Abstract
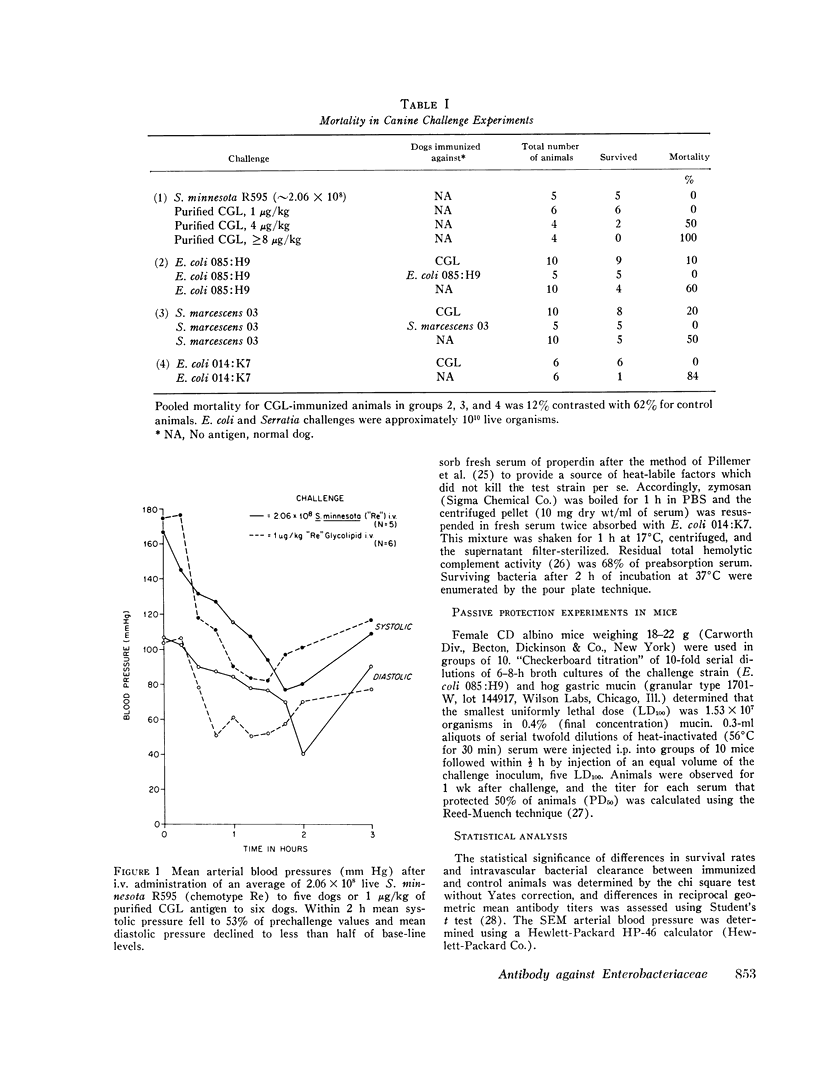
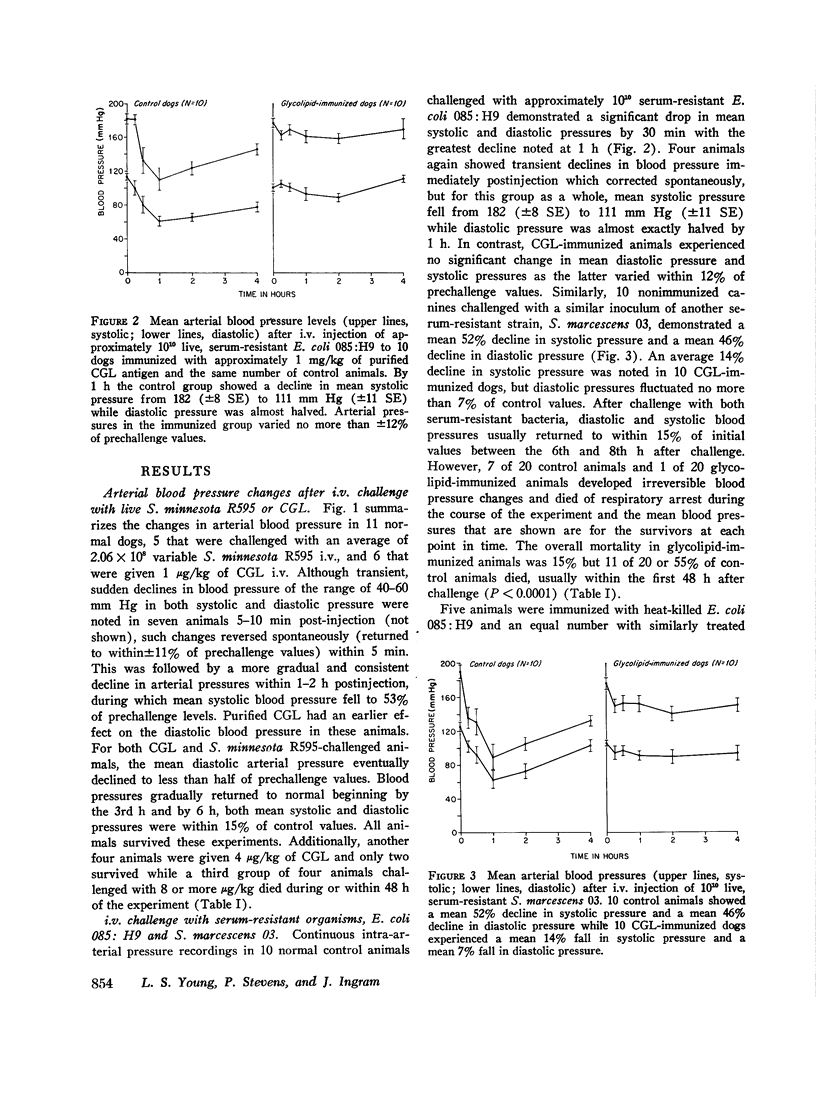
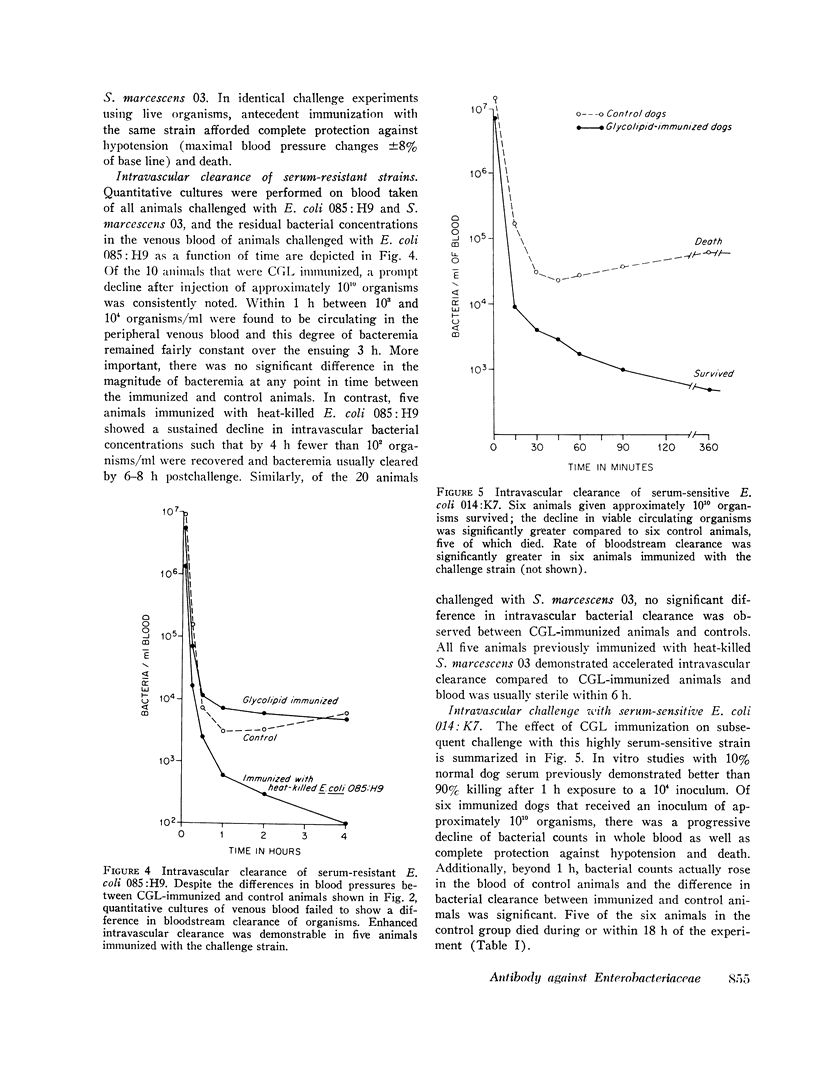
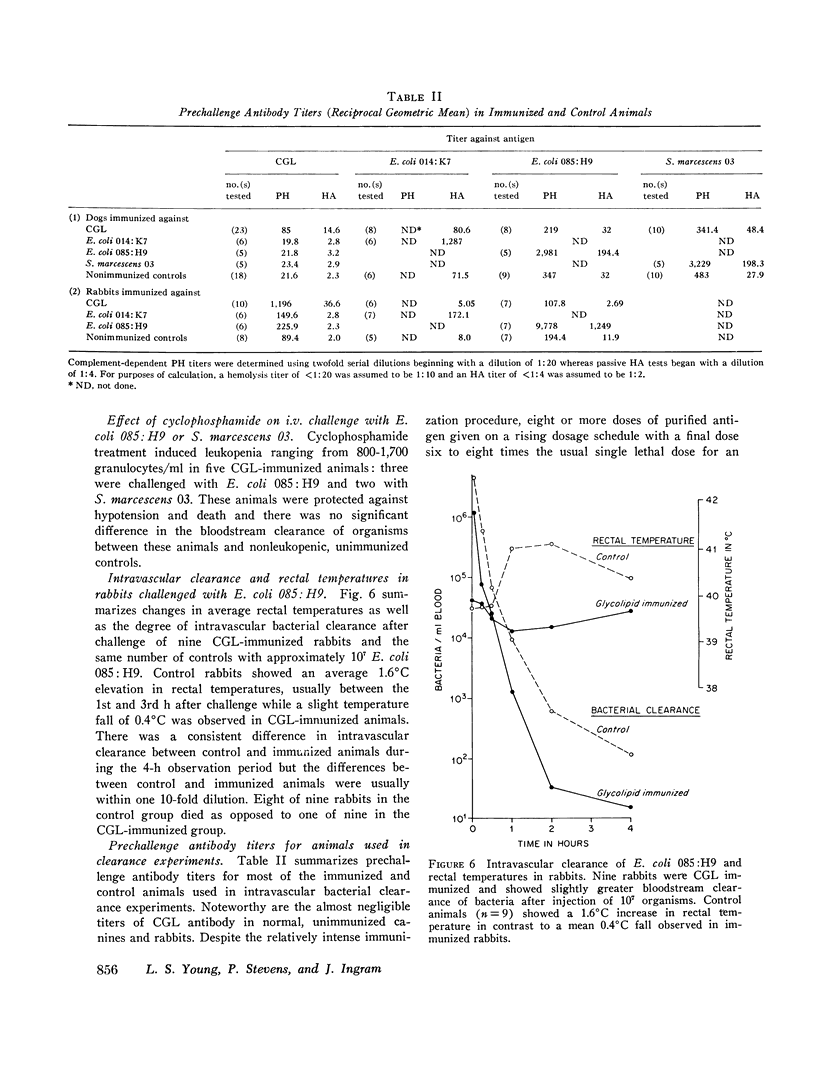
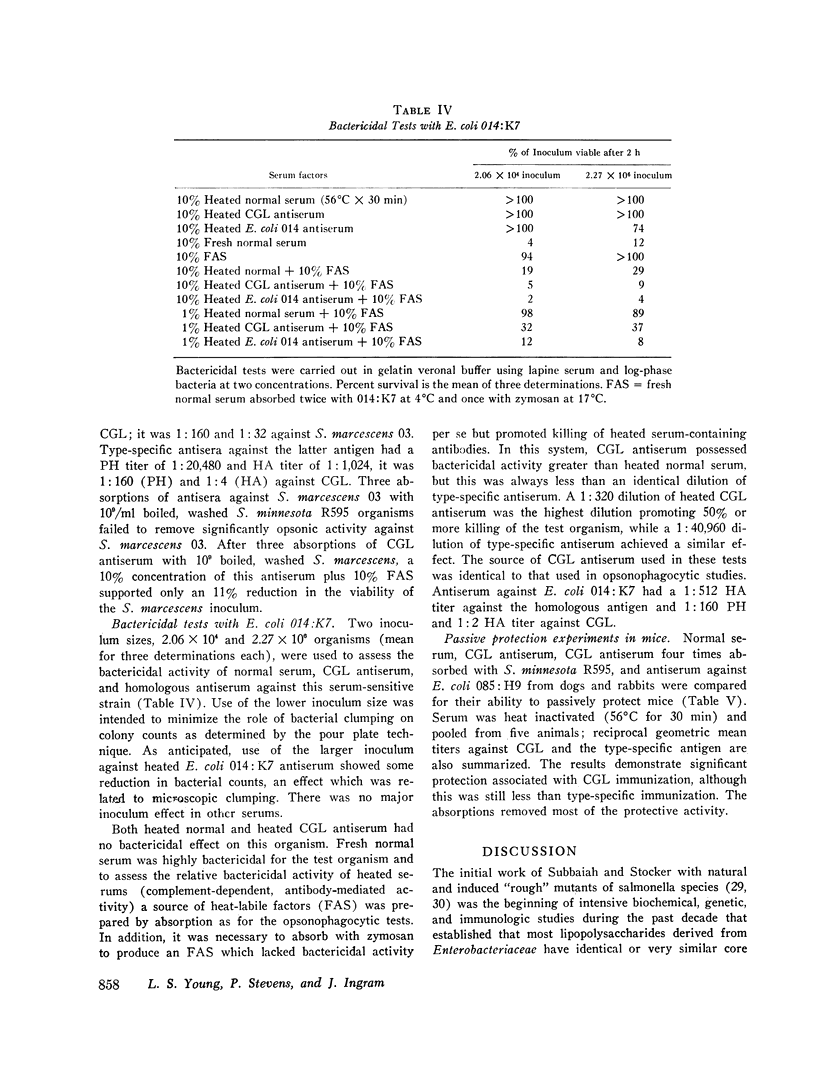
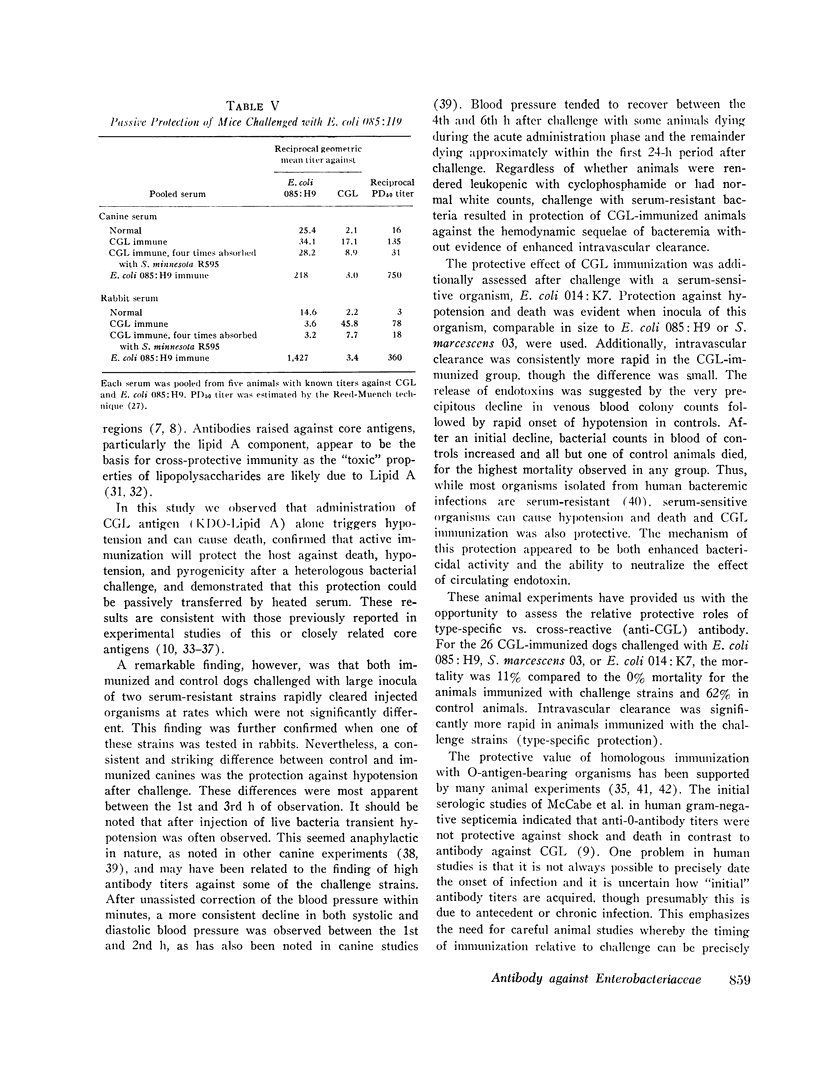
Antibodies against the "core" glycolipid of Enterobacteriaceae (2-keto, 3-deoxyoctonate-Lipid A) have been associated with protection against the sequelae of gram-negative rod bacteremia. To investigate the nature of this protection, dogs and rabbits were immunized with purified glycolipid prepared by phenol-chloroform-petroleum ether extraction of the "Re" mutant of Salmonella minnesota 595 and opsonophagocytic and bactericidal tests were carried out using lapine peritoneal granulocytes and serum factors. Whereas 1-4 mug/kg of glycolipid i.v. produced hypotension in dogs and 8 mug/kg i.v. was lethal, a rising dosage schedule of immunization with an average total dose of 1 mg/kg produced striking protection against shock and death against challenge with heterologous organisms. 20 control dogs, given approximately 10(10) live, serum-resistant Escherichia coli 0.85:H9 or Serratia marcescens 03 during a continuous intra-arterial pressure transducer recording, showed a drop in mean systolic pressure from 186 (+/- 6 SE) to 101 (+/- 12 SE) MM Hg and a fall in mean diastolic pressure from 118 (+/- 3 SE) to 64 (+/- 8 SE) mm Hg within 60-120 min. Minor pressure changes (average less than 12% of prechallenge levels) were seen in the same number of immunized dogs. In contrast, no significant difference was noted in the bloodstream clearance of these serum-resistant organisms over a period of 4-6 h between immunized and control dogs. Intravascular clearance was greater in animals immunized with the challenged strain or in glycolipid-immunized animals challenged with highly serum-sensitive E. coli 0.14:K7. Antibody against core glycolipid protected against the hemodynamic sequelae of bacillemia, augmented intravascular clearance of serum-sensitive organisms, and abrogated the pyrogenic response to enteric bacilli, but did not enhance clearance of serum-resistant organisms. Although canine and lapine antiserum against core glycolipid passively protected mice against a heterologous challenge, opsonophagocytic and bactericidal activity was at least 100-fold less than type-specific antiserum.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Fisher M. W., MacMillan B. G. Immunological control of Pseudomonas infection in burn patients: a clinical evaluation. Arch Surg. 1971 Jan;102(1):31–35. doi: 10.1001/archsurg.1971.01350010033008. [DOI] [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- DuPont H. L., Spink W. W. Infections due to gram-negative organisms: an analysis of 860 patients with bacteremia at the University of Minnesota Medical Center, 1958-1966. Medicine (Baltimore) 1969 Jul;48(4):307–332. doi: 10.1097/00005792-196907000-00003. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. Hospital-acquired infections. N Engl J Med. 1970 Dec 17;283(25):1384–1391. doi: 10.1056/NEJM197012172832507. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. The serum bactericidal reaction. IV. Phenotypic conversion of Escherichia coli from serum-resistance to serum-sensitivity by diphenylamine. J Infect Dis. 1969 Oct;120(4):437–444. doi: 10.1093/infdis/120.4.437. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F., Feingold D. S. The serum bactericidal reaction. 3. Antibody and complement requirements for killing a rough Escherichia coli. J Immunol. 1969 Jun;102(6):1379–1387. [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- LANDY M., PILLEMER L. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956 Sep 1;104(3):383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN L. D., SPINK W. W., VISSCHER M. B., WEIL M. H. Studies on the circulatory changes in the dog produced by endotoxin from gram-negative microorganisms. J Clin Invest. 1956 Nov;35(11):1191–1198. doi: 10.1172/JCI103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- McCabe W. R., Kreger B. E., Johns M. Type-specific and cross-reactive antibodies in gram-negative bacteremia. N Engl J Med. 1972 Aug 10;287(6):261–267. doi: 10.1056/NEJM197208102870601. [DOI] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Medeiros A. A., O'Brien T. F. Recent experience with bacillemia due to gram-negative organisms. J Infect Dis. 1971 Sep;124(3):239–246. doi: 10.1093/infdis/124.3.239. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., WURZ L., TODD E. W. The properdin system and immunity. III. The zymosan assay of properdin. J Exp Med. 1956 Jan 1;103(1):1–13. doi: 10.1084/jem.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. K., Snyderman R., Mergenhagen S. E. Activation of complement by endotoxin: a role for 2 globulin, C1, C4 and C2 in the consumption of terminal complement components by endotoxin-coated erythrocytes. J Immunol. 1972 Aug;109(2):334–341. [PubMed] [Google Scholar]
- ROWLEY D. Rapidly induced changes in the level of non-specific immunity in laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):223–234. [PMC free article] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINK W. W., DAVIS R. B., POTTER R., CHARTRAND S. THE INITIAL STAGE OF CANINE ENDOTOXIN SHOCK AS AN EXPRESSION OF ANAPHYLACTIC SHOCK: STUDIES ON COMPLEMENT TITERS AND PLASMA HISTAMINE CONCENTRATIONS. J Clin Invest. 1964 Apr;43:696–704. doi: 10.1172/JCI104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- Sanford J. P. The Jennerian approach to gram-negative bacillary bacteremia? N Engl J Med. 1972 Aug 10;287(6):304–305. doi: 10.1056/NEJM197208102870612. [DOI] [PubMed] [Google Scholar]
- Tate W. J., 3rd, Douglas H., Braude A. I., Wells W. W. Protection against lethality of E. coli endotoxin with "O" antiserum. Ann N Y Acad Sci. 1966 Jun 30;133(2):746–762. doi: 10.1111/j.1749-6632.1966.tb52403.x. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S., Hoffman K. R., Stevens P. "Core" glycolipid of enterobacteriaceae: immunofluorescent detection of antigen and antibody. Proc Soc Exp Biol Med. 1975 Jun;149(2):389–396. doi: 10.3181/00379727-149-38814. [DOI] [PubMed] [Google Scholar]
- Young L. S., Meyer R. D., Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973 Oct;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Braude A. I. Human antiserum for prevention of the local shwartzman reaction and death from bacterial lipopolysaccharides. J Clin Invest. 1973 Dec;52(12):3236–3238. doi: 10.1172/JCI107524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]



