ABSTRACT
BACKGROUND
Many older adults in the U.S. do not receive appropriate colorectal cancer (CRC) screening. Although primary care physicians’ recommendations to their patients are central to the screening process, little information is available about their recommendations in relation to guidelines for the menu of CRC screening modalities, including fecal occult blood testing (FOBT), flexible sigmoidoscopy (FS), colonoscopy, and double contrast barium enema (DCBE). The objective of this study was to explore potentially modifiable physician and practice factors associated with guideline-consistent recommendations for the menu of CRC screening modalities.
METHODS
We examined data from a nationally representative sample of 1266 physicians in the U.S. surveyed in 2007. The survey included questions about physician and practice characteristics, perceptions about screening, and recommendations for age of initiation and screening interval for FOBT, FS, colonoscopy and DCBE in average risk adults. Physicians’ screening recommendations were classified as guideline consistent for all, some, or none of the CRC screening modalities recommended. Analyses used descriptive statistics and polytomous logit regression models.
RESULTS
Few (19.1%; 95% CI:16.9%, 21.5%) physicians made guideline-consistent recommendations across all CRC screening modalities that they recommended. In multivariate analysis, younger physician age, board certification, north central geographic region, single specialty or multi-specialty practice type, fewer patients per week, higher number of recommended modalities, use of electronic medical records, greater influence of patient preferences for screening, and published clinical evidence were associated with guideline-consistent screening recommendations (p < 0.05).
CONCLUSIONS
Physicians’ CRC screening recommendations reflect both overuse and underuse, and few made guideline-consistent CRC screening recommendations across all modalities they recommended. Interventions that focus on potentially modifiable physician and practice factors that influence overuse and underuse and address the menu of recommended screening modalities will be important for improving screening practice.
KEY WORDS: guidelines mass screening, colorectal neoplasms/prevention & control, fecal occult blood test, flexible sigmoidoscopy, colonoscopy
INTRODUCTION
Clinical evidence1–5 and practice guidelines6–10 have long supported screening average risk adults aged 50 and older for colorectal cancer (CRC). Guidelines endorse a menu of options, including the fecal occult blood test (FOBT), flexible sigmoidoscopy (FS), colonoscopy, and double-contrast barium enema (DCBE).9,10 Guideline-recommendations for screening reflect current understanding of natural history of CRC, test characteristics, and the balance of potential benefits and harms associated with each modality. Initiating screening at age 50 is recommended for all modalities, although the recommended interval varies by modality, ranging from annual screening with FOBT, every 5 years with FS and DCBE, to every 10 years with colonoscopy. Beginning in 2004, the National Committee for Quality Assurance added this menu of CRC screening modalities to the Healthcare Effectiveness Data and Information Set (HEDIS) measures,11 which are used to evaluate health plan performance. Ideally, having multiple options for screening will allow physicians and patients to consider the risks, benefits, and other attributes of CRC screening modalities and identify the option that is most acceptable to the patient.
Many adults do not receive appropriate CRC screening,12,13 however. In national data from 2008, only 54.5% of adults aged 50-75 were up-to-date with any recommended CRC screening.14 Although many patient factors are associated with screening,12,13,15–17 previous research has shown physician recommendation to be one of the strongest predictors of CRC screening receipt.16–18 By recommending CRC screening to their patients, primary care physicians play a central role in implementing guidelines. Examination of the extent to which physicians’ recommendations for the menu of CRC screening modalities are guideline-consistent has received limited attention, however. To date, most studies have focused on individual screening modalities,19–22 rather than examining the menu of modalities. In this study, we used national data for the menu of recommended CRC screening modalities to explore physician and practice factors associated with guideline-consistent recommendations, with the goal of informing efforts to improve CRC screening.
METHODS
Physician Sample
We used data from a national survey of CRC screening practices among primary care physicians conducted by the National Cancer Institute in 2006-2007.23 The American Medical Association’s Physician Masterfile was used as the sampling frame. Eligible physicians were less than 76 years old, listed in the database with an active license, and had patient care as their major professional activity. Physicians who were retired, deceased, in residency training, or involved in full-time teaching, research, or administration were excluded. A total of 1,266 physicians completed the survey for a cooperation rate of 75% and an absolute response rate of 69%.23
Theoretical Framework
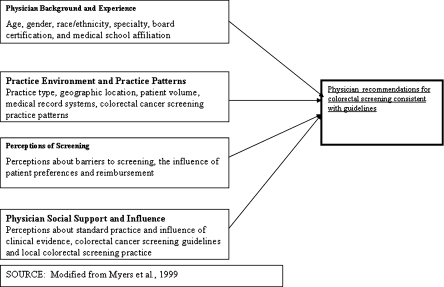
We used a theoretical model of physician behavior24,25 to guide selection of relevant physician and practice variables and inform our analysis (Fig. 1). The model, which is based on Social Cognitive Theory26 and the Theory of Reasoned Action,27 includes domains of physician background and experience; practice environment and practice patterns; physician perceptions of CRC screening; and physician social support and influence. Within the domain of physician background and experience, physician characteristics, including younger age, board certification, and medical school affiliation, have been shown to be associated with screening recommendations and practice.19,23,25,28–31 We hypothesized that these factors would also be associated with guideline-consistent recommendations. Less work has been conducted to assess associations between the domains of practice environment, perceptions of screening, and physician support, and guideline-consistent recommendations. Specifically, we hypothesized that physicians in practices with electronic medical records (EMRs) would be more likely to make guideline-consistent recommendations than those in practices with paper records. We also anticipated that physicians who indicated that clinical evidence was very influential or identified screening guidelines as being very influential would be more likely to make guideline-consistent recommendations.
Figure 1.
Conceptual model of physicians’ recommendations for guideline-consistent colorectal cancer screening.
Measures
Guideline-Consistent Screening Recommendations Our measure of guideline-consistent CRC screening recommendations was based on guidelines at the time the survey was conducted.9,32,33 All guidelines endorsed screening initiation at age 50 and specific intervals for each modality (i.e., annual home FOBT, every 5 years for FS and DCBE, and every 10 years for colonoscopy). Physicians were queried about their recommendations for age of initiation and screening interval for each modality. This information was used to create a measure of guideline-consistent recommendation for each screening modality. Because physicians may discuss multiple modalities with patients, we also created a summary guideline-consistent CRC screening recommendation measure that included all modalities that they recommended. For example, if a physician recommended only one modality, age at initiation and interval were evaluated for only that modality. If a physician recommended all four modalities, age at initiation and interval were evaluated for all four. We also measured whether all; some, but not all; or none of their recommendations were guideline-consistent (i.e., three levels). For this summary measure, physicians who did not recommend any CRC screening modality were classified as having no guideline-consistent recommendations, and physicians who recommended only one modality were classified as either having all or none of their recommendations being guideline consistent.Physician background and experience were measured by age, gender, specialty, race/ethnicity, international medical school graduate, board certification, and medical school affiliation. Specialty was measured as family medicine/general practice, internal medicine, and obstetrics and gynecology.Practice environment and practice patterns were measured with practice type, U.S. Census region (www.census.gov/geo/www/us_regdiv.pdf), number of patients seen during a typical week, number of CRC screening modalities recommended, and the modalities recommended. Monthly volume of screening and type of record system in the main practice were also measured.Physician perceptions about screening were measured with perceptions about barriers to screening and factors influencing screening practice. Patient barriers to screening were measured with responses to questions with the stem “When you talk to your asymptomatic, average risk patients about colorectal cancer screening, how often do you encounter the following? My patients...” and included “do not want to discuss CRC screening”, “have difficulty understanding the information I present about CRC screening”, “are unaware of CRC screening”, “do not perceive CRC as a serious health threat”, and “cannot afford or lack adequate insurance coverage for CRC screening”. Physicians also responded to the statement “My patients do not follow through to complete CRC screening tests” based on the question stem, “How often do you encounter the following barriers to CRC screening for asymptomatic, average-risk patients in your practice”. Responses to all six items (i.e., usually = 3, sometimes = 2, rarely = 1, never = 0) were combined into a single numeric composite measure and categorized into tertiles (<8, 8–9, ≥10).Physicians also responded to questions with the stem “To what extent are the following factors influential in your recommendations for colorectal cancer screening?” Perceptions about influences included “availability of reimbursement by third party payers, including Medicare and Medicaid” and “my patients’ preferences for colorectal cancer screening”. Response categories were very influential, somewhat influential, not influential, and not applicable or not familiar with.Physician social support and influence was defined by responses to the same stem question concerning the influence of “clinical evidence in the published literature”, “how my colleagues in my practice or local community provide colorectal cancer screening for their patients”, and screening guidelines from the U.S. Preventive Services Task Force and the American Cancer Society on the physician’s CRC screening recommendations. Exact wording of all survey items is available at http://healthservices.cancer.gov/surveys/screening_rp/screening_rp_colo_lung_inst.pdf.
Analyses
Distributions of physician and practice characteristics, perceptions of screening and social support and influence were reported with descriptive statistics. Screening recommendations were categorized as being guideline consistent for each modality and across all modalities that physicians recommended in a summary measure (i.e., all, some, or none guideline consistent). We used the summary measure of guideline-consistent recommendations as the outcome in multivariate polytomous logit regression models. We evaluated physician characteristics shown to be associated with cancer screening in other studies (i.e., age, gender, specialty, board certification, medical school affiliation) and also more novel measures within the domains of practice environment, perceptions of screening, and physician social support reported in Table 1. We used a backwards elimination approach to develop a parsimonious model where covariates with an association with the outcome at p < 0.2 using the Wald F-test were retained. Coefficients and p-values in the reduced model changed little from the full model. To further assess the impact of this p-value driven approach for hypothesis generation in our multivariate modeling, we bootstrapped 200 data sets from our sample within the sample strata and applied the backward elimination approach to obtain a reduced model for each bootstrap data set.34 Consistency between the full and reduced models and the bootstrap analysis showed that our multivariate model results were robust.35
Table 1.
Primary Care Physician and Practice Characteristics
| N = 1,266 | |
|---|---|
| Weighted percentage* | |
| Physician background and experience | |
| Age | |
| <40 | 20.1 |
| 40-49 | 30.7 |
| 50-59 | 31.9 |
| ≥60 | 17.4 |
| % Male | 68.8 |
| % White, non-Hispanic | 72.1 |
| % Board certified | 80.2 |
| % international medical school graduate | 21.7 |
| Specialty | |
| Family Medicine/ General Practice | 45.2 |
| Internal Medicine | 36.9 |
| Obstetrics Gynecology | 17.9 |
| % with medical school affiliation | 35.1 |
| Practice environment and practice patterns | |
| Practice type | |
| Solo Practice | 26.0 |
| Single specialty group | 48.1 |
| Multi-specialty group | 23.3 |
| Other/Missing | 2.5 |
| US Census region of country | |
| North central | 23.1 |
| Northeast | 20.1 |
| South | 33.2 |
| West | 22.6 |
| Missing | 0.9 |
| Typical number of patients per week | |
| <76 | 34.9 |
| 76-100 | 32.4 |
| ≥101 | 32.7 |
| Record systems | |
| Paper charts | 56.2 |
| Partial EMR/transition to EMR | 26.0 |
| Full EMR | 17.8 |
| CRC screening tests ordered, referred or performed per month | |
| Low (<21) | 30.6 |
| Intermediate (21-<45) | 35.7 |
| High (45+) | 33.7 |
| Number of CRC screening modalities recommended | |
| 0 | 0.7 |
| 1 | 17.5 |
| 2 | 58.6 |
| 3 | 18.0 |
| 4 | 5.2 |
| Modalities recommended | |
| % Recommend FOBT | 80.3 |
| % Recommend FS | 25.7 |
| % Recommend colonoscopy | 94.8 |
| % Recommend DCBE | 8.6 |
| % Recommend FOBT and colonoscopy | 76.2 |
| % Recommend FOBT and FS | 23.4 |
| % Recommend flexible sigmoidoscopy and colonoscopy | 22.7 |
| % Recommend FOBT and colonoscopy and flexible sigmoidoscopy | 20.6 |
| Perceptions of screening | |
| Patient barriers to screening (composite measure) | |
| Low (<8) | 35.2 |
| Intermediate (8-9) | 33.4 |
| High (≥10) | 31.4 |
| Provider barriers to screening | |
| Shortage of providers for screening other than FOBT or for follow up with invasive endoscopic procedures usually a barrier | 12.2 |
| Usually not enough time to discuss screening with patients | 5.2 |
| Reimbursement | |
| Very influential | 25.0 |
| Somewhat influential | 35.7 |
| Not influential/not applicable/missing | 39.3 |
| Patient preferences for CRC screening | |
| Very influential | 26.2 |
| Somewhat influential | 52.5 |
| Not influential/not applicable/missing | 21.2 |
| Physician social support and influence | |
| Clinical evidence | |
| Very influential | 69.4 |
| Somewhat influential | 27.4 |
| Not influential/ not applicable/missing | 3.2 |
| USPSTF guidelines | |
| Very influential | 63.7 |
| Somewhat influential | 28.2 |
| Not influential/not applicable/missing | 8.1 |
| ACS guidelines | |
| Very influential | 67.3 |
| Somewhat influential | 28.6 |
| Not influential/not applicable/missing | 4.2 |
| Local CRC screening practice | |
| Very influential | 15.2 |
| Somewhat influential | 38.4 |
| Not influential/not applicable/missing | 46.4 |
*Estimates are weighted for selection probability and non-response using survey design variables and SUDAAN statistical software
CRC = colorectal cancer screening EMR=electronic medical record FOBT = fecal occult blood test DCBE = double contrast barium enema USPSTF = U.S. Preventive Services Task Force ACS = American Cancer Society
National Survey of Primary Care Physicians’ Cancer Screening Recommendations and Practices, 2006–2007
Exact wording of all items is available at http://healthservices.cancer.gov/surveys/screening_rp/screening_rp_colo_lung_inst.pdf.
A sample weight that accounts for the probability of selection as well as an adjustment for non-response by sample strata was assigned to each survey respondent. The statistical software SUDAAN was used to apply the sampling weights and incorporate the stratified survey design in the statistical analysis.
RESULTS
Sample Characteristics The majority of physicians were younger than 60, male, and white, non-Hispanic (Table 1). Most were board certified. Physicians practiced in a variety of settings, including solo (26%), single specialty group (48%), and multi-specialty group practice (23%). Most physicians saw 100 or fewer patients per week and used paper charts as their record system. Most physicians recommended 2 screening modalities and about 18% recommended either 1 modality or 3 modalities. Almost all physicians recommended colonoscopy (94.8%) and most recommendations included FOBT and colonoscopy (76.2%). About one quarter identified patient preferences for screening as very influential. The majority of physicians indicated that clinical evidence or screening guidelines were very influential in their CRC screening practice.
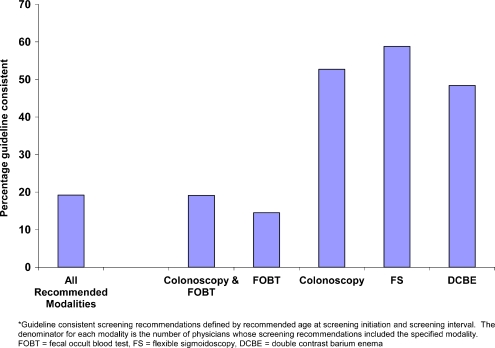
Guideline-Consistent Screening Recommendations Most physicians made guideline-consistent recommendations for initiating screening in average risk patients at age 50 and for screening intervals, although this varied by modality (Table 2). Physicians with guideline-inconsistent age at initiation recommendations mostly recommended starting at younger, rather than at older, ages. Physicians with guideline-inconsistent screening interval recommendations mostly recommended more frequent, rather than less frequent testing, particularly for colonoscopy, the modality with the longest recommended interval between screening tests. Guideline-consistent recommendations for both age at initiation and screening interval varied by modality, with approximately half of physicians making guideline-consistent recommendations for colonoscopy, FS, or DCBE and fewer than 20% for FOBT (Fig. 2). Few physicians (19%; 95% CI:17%, 22%) made guideline-consistent recommendations for all modalities that they recommended. More physicians made some guideline-consistent screening recommendations (40.4%; 95% CI: 37.9%, 43.1%), but a nearly equal proportion made recommendations for which none were guideline consistent (40.5%; 95% CI: 38.0%, 43.0%). In multivariate analysis, measures of physician background, practice environment, perceptions of screening, and social support were significantly associated with guideline-consistent recommendations (Table 3). Within the domain of physician background and experience, physicians younger than age 40 and those who were board certified were more likely than older or non-board certified physicians to make guideline-consistent recommendations. Within the domain of practice environment and practice patterns, physicians in single specialty and multi-specialty group practices were more likely to make guideline-consistent recommendations than were physicians in solo practice. Geographic variability was evident, with physicians in the North Central region of the country more likely to make guideline-consistent recommendations. Physicians in practices with a full EMR or in transition to EMR from paper records were more likely than physicians in practices with paper charts to make guideline-consistent recommendations.Physicians seeing fewer patients in a typical week and those recommending 3 or 4 CRC screening modalities were more likely to make guideline-consistent recommendations than were physicians seeing more patients or recommending fewer screening modalities. Within the domain of perceptions of CRC screening, physicians who reported that patient preferences for screening were influential were more likely to make guideline-consistent screening recommendations.Finally, in the domain of social support and influence, physicians who indicated that clinical evidence in the published literature was influential in their screening practice were more likely to make some guideline-consistent screening recommendations than were physicians who reported clinical evidence as less influential.
Table 2.
Primary Care Physicians’ Guideline-Consistent Colorectal Cancer Screening Recommendations*: Age at Initiation and Screening Interval by Modality
| Screening Test (Number of Physicians Recommending) | |||||
|---|---|---|---|---|---|
| Fecal Occult Blood Test | Flexible Sigmoidoscopy | Colonoscopy | Double Contrast Barium Enema | ||
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | ||
| Age at Initiation | N | N = 1004 | N = 323 | N = 1197 | N = 98 |
| Younger than guideline | 41.3 | 10.6 | 3.4 | 10.4 | |
| (38.6, 44.0) | (7.6, 14.4) | (2.6, 4.4) | (4.6, 22.0) | ||
| Guideline-Consistent | 58.0 | 86.9 | 93.9 | 85.1 | |
| (55.2, 60.7) | (82.7, 90.2) | (92.5, 95.0) | (74.4, 91.8) | ||
| Older than guideline | 0.8 | 2.6 | 2.8 | 4.4 | |
| (0.4, 1.5) | (1.3, 5.0) | (1.9, 3.9) | (1.9, 10.2) | ||
| Screening Interval | N = 994 | N = 317 | N = 1170 | N = 96 | |
| More frequent than guideline | 0.2 | 23.1 | 44.3 | 19.8 | |
| (0.0, 0.8) | (18.0, 29.2) | (41.7, 47.0) | (12.4, 30.1) | ||
| Guideline-Consistent | 88.5 | 67.6 | 55.7 | 63.3 | |
| (86.2, 90.4) | (61.9, 72.8) | (53.0, 58.3) | (51.8, 73.4) | ||
| Less frequent than guideline | 11.3 | 9.3 | 0.0 | 16.9 | |
| (9.4, 13.6) | (6.5, 13.2) | (10.7, 25.7) | |||
*Estimates are weighted for selection probability and non-response using survey design variables and SUDAAN statistical software Data source: National Survey of Primary Care Physicians’ Cancer Screening Recommendations and Practices, 2006-2007
Shading represents guideline-consistent recommendations
Figure 2.
Percentage of primary care physicians with guideline-consistent colorectal cancer screening recommendations.
Table 3.
Multivariate Associations between Primary Care Physician and Practice Characteristics and Guideline-Consistent Colorectal Cancer Screening Recommendations
| Guideline-consistent screening recommendations* | p-value | ||||
|---|---|---|---|---|---|
| All guideline-consistent vs. none | Some guideline-consistent vs. none | ||||
| OR | (95% CI) | OR | (95% CI) | ||
| Physician background and experience | |||||
| Age | |||||
| <40 | 1.00 | 1.00 | <0.001 | ||
| 40-49 | 0.55 | (0.34, 0.88) | 0.82 | (0.52, 1.28) | |
| 50-59 | 0.39 | (0.23, 0.67) | 0.70 | (0.43, 1.15) | |
| ≥ 60 | 0.17 | (0.09, 0.35) | 0.41 | (0.24, 0.71) | |
| Gender | |||||
| Male | 1.00 | 1.00 | 0.002 | ||
| Female | 0.94 | (0.63, 1.41) | 1.63 | (1.20, 2.21) | |
| International medical school graduate | |||||
| Yes | 1.00 | 1.00 | 0.064 | ||
| No | 1.99 | (1.11, 3.58) | 1.23 | (0.87, 1.74) | |
| Board certification | |||||
| Yes | 1.95 | (1.12, 3.38) | 1.42 | (1.00, 2.03) | 0.030 |
| No | 1.00 | 1.00 | |||
| Medical school affiliation | |||||
| Yes | 1.27 | (0.90, 1.80) | 1.32 | (0.98, 1.80) | 0.151 |
| No | 1.00 | 1.00 | |||
| Specialty | |||||
| Family medicine/General practice | 1.00 | 1.00 | 0.132 | ||
| Internal medicine | 0.63 | (0.40, 0.99) | 0.75 | (0.53, 1.06) | |
| Obstetrics/Gynecology | 0.70 | (0.46, 1.05) | 0.68 | (0.45, 1.03) | |
| Practice environment and practice patterns | |||||
| Practice type | |||||
| Solo practice | 1.00 | 1.00 | <0.001 | ||
| Single specialty group | 3.09 | (1.67, 5.69) | 1.68 | (1.12, 2.52) | |
| Multi-specialty group | 4.71 | (2.54, 8.75) | 1.45 | (0.96, 2.19) | |
| Other | 1.93 | (0.47, 7.92) | 0.71 | (0.22, 2.26) | |
| Region | |||||
| Northeast | 0.67 | (0.42, 1.07) | 0.94 | (0.66, 1.35) | 0.014 |
| North central | 1.54 | (0.98, 2.42) | 1.45 | (1.03, 2.04) | |
| South | 1.00 | 1.00 | |||
| West | 1.05 | (0.69, 1.60) | 0.99 | (0.66, 1.47) | |
| Number of patients per week | |||||
| <76 | 1.00 | 1.00 | 0.008 | ||
| 76-100 | 0.76 | (0.48, 1.23) | 1.09 | (0.72, 1.64) | |
| ≥101 | 0.45 | (0.28, 0.72) | 0.98 | (0.66, 1.44) | |
| Record systems | |||||
| Paper charts | 1.00 | 1.00 | 0.007 | ||
| Partial EMR/transition to EMR | 1.47 | (0.92, 2.35) | 1.15 | (0.77, 1.71) | |
| Full EMR | 2.31 | (1.50, 3.56) | 1.44 | (0.97, 2.14) | |
| Number of CRC screening modalities recommended | |||||
| 1 or 2 | 1.00 | 1.00 | <0.001 | ||
| 3 or 4 | 0.99 | (0.57, 1.72) | 3.43 | (2.30, 5.12) | |
| Physician perceptions of colorectal cancer screening | |||||
| Availability of third party reimbursement | |||||
| Very influential | 1.70 | (0.97, 2.96) | 1.11 | (0.74, 1.68) | 0.101 |
| Somewhat influential | 1.37 | (0.95, 1.98) | 1.28 | (0.98, 1.68) | |
| Not influential | 1.00 | 1.00 | |||
| Patients preferences for CRC screening | |||||
| Very influential | 1.96 | (1.18, 3.24) | 1.92 | (1.17, 3.15) | 0.018 |
| Somewhat influential | 1.37 | (0.89, 2.11) | 1.72 | (1.14, 2.59) | |
| Not influential | 1.00 | 1.00 | |||
| Patient barriers to screening (composite measure) | |||||
| Low (<8) | 1.00 | 1.00 | 0.168 | ||
| Intermediate (8-9) | 1.56 | (0.98, 2.46) | 1.21 | (0.86, 1.70) | |
| High (≥10) | 0.91 | (0.57, 1.43) | 0.95 | (0.67, 1.34) | |
| Physician social support and influence | |||||
| Clinical evidence in published literature | |||||
| Very or somewhat influential | 3.25 | (0.82, 12.84) | 3.75 | (1.39, 10.10 | 0.028 |
| All other | 1.00 | 1.00 | |||
| Local CRC screening practice | |||||
| Very influential | 0.45 | (0.24, 0.82) | 0.53 | (0.35, 0.79) | 0.005 |
| Somewhat influential | 0.49 | (0.33, 0.73) | 0.76 | (0.56, 1.03) | |
| Not influential | 1.00 | 1.00 | |||
*Estimates are weighted for selection probability and non-response using survey design variables and SUDAAN statistical software
CRC=colorectal cancer screening EMR=electronic medical record FOBT=fecal occult blood test DCBE=double contrast barium enema USPSTF=U.S. Preventive Services Task Force ACS=American Cancer Society
National Survey of Primary Care Physicians’ Cancer Screening Recommendations and Practices, 2006-2007
Exact wording of all items is available at http://healthservices.cancer.gov/surveys/screening_rp/screening_rp_colo_lung_inst.pdf
DISCUSSION
In this study, we used data from a nationally representative survey of primary care physicians to evaluate the extent to which physicians’ CRC screening recommendations for average risk patients were guideline-consistent. Although few physicians made recommendations that were guideline consistent for all CRC modalities they recommended (19%), a larger portion made at least some recommendations that were guideline consistent (40%). A comparable proportion (41%) made CRC screening recommendations for which none were guideline consistent. A major contribution of this study is that we evaluated whether U.S. physicians’ recommendations are guideline consistent across the menu of CRC screening modalities.
The proportion of physicians making guideline-consistent screening recommendations was higher in 2007 than reported in a prior national survey of primary care physicians conducted in 2000.35 Across individual CRC screening modalities, more physicians recommended initiating screening at age 50 and recommended intervals for most modalities were more likely to be guideline consistent in 2007 than in 2000.28 CRC screening practice patterns have changed dramatically over this period with many more physicians recommending colonoscopy in 2007 than in 2000 (95% vs. 37%) and many fewer recommending sigmoidoscopy (26% vs. 76%).23,28 In 2007, the most common combination recommended included colonoscopy and FOBT (76% vs. 35% in 2007 and 2000, respectively); whereas in 2000 the most common combination included FOBT and FS (23% vs. 73% in 2007 and 2000, respectively).28 Differences across this time period likely reflect secular changes, such as implementation of Medicare reimbursement for screening colonoscopy, HEDIS measures for CRC screening, greater consistency across guidelines, as well as changes in physician and practice characteristics.
Others have reported that physician recommendations for specific screening modalities are not consistent with guidelines.19,22,23,28 In this study, most physicians whose CRC screening recommendations were not guideline-consistent recommended initiating screening in their younger patients or at more frequent intervals than prescribed. Importantly, colonoscopy was the modality for which the highest proportion of physicians (approximately 40%) recommended screening more frequently than guidelines specify. It is also the most expensive CRC screening modality and the most commonly recommended. Overuse of screening is expensive for the health care system, and may result in unnecessary follow-up testing for patients36 and increased risk of complications.37
Some physicians recommended initiating screening in patients older than age 50 or at longer intervals than specified in guidelines. Underuse of screening results in fewer earlier stage or pre-invasive cancers being detected. Estimates of the effectiveness and cost-effectiveness of CRC screening are typically based on a specified starting age and screening intervals for each modality; our findings suggest that if CRC screening were evaluated as applied in practice, estimates of the effectiveness and cost-effectiveness might be substantially different. For example, increasing the frequency of screening colonoscopy from every 10 years to every 5 years increases costs and complications with little improvement in survival.38
As has been reported elsewhere, younger, board certified physicians in larger practices were more likely to make guideline-consistent screening recommendations.19,25,28,39 Other components of our theoretical framework were significantly associated with guideline-consistent recommendations. In the domain of practice environment and practice patterns, several variables were associated with screening performance, including geographic region. Area-level primary care physician supply has been previously reported to be associated with CRC screening utilization40 and stage of CRC diagnosis.41 Geographic variation in practice patterns and guideline-consistent CRC screening recommendations may also reflect unmeasured population characteristics, differences in state level health insurance coverage requirements, health care programs or other area-level factors. Understanding the role of these geographic characteristics on health outcomes is an important area for additional research.
Physicians in practices with a full EMR and those in practices transitioning to EMR were more likely to make guideline-consistent recommendations than were physicians in practices with paper charts. Differences between physicians in practices in transition to EMR and those with paper charts likely reflect their early adoption of measures to improve primary care practice rather than a direct effect of a partial EMR. Others have suggested that computerized office reminder systems can improve CRC screening, although the evidence about the impact of EMRs on the quality of care is mixed.42 A strength of our study is that the level of adoption of EMRs was reported by physicians, who are the main users of medical record systems. Future research might also evaluate how the EMRs are being used, namely, for medical record storage, patient-physician and physician-physician communication, or decision support,43 when evaluating quality of care outcomes.
Several of our findings suggest that physicians with more patient-centered practices are more likely to make guideline-consistent recommendations. Physicians who indicated that patient preferences were influential and who reported seeing fewer patients in a typical week were more likely to make guideline-consistent CRC screening recommendations. Others have described the many demands on primary care physicians’ time;44 seeing fewer patients may allow physicians to spend more time with each patient. Physicians who recommended three or four modalities, and might be more likely to offer more options to patients, were also more likely to make guideline-consistent recommendations than were physicians who recommended only one or two modalities. Taken together, these findings suggest that presentation and recommendation of multiple screening options and attention to patient preferences, which are hallmarks of shared decision-making, are also associated with greater quality of care.
In the domain of physician social support and influence, physicians who reported that clinical information in the published literature was influential in their CRC screening practice were more likely to make guideline-consistent recommendations, although surprisingly, we did not observe an association between the perception of guidelines being influential and guideline-consistent screening recommendations. This suggests that efforts to improve delivery of CRC screening should focus on enhancing awareness and understanding of both clinical evidence and guidelines.
Despite the strengths of using a theoretical framework with data from a national sample of primary care physicians with a high response rate and conducting detailed statistical analyses to evaluate our multivariate model development, our study has some limitations. To encourage a high response rate, the survey was relatively brief and did not include questions about factors that may influence screening recommendations, such as disagreement with or misunderstanding of guidelines, or concerns about malpractice. Earlier studies have shown that physician self-report overstates practices they believe to be recommended (e.g., patient receipt of cancer screening45), although more recent studies suggest that physician self-report of practice is reliable.46 Any idealized reports of practice would imply that guideline-consistent screening recommendations are lower than reported here.
In summary, few primary care physicians made recommendations for CRC screening that were consistent with guidelines for the menu of screening modalities. Physicians’ CRC screening recommendations reflect both overuse and underuse. Implementation of effective interventions that address overuse and underuse of screening and focus on potentially modifiable physician and practice factors will be important for improving screening practice.
Acknowledgement
The survey was conducted under contract with Westat. Funding support was provided by the National Cancer Institute (contract numbers N02-PC-51308), the Agency for Healthcare Research and Quality (inter-agency agreement numbers Y3-PC-5019-01 and Y3-PC-5019-02) and the Centers for Disease Control and Prevention (interagency agreement number Y3-PC-6017-01). Presented in part at the American Public Health Association meeting November 10, 2009.
Conflict of Interest None disclosed.
REFERENCES
- 1.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schumann LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Thomas WM, Chamberlain J, Pye G, Sheffeild J, James PD, et al. Randomized, controlled trial of faecal occult blood screening for colorectal cancer Results for first 107, 349 subjects. Lancet. 1989;1:1160–1164. doi: 10.1016/S0140-6736(89)92750-5. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst. 1993;85:1311–1318. doi: 10.1093/jnci/85.16.1311. [DOI] [PubMed] [Google Scholar]
- 6.Guide to Clinical Preventive Services: Report of the US Preventive Services Task Force. 2. Baltimore, MD: Williams & Wilkins; 1996. [Google Scholar]
- 7.Byers T, Levin B, Rothenberger D, Dodd GD, Smith RA. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer. CA Cancer J Clin. 1997;47:154–160. doi: 10.3322/canjclin.47.3.154. [DOI] [PubMed] [Google Scholar]
- 8.Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 9.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA, Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al. American Cancer Society guidelines on screening and surveillance for the early detection of adenomatous polyps and colorectal cancer: update 2001. CA Cancer J Clin. 2001;51:44–54. doi: 10.3322/canjclin.51.1.38. [DOI] [Google Scholar]
- 11.National Committee for Quality Assurance (NCQA). Accessed July 20, 2009. HEDIS® - Health Plan Employer Data and Information Set. 2009.
- 12.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomark Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomark Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN. Trends in the use and quality of colorectal cancer screening in the U.S. 2010. NIH State of the Science Conference: Enhancing Use and Quality of Colorectal Cancer Screening. 2-2-2010.
- 15.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89:1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 16.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/S0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Schenck AP, Davis WW. Barriers to colorectal cancer screening among Medicare consumers. Am J Prev Med. 2006;30:313–319. doi: 10.1016/j.amepre.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Levy BT, Dawson J, Hartz AJ, James PA. Colorectal cancer testing among patients care for by Iowa family physicians. Am J Prev Med. 2006;31:193–201. doi: 10.1016/j.amepre.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Hawley ST, Levin B, Vernon SW. Colorectal cancer screening by primary care physicians in two medical care organizations. Cancer Detect Prev. 2001;25:309–318. [PubMed] [Google Scholar]
- 20.Montano DE, Phillips WR, Kasprzyk D. Explaining physician rates of providing flexible sigmoidoscopy. Cancer Epidemiol Biomark Prev. 2000;9:665–669. [PubMed] [Google Scholar]
- 21.Schroy PC, III, Geller AC, Wood MC, Page M, Sutherland L, Holm LJ, et al. Utilization of colorectal cancer screening tests: a1997 survey of Massachusetts internists. Prev Med. 2001;33:381–391. doi: 10.1006/pmed.2001.0903. [DOI] [PubMed] [Google Scholar]
- 22.Nadel MR, Shapiro JA, Klabunde CN, Seeff LC, Uhler R. mith RA et al. A national survey of primary care physicians’ methods for screening for fecal occult blood. Ann Intern Med. 2005;142:86–94. doi: 10.7326/0003-4819-142-2-200501180-00007. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med. 2009;37:8–16. doi: 10.1016/j.amepre.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers RE, Hyslop T, Gerrity M, Schlackman N, Hanchak N, Grana J, et al. Physician intention to recommend complete diagnostic evaluation in colorectal cancer screening. Cancer Epidemiol Biomark Prev. 1999;8:587–593. [PubMed] [Google Scholar]
- 25.Yabroff KR, Klabunde CN, Myers R, Brown M. Physician recommendations for follow-up of positive fecal occult blood test. Med Care Res Rev. 2005;62:79–110. doi: 10.1177/1077558704271725. [DOI] [PubMed] [Google Scholar]
- 26.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 27.Fishbein M, Ajzen I. Understanding attitudes and predicting social behavior. Englewood, NJ: Prentice Hall; 1980. [Google Scholar]
- 28.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians’ colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–362. doi: 10.1016/S0091-7435(02)00066-X. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz JS, Lewis CE, Clancy C, Kinosian MS, Radany MH, Koplan JP. Internists’ practices in health promotion and disease prevention A survey. Ann Intern Med (US) 1991;114:46–53. doi: 10.7326/0003-4819-114-1-46. [DOI] [PubMed] [Google Scholar]
- 30.Pham HH, Schrag D, Hargraves JL, Bach PB. Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294:473–481. doi: 10.1001/jama.294.4.473. [DOI] [PubMed] [Google Scholar]
- 31.Lurie M, Slater J, McGovern P, Ekstrum J, Quam L, Margolis K. Preventive care for women: Does the sex of the physician matter? N Engl J Med. 1993;329:478–482. doi: 10.1056/NEJM199308123290707. [DOI] [PubMed] [Google Scholar]
- 32.Smith RA, Cokkinides V, Eyre HJ. American Cancer Society. American Cancer Society guidelines for the early detection of cancer, 2004. CA Cancer J Clin. 2004;54:41–52. doi: 10.3322/canjclin.54.1.41. [DOI] [PubMed] [Google Scholar]
- 33.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale - update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE., Jr . Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 35.Physicians’ Recommendations for Guideline Consistent Colorectal Cancer Screening. http://healthservicescancergov/guidelineconsistentCRCscreening/. 2010;Accessed July 22, 2010.
- 36.Lafata JE, Simpkins J, Lamerato L, Poisson L, Divine G, Johnson CC. The economic impact of false-positive cancer screens. Cancer Epidemiol Biomark Prev. 2004;13:2126–2132. [PubMed] [Google Scholar]
- 37.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 38.Lansdorp-Vogelaar I, Ballegooijen M, Zauber AG, Boer R, Wilschut J, Habbema JDF. At what costs will screening with CT colonography be competitive? a cost effectiveness approach. Int J Cancer. 2008;124:1161–1168. doi: 10.1002/ijc.24025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner BJ, Amsel Z, Lustbader E, Schwartz JS, Balshem A, Grisso JA. Breast cancer screening: effect of physician speciality, practice setting, year of medical school graduation, and sex. Am J Prev Med. 1992;8:78–85. [PubMed] [Google Scholar]
- 40.Haas JS, Fitzmaurice G, Brawarsky P, Liang S-Y, Hiatt RA, Klabunde CN, et al. Association of regional variation in primary care physicians’ colorectal cancer screening recommendations with individual use of colorectal cancer screening. Prev Chronic Dis. 2007;4:1–11. [PMC free article] [PubMed] [Google Scholar]
- 41.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Durme DJ, Ayanian JZ, et al. The effects of physician supply on the early detection of colorectal cancer. J Fam Pract. 1999;48:850–858. [PubMed] [Google Scholar]
- 42.Garg AX, Adhikari NKJ, McDonald H, Rosas-Arellano MP, Devereaux PJ, Beyene J, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA. 2005;293:1223–1238. doi: 10.1001/jama.293.10.1223. [DOI] [PubMed] [Google Scholar]
- 43.Bodenheimer T, Grumbach K. Electronic technology: a spark to revitalize primary care? JAMA. 2003;290:259–264. doi: 10.1001/jama.290.2.259. [DOI] [PubMed] [Google Scholar]
- 44.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/AJPH.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montaño DE, Phillips WR. Cancer screening by primary care physicians: a comparison of rates obtained from physician self-report, patient survey, and chart adult. Am J Public Health. 1995;85:795–800. doi: 10.2105/AJPH.85.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeazel MW, Linstrom Bremer KM, Center BA. A validated tool for gaining insight into clinicians’ preventive medicine behaviors and beliefs: the preventive medicine attitudes and activities questionnaire (PMAAQ) Prev Med. 2006;43:86–91. doi: 10.1016/j.ypmed.2006.03.021. [DOI] [PubMed] [Google Scholar]