Abstract
The genomes of Lactobacillus casei/paracasei and Lactobacillus rhamnosus strains carry two genes encoding homologues of p40 and p75 from L. rhamnosus GG, two secreted proteins which display anti-apoptotic and cell protective effects on human intestinal epithelial cells. p40 and p75 carry cysteine, histidine-dependent aminohydrolase/peptidase (CHAP) and NLPC/P60 domains, respectively, which are characteristic of proteins with cell-wall hydrolase activity. In L. casei BL23 both proteins were secreted to the growth medium and were also located at the bacterial cell surface. The genes coding for both proteins were inactivated in this strain. Inactivation of LCABL_00230 (encoding p40) did not result in a significant difference in phenotype, whereas a mutation in LCABL_02770 (encoding p75) produced cells that formed very long chains. Purified glutathione-S-transferase (GST)-p40 and -p75 fusion proteins were able to hydrolyze the muropeptides from L. casei cell walls. Both fusions bound to mucin, collagen and to intestinal epithelial cells and, similar to L. rhamnosus GG p40, stimulated epidermal growth factor receptor phosphorylation in mouse intestine ex vivo. These results indicate that extracellular proteins belonging to the machinery of cell-wall metabolism in the closely related L. casei/paracasei-L. rhamnosus group are most likely involved in the probiotic effects described for these bacteria
Key Words: Lactobacillus, Probiotic, Cell-wall hydrolases
Introduction
Lactobacilli are normal constituents of the intestinal tract microbiota and some strains have attracted intense interest due to their probiotic health-promoting effects. The adaptation to the intestinal tract of these bacteria is based on two different aspects. First, they have developed strategies to adapt to the harsh conditions found in this niche, including mechanisms to cope with stress (i.e. low pH, presence of bile), adhere to the mucosal surface (i.e. epithelial cells, mucus and extracellular matrix protein binding) and exploit the nutritional characteristics of the medium (e.g. utilization of different carbohydrates) [Lebeer et al., 2008]. Second, regular presence in the human gut provided these bacteria with multiple mechanisms to interact with the host that are at the basis of their health-promoting effects. Intestinal epithelial and immune cells are responsive to probiotic-derived products (metabolites, proteins, cell-wall constituents or DNA) that are important for the maintenance of intestinal homeostasis, acting at the level of epithelial barrier protection and immunomodulation [Corthesy et al., 2007; Lebeer et al., 2008; Vanderpool et al., 2008; Hörmannsperger and Haller, 2010]. Most of the characterized probiotic-host interactions take place through pattern recognition receptors (e.g. toll-like receptors, TLR) which recognize common molecules present at the bacterial surface (peptidoglycan, lipoteichoic acids) or derived from them (e.g. unmethylated CpG DNA, exopolysaccharides) [Lebeer et al., 2010]. In addition, other secreted or cell surface-exposed probiotic factors have been described, but their nature and targets are poorly known. The GroEL chaperone from Lactobacillus johnsonii La1 is surface located and stimulates interleukin-8 secretion in macrophages [Bergonzelli et al., 2006]. The surface-layer protein A from Lactobacillus acidophilus NCFM binds to the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) receptor in dendritic cells influencing interleukin production [Konstantinov et al., 2008]. Soluble low-molecular-weight peptides produced by Lactobacillus rhamnosus GG (LGG), one of the most intensively studied probiotic strains, activate MAP kinases and induce cytoprotective heat-shock proteins in intestinal cells [Tao et al., 2006]. Yan and Polk [2002] reported that LGG prevented cytokine-induced apoptosis in intestinal epithelial cells. In fact, the factors responsible for this were present in the LGG culture supernatant and have been identified as two proteins with approximate sizes of 40 and 75 kDa (p40 and p75, respectively). Purified p40 and p75 stimulate Akt activation, display anti-apoptotic activity, prevent epithelial barrier damage caused by oxidative agents and decrease susceptibility to dextran sodium sulfate-induced colon epithelial injury in mice [Yan et al., 2007, 2010; Seth et al., 2008]. The complete and the partial ORFs encoding p40 and p75 from LGG were determined and showed to code, respectively, for a protein of unknown function and a protein annotated as a putative cell-wall hydrolase [Yan et al., 2007]. In this work, we show that genes encoding homologues to these proteins are also present in other strains of the closely related Lactobacillus casei/paracasei-L. rhamnosus group. We have characterized p40 and p75 proteins from Lactobacillus casei BL23, a strain widely used in genetic and physiologic studies and which displays probiotic effects in animal models [Foligne et al., 2007; Monedero et al., 2007; Rochat et al., 2007]. We have shown that these proteins play a cell-wall hydrolytic role in this host.
Results
p40- and p75-Encoding Genes Are Present in the Genomes of Several Lactobacillus Strains
Yan et al. [2007] reported that Western blot analysis with anti-p40 and anti-p75 sera evidenced the presence of p40 and p75 homologue proteins in L. casei ATCC393 and L. casei ATCC334 but not in L. acidophilus. In fact, a genomic BLAST search of sequences available in the GenBank database (http://www.ncbi.nlm.nih.gov/Genbank/index.html) revealed that genes encoding p40 and p75 homologues were exclusively found in the genomes of L. casei/paracasei and L. rhamnosus strains. Sequence alignment of p40 (fig. 1a) shows that, with the exception of a serine-rich region (amino acids 256–286 in p40 from L. casei BL23), this protein exhibits more than 98% identity within L. casei/paracasei and L. rhamnosus strains. Analysis of p40 domain composition revealed a C-terminal CHAP domain (cysteine, histidine-dependent aminohydrolase/peptidase, PF05257) which is usually present in N-acetylmuranoyl-L-alanine amidases or endopeptidases involved in cell-wall metabolism [Bateman and Rawlings, 2003]. The p40 N-terminal portion comprised a domain of unknown function (COG3883) with low similarity (about 26–30% amino acid identity) to proteins showing cell-wall binding or peptidoglycan hydrolytic activity from Bacillus, Clostridium, Streptococcus or Listeria species.
Fig. 1.
a Amino acid alignment of p40 proteins from L. casei BL23 (LCABL_00230), L. casei ATCC334 (LSEI_0020), L. paracasei ATCC25302 (HMPREF0530_0772), L. paracasei 8700: 2, L. rhamnosus GG (LGG_00031), L. rhamnosus Lc705 (LC705_00025), L. rhamnosus LMS2-1 (HMPREF0539_0332) and L. rhamnosus HN001 (LRH_09303). b Amino acid alignment of p75 proteins from L. casei BL23 (LCABL_02770), L. casei ATCC334 (LSEI_0281), L. paracasei ATCC25302 (HMPREF0530_0378), L. paracasei 8700: 2, L. rhamnosus GG (LGG_00324), L. rhamnosus Lc705 (LC705_00310), L. rhamnosus LMS2-1 (HMPREF-0539_2610) and L. rhamnosus HN001 (LRH_12224). Numbers in parentheses refer to the gene locus encoding p40 or p75, except for L. paracasei 8700: 2 which does not possess an annotated genome (GenBank ABQV00000000). The position of the putative cleavage sites for signal peptidase I are marked with an arrow. The extension of the different protein domains is indicated by horizontal dark lines.
p75 homologue proteins were encoded by genes whose translation products had a molecular weight of 49 kDa. The reported size for the LGG protein was 75 kDa, for which it was assumed that p75 displayed an anomalous migration in SDS-PAGE gels. Domain analysis revealed that p75 was also a modular protein carrying a C-terminal NLPC/P60 (PF00877) domain and an N-terminal domain of unknown function (fig. 1b). The NLPC/P60 domain is usually found at the C-terminus of bacterial proteins described as putative cell-wall hydrolases of the endopeptidase class and in invasion-associated proteins from pathogens [Anantharaman and Aravind, 2003]. p75 from L. casei/paracasei strains were more than 96% identical, whereas identity of L. rhamnosus p75 was more than 98% (fig. 1b). Similarity between both groups fall to a82% identity due to a region of about 160 amino acids at the N-terminal domain which differed in L. casei/paracasei and L. rhamnosus (fig. 1b). In this region the protein from L. casei/paracasei contained a number of repetitions of the Ala-Ala-Ala-Ser sequence and a proline-rich region, whereas in p75 from L. rhamnosus several repetitions of the Ala-Ala-Ala-Ser-Gln sequence were found.
p40 and p75 were predicted to have an N-terminal signal peptide recognized by signal peptidase I and were therefore expected to be secreted. A high degree of synteny was observed in the genome regions coding for p40 and p75. However, their genome contexts did not allow hypothesizing a putative function for both proteins.
p40 and p75 Are Secreted in L. casei BL23
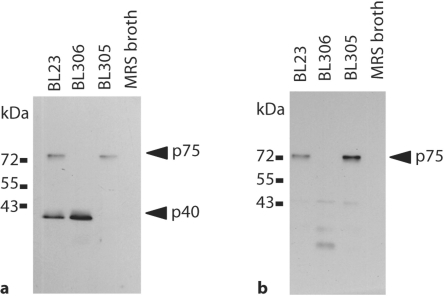
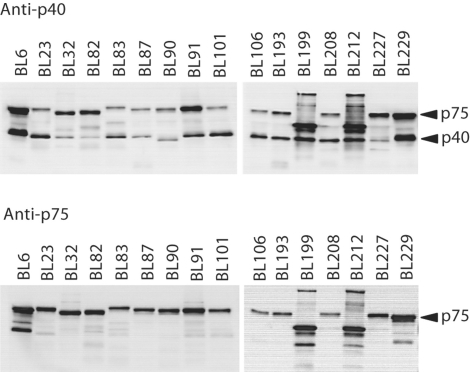
In order to determine the likely function and cellular location of p40 and p75 in L. casei BL23, mutants in LCABL_00230 (encoding p40) and LCABL_02770 (encoding p75) were constructed in this strain by integration of a plasmid carrying internal fragments of the genes. The obtained mutants BL305 (LCABL_00230::pRV300) and BL306 (LCABL_02770::pRV300) were analyzed for the presence of p40 and p75 in culture supernatants by using sera raised against LGG proteins [Yan et al., 2007] (fig. 2). LGG anti-p40 antibodies reacted against 40- and 75-kDa protein bands present in supernatants of BL23. The 40-kDa band disappeared in BL305 supernatants and the 75-kDa band disappeared in samples from the BL306 strain (fig. 2a). These results confirmed that, as previously reported for LGG, antibodies against p40 cross-reacted to p75 [Yan et al., 2007]. However, no obvious homology was identified between both proteins. LGG anti-p75 antibodies detected a 75-kDa protein which disappeared in the BL306 strain (fig. 2b). In accordance to the mutation present in BL306, faint bands of less than 30 kDa were observed in the supernatant of this strain, which were probably the result of truncated p75 derived from the gene disruption by single cross-over. The results confirmed that, as expected, p40 and p75 were secreted in L. casei BL23. A Western blot screening for the presence of p40- and p75-like proteins in the supernatants of several L. casei/paracasei strains from different origins revealed the presence of reacting bands in all the strains (fig. 3). This reinforced the idea that these proteins are common in this bacterial group. Small differences in size were detected among the strains, indicating a certain variation in the proteins. In one case, the lower molecular weight detected in p40 could be attributed to changes at the level of protein sequence: compared to the rest of L. casei/paracasei sequenced strains, p40 from ATCC334 (BL90) had a deletion of 15 amino acids (fig. 1a), which resulted in a smaller protein of 38 kDa. Only two strains (BL199 and BL212) showed a clear difference in the band pattern, suggesting the presence of different forms of p40 and p75 or proteolysis of the secreted proteins.
Fig. 2.
p40 and p75 are secreted in L. casei BL23. a Western blot with anti-p40 serum. b Western blot with anti-p75 serum. L. casei supernatants (10 μl) or fresh MRS broth (10 μl) were loaded in each well. BL23 (wild type); BL305 (LCABL_00230::pRV300); BL306 (LCABL_02770:: pRV300).
Fig. 3.
Western blot screening for the presence of p40 and p75 in the supernatants of several L. casei/paracasei strains. 20 μl of bacterial overnight culture supernatants were subjected to SDSPAGE and probed with antisera raised against LGG p40 and p75.
p75 Is Involved in Cell Separation
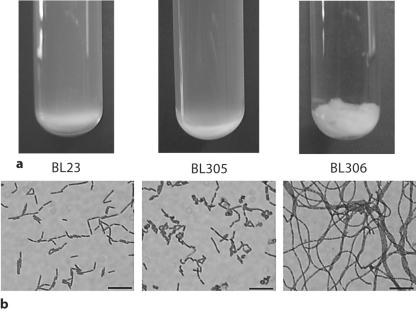
The p40-disrupted mutant (BL305) showed a growth behavior similar to the wild type. However, the p75-disrupted strain (BL306) exhibited an autoaggregating phenotype in liquid medium (fig. 4a). Under the microscope, BL305 displayed some curved forms and shorter bacilli (3.2 ± 0.7 μm) than BL23 (4.1 ± 1.2 μm), whereas BL306 (p75) cells formed very long chains of more than 100 μm (fig. 4b). These long chains were probably responsible for the macroscopic characteristics of the strain in liquid cultures, as they tended to interlace forming cords and balls, and suggested that in this mutant the capacity of cell separation after division was impaired.
Fig. 4.
Growth behavior and cellular morphology of L. casei strains lacking p40 and p75. a Overnight liquid cultures in MRS medium. b Micrographs of wild-type L. casei and different mutant strains. The bars represent 10 μm. BL23 (wild-type); BL305 (LCABL_00230::pRV300); BL306 (LCABL_02770::pRV300).
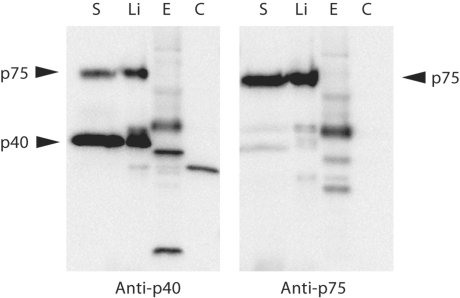
p40 and p75 could be extracted from the L. casei cell surface by a simple cold water treatment (data not shown) or by extraction with 1.5 M LiCl, whereas they were absent from the cytoplasmic fraction (fig. 5). These results indicated that a fraction of the proteins is attached to the cell surface but not tightly bound to it.
Fig. 5.
Extraction of p40 and p75 from the cell surface. A Western blot detection of p40 and p75 in different protein fractions of L. casei BL23 is shown. S = Supernatant; Li = proteins extracted with LiCl; E = cell-envelope fraction (cell-wall/membrane fraction); C = cytoplasm. The S lanes contains 25 μl of supernatants, the rest of samples contain 1 μg of protein.
p40 and p75 Are Able to Hydrolyze L. casei Muropeptides
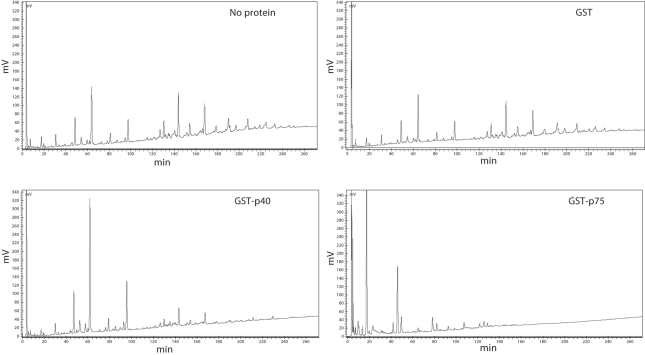
Sequence analysis, cellular location and the phenotype of an L. casei strain lacking p75 (BL306) suggested that p40 and p75 proteins are enzymes involved in the metabolism of the L. casei cell wall. p40 and p75 proteins from L. casei BL23 were purified as GST fusions and assayed for the hydrolysis of muropeptides obtained by hydrolysis of L. casei cell walls with mutanolysin. Figure 6 shows the HPLC chromatograms of L. casei muropeptides after incubation with the purified proteins. Whereas incubation with a GST control did not change the muropeptide profile, GST-p40 and GST-p75 reduced the amount of the most hydrophobic peptides and resulted in the appearance of polar products, possibly smaller peptides, which indicate hydrolytic activity on muropeptides. Therefore, since they are cell-wall-bound muramidases, genes encoding p40 and p75 will hereinafter be called cmuA and cmuB, respectively.
Fig. 6.
Hydrolysis of L. casei muropeptides by GST-p40 and GST-p75. Muropeptides isolated from L. casei BL23 cell walls were incubated without protein or with GST, GST-p40 or GST-p75 and the hydrolysis products were separated by HPLC.
p40 and p75 from L. casei Stimulate Epidermal Growth Factor Receptor Phosphorylation in Mice
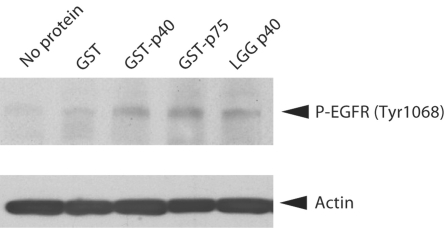
The probiotic activity of LGG p40 has been reported to involve epidermal growth factor receptor (EGFR) and this protein is able to activate the EGFR pathway in an ex vivo colon organ culture model and in vivo[Yan et al., 2010]. Therefore, we tested whether the L. casei BL23 p40 and p75 proteins exhibited a similar activity. Figure 7 shows that incubation of mouse colon fragments with either GST-p40 or GST-p75 stimulated EGFR phosphorylation similar to p40 purified from LGG.
Fig. 7.
L. casei p40 and p75 stimulates EGFR phosphorylation. Mouse colon fragments were incubated with 10 ng/ml of each protein for 2 h and phosphorylation of EGFR was detected with anti-EGFR-phospho-Tyr1068 antibodies. LGG p40 is p40 from L. rhamnosus GG. Anti-actin antibodies were used as a loading control.
L. casei p40 and p75 Bind to Extracellular Matrix Proteins and Epithelial Cells
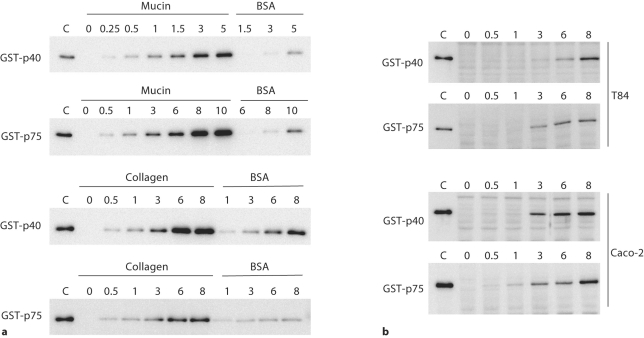
Some surface-associated proteins displaying cell-wall hydrolytic activity from staphylococci, enterococci and Listeria are adhesion molecules with extracellular matrix (ECM) and eukaryotic cell binding activity [Milohanic et al., 2001; Teng et al., 2003; Heilmann et al., 2005]. In order to test this possibility, in vitro binding to immobilized mucin, to the ECM proteins fibronectin, fibrinogen and collagen and to T84 and Caco-2 epithelial cells was assayed with GST-p40 and GST-p75. Figure 8a shows that GST-p40 and GST-p75 bound to mucin and collagen in a dose-dependent and saturable manner. No binding was detected for fibronectin or fibrinogen (data not shown). Both fusions bound to BSA much less efficiently. Control experiments in which GST activity was determined in the wells after washing demonstrated that GST by itself did not bind to these molecules (data not shown). Both proteins were also able to bind to T84 and Caco-2 epithelial cells (fig. 8b).
Fig. 8.
Binding of L. casei p40 and p75 to mucin, collagen and different intestinal epithelial cell lines. a Mucin, collagen or BSA were bound to microtiter plates and incubated with different amounts of GST-p40 and GST-p75 (indicated in micrograms by the numbers above each panel). After washing, the bound proteins were released and detected by Western blot. b Monolayers of T84 or Caco-2 cells were incubated with different quantities of GST-p40 and GST-p75 (indicated above the panels). After washing, bound proteins were detected by Western blot. Lanes marked as C contain 5 ng of purified proteins as control.
Discussion
Most proteins from probiotic bacteria identified as factors interacting with host cells are non-specialized proteins actually implicated in other bacterial processes. This included the EF-Tu translation elongation factor and the molecular chaperone GroEL from L. johnsonii [Granato et al., 2004; Bergonzelli et al., 2006], different glycolytic enzymes from Lactobacillus plantarum and Lactobacillus crispatus [Hurmalainen et al., 2007; Kinoshita et al., 2008; Ramiah et al., 2008], and the surface-layer protein A from L. acidophilus [Konstantinov et al., 2008], involved in epithelial cells and ECM attachment, plasminogen activation and immunoregulation. We showed here that two proteins, p40 and p75, identified as probiotic factors in LGG and also present in members of the L. casei/paracasei-L. rhamnosus group, fitted into the group of cell-wall hydrolases, also known as autolysins.
Bacterial autolysins comprise a group of enzymes which display the complete set of activities for peptidoglycan hydrolysis (N-acetyl-muramoyl-L-alanine amidase, N-acetylglucosaminidase, endopeptidase, carboxypeptidase and N-acetylmuramidase) and play a crucial role in bacterial cell growth, shape maintenance and division. p40 and p75 are modular proteins which carry carboxy-terminal catalytic domains with putative amidase and endopeptidase activity, respectively, and N-terminal domains that could be implicated in their interaction with peptidoglycan. In this sense, p75 is an atypical peptidoglycan hydrolase as it lacks any recognized motif involved in peptidoglycan binding in other autolysins (e.g. LysM, GW or SH3 domains [Layec et al., 2008]). On the contrary, a similar N-terminal domain of p40 (COG3883) present in CwlO, a cell-wall endopeptidase from Bacillus subtilis [Yamaguchi et al., 2004], has been shown to interact with peptidoglycan. p40 and p75 hydrolytic activity could be demonstrated on L. casei BL23 muropeptides and both proteins were secreted and bound to the bacterial surface. The lack of p40 in L. casei BL23 only resulted in small changes in cellular morphology. However, p75 was crucial for cell separation. In firmicutes, this function is species-specific and is usually carried out by autolysins carrying CHAP, NLPC/P60 or N-acetylglucosaminidase domains [Layec et al., 2008]. Lack of daughter cell separation activity leads to the formation of long chains of cells or filamentous cells which do not separate but contain fully formed septa [Wuenscher et al., 1993; Gao et al., 2006].
The biological activity of p40 and p75 proteins on intestinal epithelium is still intriguing. Similar to LGG, it has been reported that the presence of p40 and p75 correlated to the capacity of culture supernatants from L. casei ATCC393 and L. casei ATCC334 to stimulate Akt activation and to inhibit cytokine-induced apoptosis in intestinal epithelial cells. On the contrary, L. acidophilus supernatants, which do not contain p40 or p75, did not show such effect [Yan et al., 2007]. LGG p40 and p75 act on intestinal epithelium through an Akt/PI3K-dependent manner mediated by EGFR activation [Yan et al., 2007, 2010; Seth et al., 2008]. L. casei BL23 p40 and p75 were also able to stimulate EGFR phosphorylation, suggesting that the activity of p40 and p75 is not strain-specific. Sequence analysis of available genomes and Western blot with bacterial supernatants revealed that p40- and p75-like autolysins are common in L. casei-paracasei and L. rhamnosus strains, ranging from human to food isolates. It is not known whether the heterogeneity found in these proteins would confer distinct characteristics to the proteins from a particular strain.
In addition to their peptidoglycan hydrolytic activity, some autolysins from bacteria are involved in other processes. Cell-surface-associated autolysins from several pathogens play a role in ECM and epithelial cell attachment and are important virulence factors. SagA from Enterococcus faecium and Aaa from Staphylococcus aureus are autolysins with a broad spectrum of ECM protein binding, showing capacity to bind to fibrinogen, collagen, fibronectin and laminin [Teng et al., 2003; Heilmann et al., 2005] and the autolytic amidase Ami from Listeria monocytogenes participates in binding to human cells through its cell-wall binding domain [Milohanic et al., 2001]. We showed that p40 and p75 from L. casei BL23 bind to mucin, collagen and to cultured epithelial cells. L. casei BL23 shows low mucin and intestinal epithelial cells adhesion [Muñoz-Provencio et al., 2009, 2010], for which it is not expected that p40 and p75 function as adhesion factors in this strain. However, in agreement to binding experiments, oral administration of FITC-labeled LGG p40 to mice resulted in the detection of p40 on the surface of colon epithelium [Yan et al., 2010]. Whether binding of LGG p40 to the mucosa relates to its effect in mouse intestine remains to be investigated.
It is established that proteins synthesized by probiotics modify host cells responses [Corthesy et al., 2007; Lebeer et al., 2008; Vanderpool et al., 2008]. However, the characterization of these proteins and of their cellular targets is still poor. The work reported here represents new insights in the study of probiotic factors in L. casei/paracasei-rhamnosus group, as two cell-wall hydrolases are related to the described functional properties of these bacteria. The activity of p40 and p75 could reside in either the N-terminal domains or the catalytic C-terminal domains. The construction of different truncated versions of the proteins and point mutation derivatives with abolished catalytic activity will help to answer this question.
Experimental Procedures
Bacterial Strains and Growth Conditions
Lactobacillus strains are listed in table 1 and they were grown in MRS medium (Difco) at 37°C under static conditions. L. casei mutant strains obtained by gene disruption were maintained in MRS supplemented with erythromycin at 5 μg/ml. Escherichia coli DH5α was used as a host for cloning and E. coli BL21(DE3)-[pLysS] was used for protein expression and purification. They were grown in LB medium at 37°C under agitation. Recombinant plasmids in E. coli were selected with ampicillin at 100 μg/ml and chloramphenicol at 20 μg/ml. Solid medium was prepared by adding 1.8% (w/v) agar.
Table 1.
L. casei/paracasei strains used in this study
| Strain | Selected characteristics | Origin |
|---|---|---|
| BL6 | type strain | ATCCa 393 |
| BL23 | laboratory strain; sequenced genome [Acedo-Felix and Perez-Martinez, 2003] | CECTb 5275 |
| BL32 | cheese isolate | CECT4040 |
| BL82 | sour milk isolate | ATCC25598 |
| BL83 | cheese isolate | CECT4043 |
| BL87 | oral cavity isolate | AT CCI 1578 |
| BL90 | cheese isolate; sequenced genome | ATCC334 |
| BL91 | dental caries isolate | ATCC4545 |
| BL101 | isolated from commercial probiotic drink | laboratory stock |
| BL106 | isolated from commercial probiotic drink | laboratory stock |
| BL193 | isolated from commercial probiotic drink | laboratory stock |
| BL199 | exopolysaccharide producer [Mozzi et al, 1996] | CRLc 87 |
| BL208 | human intestinal isolate | laboratory stock |
| BL212 | dry cured sausage isolate [Fadda et al, 1998] | CRL686 |
| BL227 | commercial probiotic | laboratory stock |
| BL229 | commercial probiotic | laboratory stock |
| BL305 | BL23 cmuA(LCABL_00230)::pRV300, eryr | this work |
| BL306 | BL23 cmuB(LCABL_02770)::pRV300, eryr | this work |
eryr = Erythromycin resistant.
American Type Culture Collection.
Colección Española de Cultivos Tipo.
Centro de Referenda para Lactobacilos.
Construction of L. casei Mutant Strains
Internal fragments of LCABL_00230 (encoding p40) and LCABL_02770 (encoding p75) genes were obtained by PCR with primer pairs 5′-TTGAAGCTTAAAAGAATGTCACAGC/ 5′-TGAGGTACCAGGTGCACTG and 5′-CCTGGTACCCAGACAGCAA/5′-GCAGAATTCGGCGTTGG, respectively, using chromosomal DNA from L. casei BL23 as amplification template (restriction sites introduced for cloning are underlined). The fragments were cloned into the integrative vector pRV300 [Leloup et al., 1997] digested with KpnI/HindIII and KpnI/EcoRI, respectively. L. casei electrocompetent cells were obtained as described [Posno et al., 1991], transformed with these constructs and plated on MRS plates supplemented with erythromycin. Integration of the suicide vector at the correct locus was checked by PCR on chromosomal DNA isolated from erythromycin-resistant colonies using appropriate oligonucleotides. Plasmid integrations resulted in strains with 104 and 126 amino acid deletions at the C-terminus of p40 and p75, respectively.
Preparation of Cellular Fractions
L. casei bacterial cells were grown in 50 ml of MRS to late exponential phase and washed twice with PBS (pH 7.4). In order to isolate surface proteins, the pellet was resuspended in a solution containing 10 mM Tris-HCl pH 8 and 1.5 M LiCl and incubated at 4°C for 1 h. Bacteria were recovered by centrifugation at 6,000 g for 10 min and proteins in the supernatant were precipitated by adding trichloroacetic acid to 10% final concentration. After incubation at 4°C for 1 h, precipitated proteins were recovered by centrifugation at 10,000 g for 20 min, washed with cold 96% ethanol and resuspended in 7 M urea. The bacterial pellet was resuspended in PBS, disrupted with glass beads and unbroken cells were discarded by three centrifugations at 6,000 g for 5 min. The supernatant was then centrifuged at 22,000 g for 20 min at 4°C. The soluble fraction was retained as the cytoplasm fraction, whereas the pellet was washed 3 times at 22,000 g for 15 min with 50 mM Tris-HCl pH 8 + 0.5 M NaCl and retained as the cell-envelope fraction (cell-wall/membrane fragments).
Western Blotting
Samples were separated in 10% SDS-PAGE gels and electrotransferred to Hybond-ECL membranes (GE Healthcare). Membranes were blocked in a 5% (w/v) non-fat dry milk solution in 50 mM Tris-HCl pH 7.6, 150 mM NaCl (TBS) containing 0.1% (v/v) Tween-20 and incubated with a 1:10,000 dilution of rabbit anti-p40 or anti-p75 serum [Yan et al., 2007] for 1 h. After three 15-min washes with TBS + 0.1% Tween-20, bound antibodies were detected with a 1:20,000 dilution of goat anti-rabbit HRP-conjugated antibody (GE Healthcare) and the ECL-advance chemiluminescent reagents as described by the supplier (GE Healthcare). Light emission from the blots was quantified with a LAS-1000 image analysis system (Fuji, Tokyo, Japan).
Protein Purification
L. casei BL23p40 and p75 proteins were purified as glutathione-S-transferase (GST) fusion proteins after expression in E. coli. LCABL_00230 was amplified with the primer pair 5′-GTTGGATCCGATACAAGCGACAG/5′-GTGGGTACCTTACCGGTGGATATAA and L. casei BL23 chromosomal DNA. The obtained fragment was digested with BamHI and KpnI and cloned into the pGEX2t vector (GE Healthcare) digested with the same enzymes. LCABL_02770 was amplified with the primer pair 5′-GTTAGATCTTCAACGGGGACA/5′-TAGGGTACCTTATAGTGAAGGACG. The PCR product was digested with BglII and KpnI and cloned into pGEX2t. Both recombinant plasmids were sequenced and transformed into E. coli BL21(DE3)[pLysS]. Recombinant bacteria were grown in 500 ml of LB supplemented with ampicillin and chloramphenicol at 37°C. When bacterial cells reached an OD600 nm of 0.4, 1 mM IPTG was added and growth continued for 3 h. The bacterial pellet was recovered by centrifugation and bacteria were broken by sonication. GST-fusion proteins were purified in glutathione-Sepharose columns (1 ml bed volume) as recommended by the manufacturer (GE Healthcare). Purified proteins were dialyzed against a buffer containing 50 mM Tris-HCl pH 8, 100 mM NaCl, 1 mM EDTA, and 15% (v/v) glycerol at 4°C and aliquots were stored at −80°C.
HPLC Analysis of the Hydrolysis of L. casei Muropeptides
In order to isolate L. casei BL23 muropeptides, cell-wall peptidoglycan was prepared as described previously [Meyrand et al., 2007]. Briefly, a 500-ml culture (OD600 nm of 0.4, 2.6 × 108 cfu/ml) was chilled on ice and cells were harvested by centrifugation. Cells were boiled in 20 ml of deionized water for 10 min. After centrifugation, they were resuspended and boiled in 2 ml of 5% SDS for 25 min. Insoluble material was recovered and boiled again in 4% SDS for 15 min. The resulting cell-wall pellet was washed at least 6 times with hot deionized water to eliminate the SDS. Proteins were removed by treatment with 2 mg/ml of pronase (90 min, 60°C) and 200 μg/ml trypsin (16 h, 37°C). Cell walls were collected by centrifugation (22,000 g, 10 min), washed once in deionized water, resuspended in 800 μl of hydrofluoric acid (48% v/v solution) and incubated at 4°C for 24 h. The resulting insoluble material was centrifuged (22,000 g, 4°C) and washed twice with 250 mM Tris-HCl pH 8 and at least 4 times with deionized water.
Purified peptidoglycan was digested with mutanolysin (2,500 U/ml; Sigma) for 19 h at 37°C under end-over-end agitation. The enzyme was inactivated by boiling the samples for 3 min and the soluble muropeptides were reduced with sodium borohydride [Dougherty, 1985]. To determine hydrolytic activity of recombinant GST-p40 and GST-p75 on soluble muropeptides, pH of the muropeptide solution was adjusted to 7 and 50 μg of purified protein was added to 100 μl of muropeptides. This mixture was incubated for 20 h at 37°C and then separated by reverse-phase HPLC as previously described [Atrih et al., 1999] using a Supelcosil LC-18-DB column at 52°C (4.6 × 150 mm, 5 μm particle size; Sulpeco) and a JascoPU-2080Plus system with detection at 202 nm.
Ex vivo Mouse Colon Organ Culture
Colon organ culture was performed as described before [Yan et al., 2007]. Briefly, colon explants were isolated from 6- to 8-week-old C57BL/6 mice, washed with sterile PBS and DMEM (Gibco) media and then cut into 4 × 4-mm pieces. These colon pieces were cultured on Netwell™ inserts (Corning Life Sciences Inc.) in DMEM (Gibco) containing 0.5% fetal bovine serum and incubated at 37°C with 5% CO2 for 2 h before treatment. Proteins (GST fusions and p40 isolated from LGG supernatant [Yan et al., 2007]) were added at a final concentration of 10 ng/ml and explants were further incubated for 2 h. At the end of the experiment, the colonic mucosa was scraped into homogenization buffer and tissue lysed [Polk, 1994]. The protein concentration was determined using the DC protein assay (Bio-Rad Laboratories). Equal amounts of cellular lysate proteins were mixed with Laemmli sample buffer and separated by SDS-polyacrylamide gel electrophoresis for Western blot analysis with anti-EGFR-phospho-Tyr1068 (Cell Signaling Technology) and anti-actin (Upstate) polyclonal antibodies.
Binding to ECM Proteins and Epithelial Cells
Binding of GST-p40 and GST-p75 to immobilized fibronectin (human plasma; Sigma), fibrinogen (fraction I from pig plasma; Sigma), collagen (type I; Roche) and mucin (porcin stomach; Sigma) was assayed in 96-well immunoplates. Polysorp plates (Nunc) were covered overnight at 4°C with 10 μg/ml fibronectin or fibrinogen in 50 mM carbonate/bicarbonate buffer pH 9.6, whereas Maxisorp plates (Nunc) were used for immobilization of 50 μg/ml collagen in PBS pH 5.5 or 500 μg/ml mucin in 50 mM carbonate/bicarbonate buffer pH 9.6. The plates were washed 3 times with PBS and blocked with 1% BSA for at least 1 h at 37°C. 100 μl of GST, GST-p40 and GST-p75 at different concentrations were added to each well and incubated for 1 h at 37°C. The wells were washed 8 times with PBS containing 0.05% Tween-20 in order to eliminate unbound proteins. Bound proteins were solubilized by addition of 40 μl of Laemmli buffer. 5-μl aliquots were subjected to SDS-PAGE and the amount of bound GST-p40 and GST-p75 was detected by Western blot. In order to assess unspecific binding of GST to ECM components, GST, GST-p40 and GST-p75 were incubated with immobilized ECM components as described above. After washing of unbound proteins, GST activities of bound proteins were determined using the substrate 1-chloro-2,4-dinitrobenzene together with reduced glutathione and the reaction was monitored by measuring the absorbance at 340 nm.
For binding assays to intestinal epithelial cells, Caco-2 cells were seeded in 96-well plates at 25,000 cells/well in DMEM (Gibco) medium supplemented with 10% fetal bovine serum, 10 mM Hepes, 0.1 mM non-essential amino acids (Gibco), 1 mM sodium pyruvate (Gibco) and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin (Gibco)). T84 cells were seeded in 96-well plates at 65,000 cells/well and grown in DMEM/F-12 medium (Gibco) containing 10% fetal bovine serum and antibiotics. Cells were used for experiments after 16–18 days (Caco-2) or 11–13 days (T84) of culture. Different concentrations of GST-p40 and GST-p75 were diluted in the respective cell culture medium and incubated for 1 h at 37°C in a humidified atmosphere containing 5% CO2. After 8 washing steps with PBS, bound GST-p40 and GST-p75 were eluted with Laemmli buffer and detected by Western blot as described above.
Acknowledgements
This work was financed by projects AGL2004-00176/ALI and Consolider Fun-C-Food CSD2007-00063 from the Spanish Ministry of Science and Innovation and by NIH grants DK54993 to D.B.P. and DK065788 to F.Y. C.B. was supported by an I3P predoctoral fellowship from the Consejo Superior de Investigaciones Científicas. We are grateful to José Coll and Gabriele Hörmannsperger for technical assistance.
References
- Acedo-Felix E, Perez-Martinez G. Significant differences between Lactobacillus casei subsp. casei ATCC 393T and a commonly used plasmid-cured derivative revealed by a polyphasic study. Int J Syst Evol Microbiol. 2003;53:67–75. doi: 10.1099/ijs.0.02325-0. [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. Evolutionary history, structural features and biochemical diversity of the NLPC/P60 superfamily of enzymes. Genome Biol. 2003;4:R11. doi: 10.1186/gb-2003-4-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrih A, Bacher G, Allmaier G, Williamson MP, Foster SJ. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J Bacteriol. 1999;181:3956–3966. doi: 10.1128/jb.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Rawlings ND. The CHAP domain: a large family of amidases including GSP amidase and peptidoglycan hydrolases. Trends Biochem Sci. 2003;28:234–237. doi: 10.1016/S0968-0004(03)00061-6. [DOI] [PubMed] [Google Scholar]
- Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74:425–434. doi: 10.1128/IAI.74.1.425-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy B, Gaskins HR, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–790S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- Dougherty TJ. Analysis of Neisseria gonorrhoeae peptidoglycan by reverse-phase, high-pressure liquid chromatography. J Bacteriol. 1985;163:69–74. doi: 10.1128/jb.163.1.69-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda S, Vignolo G, Holgado APR, Oliver G. Proteolytic activity of Lactobacillus strains isolated from dry fermented sausages on muscle sarcoplasmic proteins. Meat Sci. 1998;49:11–18. doi: 10.1016/s0309-1740(97)00097-1. [DOI] [PubMed] [Google Scholar]
- Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13:236–243. doi: 10.3748/wjg.v13.i2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LY, Pak M, Kish R, Kajihara K, Brown EJ. A mycobacterial operon essential for virulence in vivo and invasion and intracellular persistence in macrophages. Infect Immun. 2006;74:1757–1767. doi: 10.1128/IAI.74.3.1757-1767.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D, Bergonzelli GE, Pridmore RD, Marvin L, Rouvet M, Corthesy-Theulaz IE. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect Immun. 2004;72:2160–2169. doi: 10.1128/IAI.72.4.2160-2169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann C, Hartleib J, Hussain MS, Peters G. The multifunctional Staphylococcus aureus autolysin Aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect Immun. 2005;73:4793–4802. doi: 10.1128/IAI.73.8.4793-4802.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörmannsperger G, Haller D. Molecular cross-talk of probiotic bacteria with the intestinal immune system: clinical relevance in the context of inflammatory bowel disease. Int J Med Microbiol. 2010;300:63–73. doi: 10.1016/j.ijmm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hurmalainen V, Edelman S, Antikainen J, Baumann M, Lahteenmaki K, Korhonen TK. Extracellular proteins of Lactobacillus crispatus enhance activation of human plasminogen. Microbiology. 2007;153:1112–1122. doi: 10.1099/mic.0.2006/000901-0. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Wakahara N, Watanabe M, Kawasaki T, Matsuo H, Kawai Y, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T. Cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPdh) of Lactobacillus plantarum La 318 recognizes human A and B blood group antigens. Res Microbiol. 2008;159:685–691. doi: 10.1016/j.resmic.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. S layer protein a of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T-cell functions. Proc Natl Acad Sci USA. 2008;105:19474–19479. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec S, Decaris B, Leblond-Bourget N. Diversity of firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Res Microbiol. 2008;159:507–515. doi: 10.1016/j.resmic.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, De Keersmaecker SC. Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev. 2008;72:728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- Leloup L, Ehrlich SD, Zagorec M, Morel-Deville F. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl Environ Microbiol. 1997;63:2117–2123. doi: 10.1128/aem.63.6.2117-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand M, Boughammoura A, Courtin P, Mezange C, Guillot A, Chapot-Chartier MP. Peptidoglycan N-acetylglucosamine deacetylation decreases autolysis in Lactococcus lactis. Microbiology. 2007;153:3275–3285. doi: 10.1099/mic.0.2007/005835-0. [DOI] [PubMed] [Google Scholar]
- Milohanic E, Jonquieres R, Cossart P, Berche P, Gaillard JL. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol Microbiol. 2001;39:1212–1224. doi: 10.1111/j.1365-2958.2001.02208.x. [DOI] [PubMed] [Google Scholar]
- Monedero V, Maze A, Boël G, Zuñiga M, Beaufils S, Hartke A, Deutscher J. The phosphotransferase system of Lactobacillus casei: regulation of carbon metabolism and connection to cold shock response. J Mol Microbiol Biotechnol. 2007;12:20–32. doi: 10.1159/000096456. [DOI] [PubMed] [Google Scholar]
- Mozzi F, Savoy de Giori G, Oliver G, Font de Valdez G. Exopolysaccharide production by Lactobacillus casei under controlled pH. Biotechnol Lett. 1996;18:435–439. [Google Scholar]
- Muñoz-Provencio D, Llopis M, Antolin M, de Torres I, Guarner F, Perez-Martinez G, Monedero V. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch Microbiol. 2009;191:153–161. doi: 10.1007/s00203-008-0436-9. [DOI] [PubMed] [Google Scholar]
- Muñoz-Provencio D, Perez-Martinez G, Monedero V. Characterization of a fibronectin-binding protein from Lactobacillus casei BL23. J Appl Microbiol. 2010;108:1050–1059. doi: 10.1111/j.1365-2672.2009.04508.x. [DOI] [PubMed] [Google Scholar]
- Polk DB. Ontogenic regulation of phospholipase C-γ1 activity and expression in the rat small intestine. Gastroenterology. 1994;107:109–116. doi: 10.1016/0016-5085(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Posno M, Leer RJ, van Luijk N, van Giezen MJ, Heuvelmans PT, Lokman BC, Pouwels PH. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl Environ Microbiol. 1991;57:1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiah K, van Reenen CA, Dicks LM. Surface-bound proteins of Lactobacillus plantarum 423 that contribute to adhesion of Caco-2 cells and their role in competitive exclusion and displacement of Clostridium sporogenes and Enterococcus faecalis. Res Microbiol. 2008;159:470–475. doi: 10.1016/j.resmic.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, Langella P. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on dextran sodium sulfate-induced colitis in mice. Microb Cell Fact. 2007;6:22. doi: 10.1186/1475-2859-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol. 2008;294:G1060–G1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat-shock proteins in intestinal epithelial cells. Am J Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- Teng F, Kawalec M, Weinstock GM, Hryniewicz W, Murray BE. An Enterococcus faecium secreted antigen, SagA, exhibits broad-spectrum binding to extracellular matrix proteins and appears essential for E. faecium growth. Infect Immun. 2003;71:5033–5041. doi: 10.1128/IAI.71.9.5033-5041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpool C, Yan F, Polk DB. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1585–1596. doi: 10.1002/ibd.20525. [DOI] [PubMed] [Google Scholar]
- Wuenscher MD, Kohler S, Bubert A, Gerike U, Goebel W. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993;175:3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Furuhata K, Fukushima T, Yamamoto H, Sekiguchi J. Characterization of a new Bacillus subtilis peptidoglycan hydrolase gene, yvcE (named cwlO), and the enzymatic properties of its encoded protein. J Biosci Bioeng. 2004;98:174–181. doi: 10.1016/S1389-1723(04)00262-2. [DOI] [PubMed] [Google Scholar]
- Yan F, Cao H, Cover TL, Chaturvedi MH, Washington K, Shi Y, Liu L, Wilson KT, Polk DB: Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGF receptor-dependent mechanism. 2010 (manuscript under revision). [DOI] [PMC free article] [PubMed]
- Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]