloplasty partial bands (Cosgrove bands [Edwards Life-sciences]), 13 for ischemic and 25 for myxomatous mitral valves (Table 4). Seven Medtronic (Minneapolis, MN) annuloplasty devices were used, 4 Collvin-Galloway full rings (CG-Future ring), all for ischemic mitral valves, and 3 Duran partial bands, all for myxomatous mitral valves (Table 5).
Table 3.
Comparison of Before and After Repair Geometric Data for CE-Physio Rings (Full) for Ischemic and Myxomatous Mitral Valves
| Nonplanarity Angle (Degrees) |
Circularity Index (AP/AL-PM Diameter) |
|||
|---|---|---|---|---|
| CE-Physio Rings | Before Repair | After Repair | Before Repair | After Repair |
| All cases, n = 24 (ischemic/nonischemic) | 136.16 ± 12.37 | 149.03 ± 14.88 | 0.94 ± 0.08 | 0.84 ± 0.11 |
| p = 0.00a | p = 0.001a | |||
| Nonischemic, n = 12 | 136.87 ± 12.81 | 147.71 ± 15.3 | 0.93 ± 0.09 | 0.82 ± 0.09 |
| p = 0.043a | p = 0.043a | |||
| Ischemic, n = 12 | 135.45 ± 12.45 | 150.40 ± 15.00 | 0.96 ± 0.06 | 0.87 ± 0.12 |
| p = 0.00a | p = 0.030a | |||
Statistically significant.
AL = anterolateral; AP = anteroposterior; CE = Carpentier-Edwards; ML = mediolateral; PM = posteromedial.
Table 4.
Changes in Mitral Valve Geometry With Use of Cosgrove Partial Bands (n = 38) for Ischemic and Myxomatous Mitral Valves
| Nonplanarity Angle (Degrees) |
Circularity Index (AL/PM Diameter) |
|||
|---|---|---|---|---|
| Before Repair | After Repair | Before Repair | After Repair | |
| All cases (ischemic/nonischemic) | 134.12 ± 16.66 | 136.96 ± 15.66 | 0.86 ± 0.08 | 0.88 ± 0.10 |
| p = 0.316 | p = 0.300 | |||
| Nonischemic, n = 25 | 131.37 ± 16.79 | 136.46 ± 17.44 | 0.86 ± 0.09 | 0.88 ± 0..09 |
| p = 0.198 | p = 0.551 | |||
| Ischemic, n = 13 | 139.20 ± 15.79 | 137.90 ± 12.38 | 0.85 ± 0.08 | 0.88 ± 0.12 |
| p = 0.716 | p = 0.363 | |||
AL = anterolateral; PM = posteromedial.
Table 5.
Changes in Mitral Valve Geometry With Use of Medtronics Duran Partial Bands and CG-Future Full Rings
| Nonplanarity Angle (Degrees) |
Circularity Index (AP/AL-PM Diameter) |
|||
|---|---|---|---|---|
| Medtronic Rings (All Patients, n = 7) | Before Repair | After Repair | Before Repair | After Repair |
| Duran bands (nonischemic only), n = 3 | 129.0 ± 2.9 | 138.80 ± 3.19 | 0.93 ± 0.15 | 0.96 ± 0.11 |
| p = 0.109 | p = 0.317 | |||
| CG-Future rings (ischemic only), n = 4 | 129.0 ± 2.9 | 147.71 ± 15.3 | 0.93 ± 0.09 | 0.82 ± 0..09 |
| p = 0.043 | p = 0.043 | |||
AL = anterolateral; AP = anteroposterior; CG = Collvin-Galloway; PM = posteromedial.
The data were further subanalyzed based on the effects of specific annuloplasty devices on the valve geometry. The CE-Physio was the most frequently used full ring, for 34% of the cases. The use of this device resulted in a significant increase in the NPA angle, namely, the annulus became less saddle shaped whether it was used for ischemic (135.45 ± 12.45 versus 150.40 ± 15.00, p = 0.00) or myxomatous MV disease (136.87 ± 12.81 versus 147.71 ± 15.3, p = 0.043; Table 3). The use of CG-Future bands (55% of the cases), however, did not change the NPA significantly for ischemic MVs (n = 13 [139.20 ± 15.79 versus 137.90 ± 12.38, p = 0.176]) or myxomatous MVs (n = 25 [131.37 ± 16.79 versus 136.46 ± 17.44, p = 0.198; Table 4). A total of 7 Medtronic annuloplasty devices (11% of the cases) were used, 3 Duran partial bands for myxomatous MV disease and 4 CG-Future rings for ischemic MV disease (Table 5). A total of 24 patients underwent posterior leaflet resection during MV repair. Of these patients, 20 had partial bands and 4 had complete rings used for MV repair.
There was a significant reduction in the circularity index (anteroposterior diameter/anterolateral-posteromedial diameter) after MV repair with CE-Physio rings for ischemic MVs (0.96 ± 0.06 versus 0.87 ± 0.12, p = 0.030) and myxomatous MVs (0.96 ± 0.06 versus 0.82 ± 0.09, p = 0.043; Table 3). In patients with MV repair with partial CG-Future annuloplasty bands, there was no significant change in the circularity index after repair for either ischemic or myxomatous MVs (Table 4).
Comment
Our study shows that the three-dimensional geometric alterations in the mitral annulus of the repaired valve structure may be determined by the nature of the implanted prosthetic device. Our results also indicate that complete annuloplasty rings increase the NPA, namely, make the mitral annulus more planar and less saddle shaped and decrease the circularity index. Furthermore, the results of this three-dimensional reconstruction were available within minutes of the acquisition of the data in the operating room.
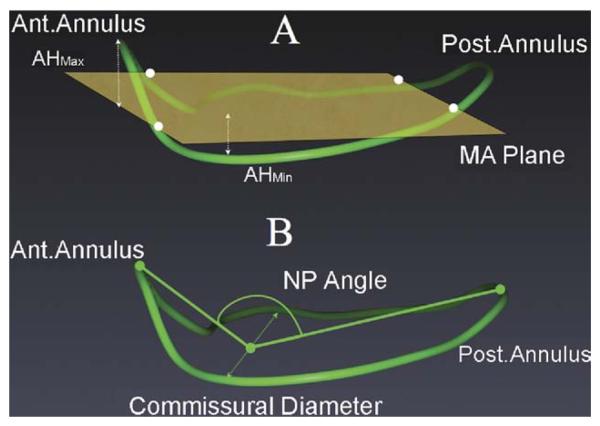
The three-dimensional geometric reconstruction of mitral annulus in earlier studies has either been performed in animals by tracking surgically placed piezoelectric crystals or in humans with real-time three-dimensional transthoracic echocardiography or TEE [6, 8, 11, 12, 20, 21, 29]. The mitral annular nonplanarity in these studies is calculated as the ratio of annular heights (maximum and minimum) and commissural width (AHCWR), namely, the AHCWR measured relative to a plane of least-square fit through the three-dimensional annulus (Fig 5) [8, 9, 13, 23]. We used the NPA as a measure of the degree of the saddle shape of the annulus. The NPA has been used as for this purpose in other studies as well [30, 31]. Our observation of an unfavorable change in planarity of the native mitral annulus with application of complete annuloplasty rings during repair is consistent with a recent study assessing the annular planarity with a different methodology, the AHCWR [12, 29].
Fig 5.
Comparison of the nonplanarity (NP) angle and the annular height commissural width ratio methods of assessment of the saddle shape of the mitral annulus. A: (AHMax = annular height maximum; AHMin = annular height minimum; Ant. = anterior annulus; MA plane = mitral annular plane of least square fit; Post. = posterior.) B: Assessment of mitral annular saddle shape by the nonplanarity angle.
Our methodology calculates the NPA as the angle subtended between the anterior and posterior annular landmarks (Figs 4, 5) and the middle point of the commissural diameter at end-systole (Fig 4) [30, 31]. A decrease in the NPA implies a more nonplanar annulus, with a corresponding increase in AHCWR (Fig 5). Similar findings were also recently reported in an animal study model in which authors reported almost a 50% reduction in the AHCWR with application of a full ring in mitral position [29].
Maintenance of annular nonplanarity has been shown to reduce leaflet stress in a finite model analysis of mitral leaflet structure [13]. Since the mitral annulus tends to become more flat during ischemic mitral regurgitation [32], repair techniques that restore its saddle shape can potentially prolong durability. Ring annuloplasty in conjunction with MV repair has been shown to prolong the durability of repair [33]. However, MV geometry (size and planarity) is significantly altered by the architecture and design of annuloplasty devices (complete versus partial and rigid versus flexible) [11, 29, 34]. A recent animal study has also demonstrated that the newly developed saddle shaped MV annuloplasty rings optimize leaflet stress and force distribution by increasing the nonplanarity of the native annulus [29]. Although there are no clinical data to support this claim, it is quite possible that annuloplasty ring selection may affect short- and long-term durability of MV repair by alteration of leaflet stress.
Further research would be needed to establish the significance and impact of these geometric alterations on leaflet stress and repair durability. The clinical significance of the availability of this information in the operating room and its impact on surgical decision making also remains to be determined. In the past, such three-dimensional geometric analysis was limited by cumbersome, time-consuming acquisition and off-line analysis. Our study describes that the three-dimensional acquisition and analysis can both be performed within minutes, with a software package tailored specifically to MV geometry [27]. Preoperative availability of information about the three-dimensional structure of the MV raises the possibility of the suitable selection of annuloplasty devices to achieve best results in individual patients.
Limitations of the Study
We have utilized a new methodology of assessment of planarity of the MV annulus, which would need further studies for validation. However, our results as well as prior results of application of NPA are consistent with studies utilizing the conventional methods of three-dimensional reconstruction. Our three-dimensional evaluation is based on R-wave- gated scan plane rotation, which can make the volumetric acquisition time consuming and difficult. However, the system can be adjusted to customize the R-R intervals to facilitate the acquisition, slightly prolonging the time to acquire the data sets.
In conclusion, the application of annuloplasty rings during MV repair surgery results in profound geometric changes in the architecture of the MV. The geometric changes can be measured before and after valve repair utilizing three-dimensional TEE in the operating room. Complete rings increase the NPA of the MV (namely, make the native mitral annulus less saddle shaped) as compared with the partial rings. Neither of the two types of rings restores the NPA to the normal range.
References
- 1.Fedak PW, McCarthy PM, Bonow RO. Evolving concepts and technologies in mitral valve repair. Circulation. 2008;117:963–74. doi: 10.1161/CIRCULATIONAHA.107.702035. [DOI] [PubMed] [Google Scholar]
- 2.Flachskampf FA, Chandra S, Gaddipatti A, et al. Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction. J Am Soc Echocardiogr. 2000;13:277–87. doi: 10.1067/mje.2000.103878. [DOI] [PubMed] [Google Scholar]
- 3.Gillinov AM, Cosgrove DM, Shiota T, et al. Cosgrove-Edwards annuloplasty system: midterm results. Ann Thorac Surg. 2000;69:717–21. doi: 10.1016/s0003-4975(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 4.Glasson JR, Komeda M, Daughters GT, Bolger AF, Ingels NB, Miller DC. Loss of three-dimensional canine mitral annular systolic contraction with reduced left ventricular volumes. Circulation. 1996;94:II152–8. [PubMed] [Google Scholar]
- 5.Glasson JR, Komeda M, Daughters GT, et al. Three-dimensional dynamics of the canine mitral annulus during ischemic mitral regurgitation. Ann Thorac Surg. 1996;62:1059–68. doi: 10.1016/0003-4975(96)00477-8. [DOI] [PubMed] [Google Scholar]
- 6.Gorman JH, Gorman RC, Jackson BM, Enomoto Y, John-Sutton MG, Edmunds LH. Annuloplasty ring selection for chronic ischemic mitral regurgitation: lessons from the ovine model. Ann Thorac Surg. 2003;76:1556–63. doi: 10.1016/s0003-4975(03)00891-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SR, Bashein G, Sheehan FH, et al. Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am Heart J. 2000;139:378–87. doi: 10.1016/s0002-8703(00)90077-2. [DOI] [PubMed] [Google Scholar]
- 8.Levine RA, Handschumacher MD, Sanfilippo AJ, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80:589–98. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 9.Ryan L, Jackson B, Parish L, et al. Quantification and localization of mitral valve tenting in ischemic mitral regurgitation using real-time three-dimensional echocardiography. Eur J Cardiothorac Surg. 2007;31:839–4. doi: 10.1016/j.ejcts.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe N, Ogasawara Y, Yamaura Y, et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation. 2005;112:I458–62. doi: 10.1161/CIRCULATIONAHA.104.524595. [DOI] [PubMed] [Google Scholar]
- 11.Yamaura Y, Yoshikawa J, Yoshida K, Hozumi T, Akasaka T, Okada Y. Three-dimensional analysis of configuration and dynamics in patients with an annuloplasty ring by multiplane transesophageal echocardiography: comparison between flexible and rigid annuloplasty rings. J Heart Valve Dis. 1995;4:618–22. [PubMed] [Google Scholar]
- 12.Ryan LP, Jackson BM, Enomoto Y, et al. Description of regional mitral annular nonplanarity in healthy human subjects: a novel methodology. J Thorac Cardiovasc Surg. 2007;134:644–8. doi: 10.1016/j.jtcvs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Salgo IS, Gorman JH, Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–7. doi: 10.1161/01.cir.0000025426.39426.83. [DOI] [PubMed] [Google Scholar]
- 14.Liddicoat JR, Mac Neill BD, Gillinov AM, et al. Percutaneous mitral valve repair: a feasibility study in an ovine model of acute ischemic mitral regurgitation. Catheter Cardiovasc Interv. 2003;60:410–6. doi: 10.1002/ccd.10662. [DOI] [PubMed] [Google Scholar]
- 15.Garwood S. Con: single-plane echocardiography does not provide an accurate and adequate examination of the native mitral valve. J Cardiothorac Vasc Anesth. 2002;16:515–20. doi: 10.1053/jcan.2002.125156. [DOI] [PubMed] [Google Scholar]
- 16.Pepi M, Barbier P, Doria E, Bortone F, Tamborini G. Intra-operative multiplane vs biplane transesophageal echocardiography for the assessment of cardiac surgery. Chest. 1996;109:305–11. doi: 10.1378/chest.109.2.305. [DOI] [PubMed] [Google Scholar]
- 17.Pu M, Griffin BP, Vandervoort PM, Leung DY, Cosgrove DM, Thomas JD. Intraoperative validation of mitral inflow determination by transesophageal echocardiography: comparison of single-plane, biplane and thermodilution techniques. J Am Coll Cardiol. 1995;26:1047–53. doi: 10.1016/0735-1097(95)00259-2. [DOI] [PubMed] [Google Scholar]
- 18.Stewart WJ, Griffin B, Thomas JD. Multiplane transesophageal echocardiographic evaluation of mitral valve disease. Am J Card Imaging. 1995;9:121–8. [PubMed] [Google Scholar]
- 19.Glasson JR, Komeda MK, Daughters GT, et al. Three-dimensional regional dynamics of the normal mitral anulus during left ventricular ejection. J Thorac Cardiovasc Surg. 1996;111:574–85. doi: 10.1016/s0022-5223(96)70309-4. [DOI] [PubMed] [Google Scholar]
- 20.Tsakiris AG, Von Bernuth G, Rastelli GC, Bourgeois MJ, Titus JL, Wood EH. Size and motion of the mitral valve annulus in anesthetized intact dogs. J Appl Physiol. 1971;30:611–8. doi: 10.1152/jappl.1971.30.5.611. [DOI] [PubMed] [Google Scholar]
- 21.Komoda T, Hetzer R, Uyama C, et al. Mitral annular function assessed by 3D imaging for mitral valve surgery. J Heart Valve Dis. 1994;3:483–90. [PubMed] [Google Scholar]
- 22.Young AA, Kramer CM, Ferrari VA, Axel L, Reichek N. Three-dimensional left ventricular deformation in hypertrophic cardiomyopathy. Circulation. 1994;90:854–67. doi: 10.1161/01.cir.90.2.854. [DOI] [PubMed] [Google Scholar]
- 23.Levine RA, Triulzi MO, Harrigan P, Weyman AE. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation. 1987;75:756–67. doi: 10.1161/01.cir.75.4.756. [DOI] [PubMed] [Google Scholar]
- 24.Ormiston JA, Shah PM, Tei C, Wong M. Size and motion of the mitral valve annulus in man. I. A two-dimensional echocardiographic method and findings in normal subjects. Circulation. 1981;64:113–20. doi: 10.1161/01.cir.64.1.113. [DOI] [PubMed] [Google Scholar]
- 25.Ryan LP, Jackson BM, Parish LM, et al. Regional and global patterns of annular remodeling in ischemic mitral regurgitation. Ann Thorac Surg. 2007;84:553–9. doi: 10.1016/j.athoracsur.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Tibayan FA, Rodriguez F, Langer F, et al. Annular remodeling in chronic ischemic mitral regurgitation: ring selection implications. Ann Thorac Surg. 2003;76:1549–55. doi: 10.1016/s0003-4975(03)00880-4. [DOI] [PubMed] [Google Scholar]
- 27.Mahmood F, Karthik S, Subramaniam B, et al. Intraoperative application of geometric three-dimensional mitral valve assessment package: a feasibility study. J Cardiothorac Vasc Anesth. 2008;22:292–8. doi: 10.1053/j.jvca.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography council for intraoperative echocardiography and the Society of Cardiovascular Anesthesiologists task force for certification in perioperative transesophageal echocardiography. Anesth Analg. 1999;89:870–84. doi: 10.1097/00000539-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Ryan LP, Jackson BM, Hamamoto H, et al. The influence of annuloplasty ring geometry on mitral leaflet curvature. Ann Thorac Surg. 2008;86:749–60. doi: 10.1016/j.athoracsur.2008.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan J, Qin JX, Popovic ZB, Agler DA, Thomas JD, Shiota T. Geometric changes of mitral annulus assessed by real-time 3-dimensional echocardiography: becoming enlarged and less nonplanar in the anteroposterior direction during systole in proportion to global left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:1179–84. doi: 10.1016/j.echo.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Kwan J, Yeom BW, Jones M, et al. Acute geometric changes of the mitral annulus after coronary occlusion: a real-time 3D echocardiographic study. J Korean Med Sci. 2006;21:217–23. doi: 10.3346/jkms.2006.21.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaji S, Nasu M, Yamamuro A, et al. Annular geometry in patients with chronic ischemic mitral regurgitation: three-dimensional magnetic resonance imaging study. Circulation. 2005;112:I409–14. doi: 10.1161/CIRCULATIONAHA.104.525246. [DOI] [PubMed] [Google Scholar]
- 33.Cohn LH, Couper GS, Aranki SF, Rizzo RJ, Kinchla NM, Collins JJ. Long-term results of mitral valve reconstruction for regurgitation of the myxomatous mitral valve. J Thorac Cardiovasc Surg. 1994;107:143–51. [PubMed] [Google Scholar]
- 34.van Rijk-Zwikker GL, Mast F, Schipperheyn JJ, Huysmans HA, Bruschke AV. Comparison of rigid and flexible rings for annuloplasty of the porcine mitral valve. Circulation. 1990;82:IV58–64. [PubMed] [Google Scholar]