ABSTRACT
BACKGROUND
The epidemiology of adverse drug events (ADEs) and medication errors has received little evaluation outside the U.S. and Europe, and extrapolating from these data might not be valid, especially regarding selecting and prioritizing solutions.
OBJECTIVE
To assess the incidence and preventability of ADEs and medication errors in Japan.
DESIGN
The Japan Adverse Drug Events (JADE) study was a prospective cohort study.
PATIENTS
A cohort of 3,459 adults admitted to a stratified random sample of seven medical and eight surgical wards and three intensive care units in three tertiary care hospitals over 6 months.
MAIN MEASURES
We measured ADE and medication error rates from daily reviews of charts, laboratories, incident reports, and prescription queries by on-site reviewers; presence of a signal was considered an incident. Two independent physicians reviewed incidents to determine whether they were ADEs or medication errors and to assess severity and preventability.
KEY RESULTS
We identified 1,010 ADEs and 514 medication errors (incidence: 17.0 and 8.7 per 1,000 patient-days, respectively) during the study period. Among ADEs, 1.6%, 4.9% and 33% were fatal, life-threatening and serious, respectively. Among ADEs, 14% were preventable. The rate per admission was 29 per 100 admissions, higher than in U.S. studies because associated with of the long length of hospital stay in Japan (mean, 17 days).
CONCLUSIONS
The epidemiology and nature of ADEs and medication errors in Japan were similar to other countries, although more frequent per admission. Solutions that worked in these countries might thus improve medication safety in Japan, as could shortening hospital length of stay.
KEY WORDS: adverse drug events, epidemiology, medication errors, patient safety
INTRODUCTION
Injuries due to medications, referred to as adverse drug events (ADEs)1, represent the most frequent cause of injuries due to medical care in hospitals in developed countries2,3. Studies have found that 6.5% of adult inpatients4, 27.4% of adult outpatients5, and 2.3% of pediatric inpatients developed ADEs6, while a meta-analysis on adult inpatients found a rate of 6.7% for adverse drug reactions 7. The consequences of ADEs range from relatively minor symptoms such as a rash to death1,4, and ADEs also result in important consequences including hospital admission, prolonged hospital stay and additional resource utilization8. Similar to other injuries due to medical care, ADEs can be associated with errors and preventable, or can be non-preventable. They can occur at any stage in the medication use process, including ordering, transcribing, dispensing, administering and monitoring1. Medication errors are any error in the medication process; they are much more common than ADEs with one study finding them in 5.3% of medication orders, although they often do not result in harm4.
The epidemiology and nature of ADEs and medication errors in hospitals have been described in detail in some Western countries, but almost all the available data come from these nations3. Many of the studies from outside the U.S. which addressed this issue were from many years ago9–11. Without such basic data from all parts of the world, the effectiveness of various solutions attested in some Western countries cannot necessarily be extrapolated to local settings worldwide12. In addition, patient safety has become a global concern. The World Health Organization thus launched the World Alliance for Patient Safety to investigate the impact of patient safety issues13. Thus, investigating the epidemiology and nature of ADEs and medication errors in local settings is essential for patient safety from both the local and global perspectives.
In particular, to have more information from outside the Western countries would be very helpful for understanding the differences by nation and region, as well as to suggest what interventions may be most helpful. To address these issues, we therefore conducted the Japan Adverse Drug Events (JADE) Study, a prospective cohort study to estimate the incidence and characteristics of ADEs and medication errors in Japan.
METHODS
Study Design and Patient Population
The JADE Study was a prospective cohort study involving three urban tertiary care hospitals in Japan. Two hospitals had electronic medical records and one did not, but none had decision support systems for prescribing or other clinical domains. All were teaching hospitals and resident physicians defined as having <3 years of training after obtaining a license were in charge of some of the patients under the supervision of attending physicians, while attending physicians directly cared for other patients without resident physicians. The total number of beds among the three hospitals was 2,224, and they were spread among 26 adult medical wards, 30 surgical wards, and three intensive care units (ICUs). The hospitals also included obstetrics/gynecology, and pediatrics wards, but we excluded these wards because they have low rates of medication use. The 56 medical and surgical wards were stratified according to hospital and whether they were medical or surgical wards, and study wards were randomly selected within a stratum using a random number generator. We, thus included seven medical and eight surgical wards as well as all three ICUs so that the study design and sample size were similar to a previous report4.
We included all adult patients aged ≥15 years who were admitted to any of the 18 study wards over a 6-month period from January through June 2004. The main units of evaluation were patient-day and admission number. The institutional review boards of three participating hospitals and Kyoto University Graduate School of Medicine approved the study.
Data Collection and Classification
Based on the reported methods1, trained nurses or nursing students placed at each participating hospital reviewed all charts daily on weekdays, along with laboratories, incident reports, and prescription queries. They also collected the characteristics of the patients in the cohort.
The primary outcome of the study was the ADE, defined as an injury due to a medication. For example, cough after receiving angiotensin-converting enzyme (ACE) inhibitors without other reasons is considered an ADE. We also identified medication errors, and we refer to ADEs with medication errors as preventable ADEs, and those without medication errors as non-preventable ADEs. Some ADEs are associated with medication errors and they can be prevented if such errors were intercepted. Medication errors could occur at any step of the medication use process. Medication errors may or may not cause ADEs. For example, cough due to an ACE inhibitor in a patient without a history of ACE inhibitor-induced cough would not be the result of a medication error, but it would be if the patient had a history of such cough. Minor errors that had little or no potential for harm were not considered potential ADEs but as medication errors (for example, a dose of noncritical medication such as docusate being administered several hours late). An incident that had potential for harm was considered both a medication error and a potential ADE (for example, a dose of critical medication such as an intravenous antibiotic not being administered). A potential ADE was a medication error with the potential to cause an injury but which did not actually do so, either because of specific circumstances, chance, or because the error was intercepted and corrected (for example, an order was written for an overdose of medication but the error was intercepted by the pharmacist).
Two independent physician reviewers evaluated all incidents and classified them according to whether they were ADEs or medication errors, as well as to their severity and preventability. If a medication error was found, then the type of error and stage in the process at which it occurred were also classified. Reviewers considered ADEs as preventable if they were due to an error or were ameliorable by any means available. Categories of severity were fatal, life-threatening, serious, and significant 1. Briefly, fatal ADEs resulted in death; life-threatening ADEs caused such issues as transfer to ICU or anaphylactic shock; serious ADEs included gastrointestinal bleeding, altered mental status, excessive sedation, increased creatinine, or a decrease in blood pressure; and significant ADEs included for example cases with rash, diarrhea or nausea. The stages of the medication use process were: ordering by physicians, transcription by nurses, dispensing by pharmacists, administration by nurses or patients themselves, and monitoring by physicians or other health professionals. When disagreement affected classification of an event, the reviewers reached consensus through discussion.
Inter-rater reliabilities were assessed using kappa statistics. The kappa scores regarding presence of an ADE between reviewers were 0.75 (ADE vs. potential ADE or exclude) and 0.77 (Exclude vs. ADE or potential ADE). The kappa for preventability was 0.86 (preventable vs. non-preventable), while kappas for severity were 0.31 (life-threatening vs. serious or significant) and 0.64 (significant vs. serious or life-threatening). These values were similar to a previous report4.
Statistical Analyses
Incidence per 1,000 patient-days, crude rates per 100 admissions and their 95% confidence intervals [CIs] were calculated as a whole and by ward type (medical, surgical, or ICU). To extrapolate to total annual rates in the three hospitals, we assumed admissions to all wards at these hospitals excluding obstetrics/gynecology and pediatrics based on data from the previous year. The observed rate for each ward type was applied to all wards of that type.
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), and categorical variables are shown as numbers and percentages. We used the logistic regression models to assess the relationships between potential risk factors and ADEs or medication errors. The potential risk factors included were elderly (≥65 years), gender, ward type (admitted to ICU, medical or surgical ward), whether the physician in charge was a resident, admission pathway (scheduled, emergency, transferred from other ward), history of allergy, and the number of medication use on admission. We used SAS 9.2 (SAS Institute Inc., Cary, NC) for all statistical analyses.
RESULTS
During the study period, there were 3,459 admissions with 59,383 patient-days on the study wards. Based on hospital administrative data, 27,156 admissions per year were predicted at the three hospitals excluding the obstetrics/gynecology and pediatric wards, and 13% of all patients were sampled in this study. Among the 3,459 patients, 1,958 (57%) were male and the mean age was 66 (SD 17) years; 62% were aged ≥65 years. The median hospital stay was 10 (interquartile range 4-19) days. The medical and surgical wards and the ICUs admitted 1,531 (44%), 1,469 (42%), and 459 (13%) patients, respectively. The median number of medications on admission was 4 (range 0-17).
Adverse Drug Events
The on-site reviewers identified 4,581 incidents during the study period. Among these incidents, reviewers judged that there were 1,010 ADEs in 726 patients, for an incidence of 17.0 [95%CI 16.0-18.1] per 1,000 patient-days and a crude rate per 100 admissions of 29.2 [95%CI 27.7-30.7]. Based on these data and information from the three hospitals, 8,000 ADEs are estimated to occur annually among the three hospitals. The incidence was higher in ICUs, with 30.7 ADEs per 1,000 patient-days, whereas the crude rate was higher in medical wards, with 32.9 events per 100 admissions (Table 1). The median hospital stay from admission to ADE was 7 (interquartile range 3-14) days.
Table 1.
Incidence of Adverse Drug Events
| Ward | n | Patient-days | ADEs | Incidence* | 95% CI | Crude rate† | 95% CI | Annual ADEs‡ |
|---|---|---|---|---|---|---|---|---|
| Medicine | 1,531 | 25,734 | 504 | 19.6 | 17.9-21.3 | 32.9 | 30.6-35.3 | 4,148 |
| Surgery | 1,469 | 30,419 | 407 | 13.4 | 12.1-14.7 | 27.7 | 25.4-30.0 | 3,218 |
| ICU | 459 | 3,230 | 99 | 30.7 | 24.6-36.7 | 21.6 | 17.8-25.3 | 634 |
ADE, adverse drug event; ICU, intensive care unit; CI, confidence interval; *per 1,000 patient-days; †per 100 admissions; ‡Extrapolated from number of ADEs and information from three hospitals
Fourteen patients suffered fatal ADEs during the study; in this group, two patients suffered two ADEs (Table 2). Fatal and life-threatening ADEs accounted for 1.6% and 4.9% of all ADEs, respectively. Ten of 14 patients with fatal ADEs died from antibiotics-associated ADEs. Sixty percent of ADEs were significant and few caused permanent disability (Table 2).
Table 2.
Severity of Adverse Drug Events
| Severity | n (patients) | Rate (%) | 95% CI |
|---|---|---|---|
| Death | 16 (14) | 1.6 | 0.8-2.4 |
| Life-threatening | 49 (46) | 4.9 | 3.5-6.2 |
| Serious | 330 (272) | 32.7 | 29.8-35.6 |
| Significant | 615 (521) | 60.9 | 57.9-63.9 |
CI, confidence interval
Antibiotics accounted for one-third of all ADEs and thus represented the most frequent drug class associated with ADEs. Sedatives, non-steroidal antiinflammatory drugs (NSAIDs) and laxatives caused 9%, 8%, and 7% of ADEs, respectively. Sedatives, NSAIDs, and electrolytes were the most frequent drug classes involved in preventable ADEs, whereas antibiotics were the class most frequently associated with non-preventable ADEs (Table 3).
Table 3.
Frequency of Adverse Drug Events According to Drug Classes
| Drug class | ADEs, n (%) (n = 1010) | Preventable ADEs, n (%) (n = 141) | Non-preventable ADEs, n (%) (n = 869) | Potential ADEs, n (%) (n = 339) | Intercepted potential ADE, n (%) (n = 98) | Nonintercepted potential ADEs, n (%) (n = 241) |
|---|---|---|---|---|---|---|
| Antibiotics | 365 (36) | 19 (13) | 346 (40) | 17 (5.0) | 8 (8.2) | 9 (3.7) |
| Antitumor agents | 26 (2.6) | 3 (2.1) | 23 (2.7) | 1 (0.3) | 0 (0) | 1 (0.4) |
| Diuretics | 20 (2.0) | 4 (2.8) | 16 (1.8) | 11 (3.2) | 3 (3.1) | 8 (3.3) |
| Antihypertensives | 52 (5.1) | 9 (6.4) | 43 (5.0) | 40 (12) | 12 (12) | 28 (12) |
| Antiarrhythmics | 2 (0.2) | 0 (0) | 2 (0.2) | 3 (0.9) | 3 (3.1) | 0 (0) |
| Cardiovascular | 14 (1.4) | 2 (1.4) | 12 (1.4) | 7 (2.1) | 4 (4.1) | 3 (1.2) |
| Anticoagulants | 30 (3.0) | 4 (2.8) | 26 (3.0) | 6 (1.8) | 2 (2.0) | 4 (1.7) |
| Dyslipidemic agents | 14 (1.4) | 0 (0) | 14 (1.6) | 1 (0.3) | 1 (1.0) | 0 (0) |
| Antidiabetics | 12 (1.2) | 2 (1.4) | 10 (1.2) | 12 (3.5) | 3 (3.1) | 9 (3.7) |
| Antiasthmatics | 7 (0.7) | 0 (0) | 7 (0.8) | 7 (2.1) | 3 (3.1) | 4 (1.7) |
| Peptic ulcer drugs | 40 (4.0) | 2 (1.4) | 38 (4.4) | 22 (6.5) | 7 (7.1) | 15 (6.2) |
| Laxatives | 73 (7.2) | 2 (1.4) | 71 (8.2) | 4 (1.2) | 3 (3.1) | 1 (0.4) |
| Antidepressants | 3 (0.3) | 1 (0.7) | 2 (0.2) | 4 (1.2) | 2 (2.0) | 2 (0.8) |
| Sedatives | 87 (8.6) | 24 (17) | 63 (7.3) | 6 (1.8) | 6 (6.1) | 0 (0) |
| Antipsychotics | 22 (2.2) | 3 (2.1) | 19 (2.2) | 1 (0.3) | 0 (0) | 1 (0.4) |
| Antiseizure | 13 (1.3) | 0 (0) | 13 (1.5) | 3 (0.9) | 2 (2.0) | 1 (0.4) |
| Antiparkinson’s drugs | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) | 1 (1.0) | 0 (0) |
| NSAIDs | 78 (7.7) | 25 (18) | 53 (6.1) | 125 (37) | 6 (6.1) | 119 (49) |
| Other analgesics | 49 (4.9) | 6 (4.3) | 43 (5.0) | 13 (3.8) | 2 (2.0) | 11 (4.6) |
| Corticosteroids | 32 (3.2) | 0 (0) | 32 (3.7) | 4 (1.2) | 3 (3.1) | 1 (0.4) |
| Antihistamines | 1 (0.1) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Electrolytes or fluids | 27 (2.7) | 26 (18) | 1 (0.1) | 11 (3.2) | 5 (5.1) | 6 (2.5) |
| Experimental drugs | 1 (0.1) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 0 (0) |
| Others | 42 (4.2) | 9 (6.4) | 33 (3.8) | 40 (12) | 22 (22) | 18 (7.5) |
ADEs, adverse drug events; NSAIDs, Non-steroidal antiinflammatory drugs
Several factors were associated with ADEs (Table 4). Those aged ≥65 years had a significantly higher rate of ADEs than younger patients. Those admitted to ICUs were at lower risk for ADEs; however, having a resident physician as the doctor in charge increased risk. The history of allergy was a correlated with ADE risk.
Table 4.
Factors Associated with Adverse Drug Events and Medication Errors
| Factors | No. of patients | Adverse drug events | Medication errors | ||||
|---|---|---|---|---|---|---|---|
| n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)* | n (%) | Crude OR (95% CI) | Adjusted OR (95% CI)* | ||
| Age (y) | |||||||
| <65 | 1304 | 203 (16) | – | – | 149 (11) | – | – |
| ≥65 | 2155 | 523 (24) | 1.7 (1.5-2.1) | 1.7 (1.5-2.1) | 284 (13) | 1.2 (0.95-1.5) | 1.2 (0.9-1.5) |
| Gender | |||||||
| Female | 1501 | 318 (21) | – | – | 202 (13) | – | – |
| Male | 1958 | 408 (21) | 1.0 (0.8-1.2) | 1.1 (0.9-1.3) | 231 (12) | 0.9 (0.7-1.05) | 1.0 (0.8-1.3) |
| Admitted ward | |||||||
| Medicine | 1531 | 350 (23) | – | – | 182 (12) | – | – |
| Surgery | 1469 | 306 (21) | 0.9 (0.7-1.06) | 1.0 (0.8-1.2) | 201 (14) | 1.2 (0.9-1.5) | 1.7 (1.4-2.2) |
| ICU | 459 | 70 (15) | 0.6 (0.5-0.8) | 0.6 (0.5-0.9) | 50 (11) | 0.9 (0.7-1.3) | 1.2 (0.9-1.7) |
| Doctor in charge | |||||||
| Not resident physician | 2526 | 499 (20) | – | – | 205 (8) | – | – |
| Resident physician | 933 | 227 (24) | 1.3 (1.1-1.6) | 1.2 (1.01-1.5) | 228 (24) | 3.7 (3.0-4.5) | 3.9 (3.1-4.9) |
| Admission pathway | |||||||
| Scheduled | 1609 | 320 (20) | – | – | 215 (13) | – | – |
| Emergency admission | 1810 | 391 (22) | 1.1 (0.9-1.3) | 1.2 (0.97-1.4) | 201 (11) | 0.8 (0.7-0.99) | 0.8 (0.6-0.9) |
| Transferred from other ward | 40 | 15 (38) | 2.4 (1.3-4.6) | 1.8 (0.9-3.6) | 17 (43) | 4.8 (2.5-9.1) | 2.7 (1.2-4.8) |
| History of allergy | |||||||
| Absent | 3117 | 640 (21) | – | – | 368 (12) | – | – |
| Present | 342 | 86 (25) | 1.3 (1.003-1.7) | 1.4 (1.04-1.8) | 65 (19) | 1.8 (1.3-2.3) | 1.6 (1.1-2.1) |
| Medication on admission (No.) | |||||||
| <4 | 1532 | 316 (21) | – | – | 138 (9) | – | – |
| ≥4 | 1927 | 410 (21) | 1.04 (0.9-1.2) | 1.0 (0.9-1.2) | 295 (15) | 1.8 (1.5-2.3) | 1.7 (1.4-2.2) |
* Adjusted OR was calculated from multivariate logistic regression model with all listed variables.
ADE, adverse drug event; ICU, intensive care unit; OR, odds ratio; CI, confidence interval; -, reference
Medication Errors and Potential Adverse Drug Events
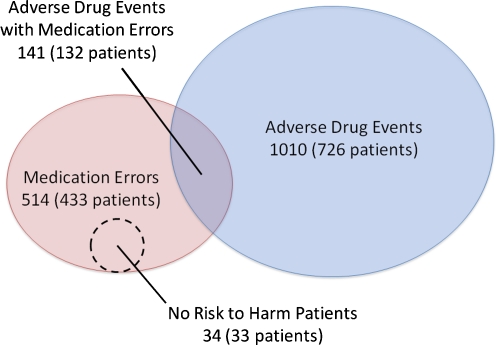
This study identified 514 medication errors among 433 patients (incidence: 8.7 [95%CI 7.9-9.4] per 1,000 patient-days) and a crude rate of 14.9 [95%CI 13.7-16.0] per 100 admissions. Based on a similar calculation for ADEs, an estimated 4,052 medication errors occur annually among the three hospitals. The incidence was higher in ICUs with 17.0 medication errors per 1,000 patient-days and the crude rate was higher in surgical wards with 16.4 events per 100 admissions. The median hospital stay from admission to medication error was 3 (interquartile range 1-10) days. Among the 514 medication errors, 141 actually resulted in ADEs and were preventable ADEs whereas 339 had the potential to cause harm (Fig. 1). The incidence of preventable ADEs and non-preventable ADEs were 2.4 [95%CI 2.0-2.8] and 14.6 [95%CI 13.7-15.6] per 1,000 patient-days, respectively. Thus, 14% of ADEs were considered preventable. The incidence of potential ADEs was thus 5.7 [95%CI 5.1-6.3] per 1,000 patient-days. Thirty-four were errors but not considered potential ADEs because of no risk of harm to patients. Three of 10 potential ADEs (98 cases) were intercepted before a drug was administered and were thus intercepted potential ADEs. Of the rest, the patient did not actually take the drug without need in 70 cases, or took the drug but no consequences were identified in 171 cases. These 241 cases were non-intercepted potential ADEs. The incidence of intercepted and non-intercepted potential ADEs were 1.7 [95%CI 1.3-2.0] and 4.1 [95%CI 3.5-4.6] per 1,000 patient-days, respectively.
Figure 1.
Relationship between adverse drug events and medication errors.
Two-thirds of preventable and potential ADEs that were associated with medication errors occurred at the ordering stage (Table 5). Among them, half of the preventable ADEs arose at the monitoring stage, but most potential ADEs occurred at the ordering stage (Table 5). Intercepted potential ADEs occurred at earlier stages whereas non-intercepted and actual but preventable ADEs occurred at later stages. Factors associated with medication errors included being admitted to a surgical ward and having a resident physician as the doctor in charge (Table 4). Those transferred from other wards, prescribed more medication on admission, and with history of allergy also had higher risk of a medication error.
Table 5.
Stages of Primary Errors Associated with Preventable and Potential Adverse Drug Events
| Event | Ordering n (%) | Transcription n (%) | Dispensing n (%) | Administration n (%) | Monitoring n (%) |
|---|---|---|---|---|---|
| Preventable ADEs | 49 (35) | 0 (0) | 0 (0) | 15 (11) | 77 (55) |
| Intercepted potential ADEs | 88 (90) | 0 (0) | 6 (6) | 4 (4) | 0 (0) |
| Nonintercepted potential ADEs | 182 (76) | 2 (0.8) | 2 (0.8) | 49 (20) | 6 (2) |
| All above events | 319 (66) | 2 (0.4) | 8 (2) | 68 (14) | 83 (17) |
ADEs, adverse drug events
DISCUSSION
The JADE study used the same methodology as that described by Bates et al in 19954. The incidence of ADEs in the present study was 17.0 per 1,000 patient-days, which was fairly similar to the 11.5 that reported by Bates et al. However, the rate per admission differed substantially between the present study and that of 1995 Bates study--29 vs. 6 ADEs per 100 admissions. This gap was primarily due to the large difference in the mean length of hospital stay between Japan and the U.S., which was 17 vs. 5 days, respectively. However, our results were consistent with another recent epidemiological report on inpatients from the U.S. which found an incidence of 15 ADEs per 100 admissions14. Hospitals can be dangerous places, and shortening stays in Japanese hospitals could potentially reduce the frequency of ADEs. The reasons for the differences in length of stay are largely cultural and relate in part to patient expectations15,16. Physicians and even patients, can determine the timing of discharge more freely in Japan compared to the U.S16,17. In addition, the reimbursement from government-run health insurance is generally based on the length of hospital stay, and because of shortages of ambulatory care, the physicians, patients, and their families are all inclined to keep patients in hospital longer17. Thus, the findings that longer hospital stay is substantially associated with ADEs represents one incentive to shorten the length of hospital stay, though many factors are clearly involved.
We found many common epidemiological characteristics of ADEs and medication errors between Japan and the U.S., including the severity and drug class of ADEs, ward type, stage of medication errors, and proportion of interception of potential ADEs. For example, nearly half of all medication errors occurred at the ordering stage (66% in Japan and 49% in the U.S.), followed by the administration or monitoring stages. Both studies also found that almost half of potential ADEs were intercepted before the drugs reached patients. These findings support the notion that ADEs and medication errors may represent similar processes despite major differences in medical systems and cultures, although the situation might also be quite different especially in settings such as developing countries.
We assessed the frequency of ADEs and medication errors in daily practice in hospitals in Japan and found that they occur often and cause substantial harm. Based on these data, healthcare professionals, policy makers, patients, and even the general population should be aware of the risk of medical care and drugs. Because the epidemiology and characteristics of ADEs and medication errors were quite similar despite differences in healthcare systems, extrapolation from state-of-art solutions in the U.S. such as computerized physician order entry, bar-coding, and having pharmacists round with teams in the intensive care units should be evaluated in Japan and perhaps other developed countries, with public support and investment18,19.
In addition, we identified several specific factors that were associated with ADEs in Japan. Older patients, those in ICUs, those transferred from other wards, and those with a history of allergy as well as those cared for by resident physicians were all at higher risk for ADEs. Thus, solutions targeting these groups could be especially effective locally in Japan. Transition from other wards was considered a particularly high risk for any kind of harm from medical care and a top priority of patient safety in developed countries13. Although regulating work hours in the U.S. does not apparently reduce ADEs20, other interventions for workplace and education for resident physicians could also be a focus of research.
Our study has several limitations. First, we analyzed data generated by the random sampling of wards from only three urban tertiary care hospitals. Therefore, our results might not be representative of Japanese inpatients in general. Also, healthcare providers might have been aware of this prospective cohort study, but a Hawthorne effect if present would suggest that our estimates are if anything conservative. Similarly, some ADEs may not have been noted in the charts and may thus have been missed, which would also make our estimates a lower bound. In addition, data collection was conducted in 2004, so that the current situation might be different. The main outcomes of our study were ADEs and medication errors, which required implicit judgment. However, the interrater reliability levels were reasonable and more robust alternatives to measure ADEs and medication errors have not yet been developed1, so that the approach we used is the standard one.
In conclusion, we showed that ADEs and medication errors were quite frequent in Japanese acute care hospitals, and that they were of a similar nature to those arising in the Western countries. The proportion of preventable ADEs and of potential ADEs with medication errors among all incidents was significant, and most of the errors occurred at the ordering, administration and monitoring stages. Interventions to support healthcare providers during ordering and administering to patients may improve drug safety among hospital inpatients, as could reducing length of stay. Future studies should assess the epidemiology in other settings in other countries, and the effectiveness of interventions that have been successful in the Western countries, such as the updating of information technologies, should be tested in other nations.
ACKNOWLEDGMENTS
We are indebted to Ms. Makiko Ohtorii, Ms. Ai Mizutani, Ms. Kimiko Sakamoto, Ms. Eri Miyake, Ms. Takako Yamaguchi, Ms. Yoko Oe, Ms. Kyoko Sakaguchi, Ms. Kumiko Matsunaga, Ms. Yoko Ishida, Ms. Kiyoko Hongo, Ms. Masae Ohtani, Ms. Yasuko Ito, Ms. Ayumi Samejima, and Ms. Shinobu Tanaka for their assistance.
This study was funded by grants 17689022 and 18659147 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Pfizer Health Research Foundation.
Presented in part at the 26th International Conference of the International Society for Quality in Health Care, Dublin, Ireland. October 13, 2009
Conflict of Interest None disclosed.
Footnotes
This study was funded by grants 17689022 and 18659147 from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Pfizer Health Research Foundation.
REFERENCES
- 1.Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306–314. doi: 10.1136/qshc.2004.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard medical practice study II. N Engl J Med. 1991;324(6):377–384. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 3.Jha AK, Prasopa-Plaizier N, Larizgoitia I, Bates DW. Patient safety research: an overview of the global evidence. Qual Saf Health Care. 2010;19(1):42–47. doi: 10.1136/qshc.2008.029165. [DOI] [PubMed] [Google Scholar]
- 4.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 5.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 6.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. Jama. 2001;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 7.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Jama. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 8.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse drug events prevention study group. Jama. 1997;277(4):307–311. doi: 10.1001/jama.277.4.307. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie RI, Ruedy J. Adverse drug reactions during hospitalization. Can Med Assoc J. 1967;97(24):1450–1457. [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz N, Wade OL. Intensive hospital monitoring of adverse reactions to drugs. Br Med J. 1969;1(5643):531–536. doi: 10.1136/bmj.1.5643.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smidt NA, McQueen EG. Adverse drug reactions in a general hospital. N Z Med J. 1973;78(494):39. [PubMed] [Google Scholar]
- 12.Morimoto T, Fukui T, Lee TH, Matsui K. Application of U.S. guidelines in other countries: aspirin for the primary prevention of cardiovascular events in Japan. Am J Med. 2004;117(7):459–468. doi: 10.1016/j.amjmed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Bates DW, Larizgoitia I, Prasopa-Plaizier N, Jha AK. Global priorities for patient safety research. BMJ. 2009;338:b1775. doi: 10.1136/bmj.b1775. [DOI] [PubMed] [Google Scholar]
- 14.Hug BL, Witkowski DJ, Sox CM, et al. Adverse drug event rates in six community hospitals and the potential impact of computerized physician order entry for prevention. J Gen Intern Med. 2010;25(1):31–38. doi: 10.1007/s11606-009-1141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anbacken O. Japanese hospitals–culture and competition: a study of ten hospitals. Int J Health Plann Manage. 1994;9(1):87–101. doi: 10.1002/hpm.4740090107. [DOI] [PubMed] [Google Scholar]
- 16.Muramatsu N, Liang J. Hospital length of stay in the United States and Japan: a case study of myocardial infarction patients. Int J Health Serv. 1999;29(1):189–209. doi: 10.2190/8A4W-83KG-J5MU-CVV2. [DOI] [PubMed] [Google Scholar]
- 17.Nomura H, Nakayama T. The Japanese healthcare system. BMJ. 2005;331(7518):648–649. doi: 10.1136/bmj.331.7518.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates DW. Using information technology to reduce rates of medication errors in hospitals. BMJ. 2000;320(7237):788–791. doi: 10.1136/bmj.320.7237.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bates DW, Gawande AA. Improving safety with information technology. N Engl J Med. 2003;348(25):2526–2534. doi: 10.1056/NEJMsa020847. [DOI] [PubMed] [Google Scholar]
- 20.Mycyk MB, McDaniel MR, Fotis MA, Regalado J. Hospitalwide adverse drug events before and after limiting weekly work hours of medical residents to 80. Am J Health Syst Pharm. 2005;62(15):1592–1595. doi: 10.2146/ajhp040527. [DOI] [PubMed] [Google Scholar]