Abstract
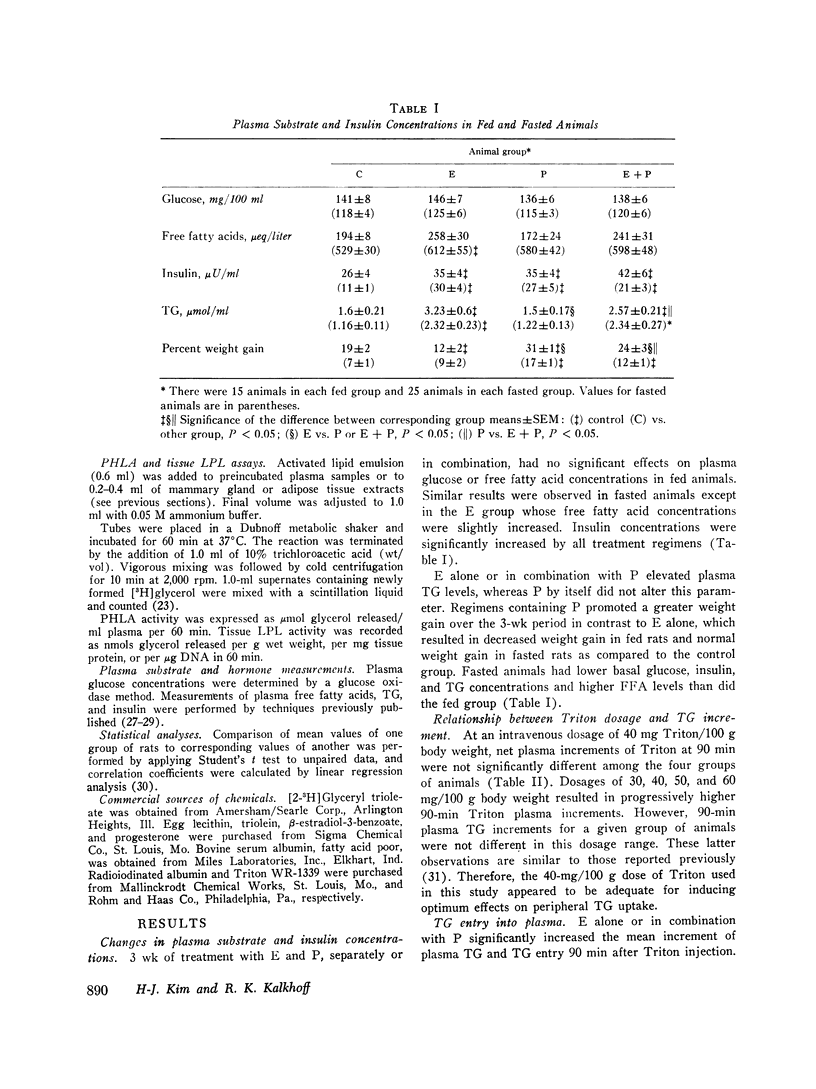
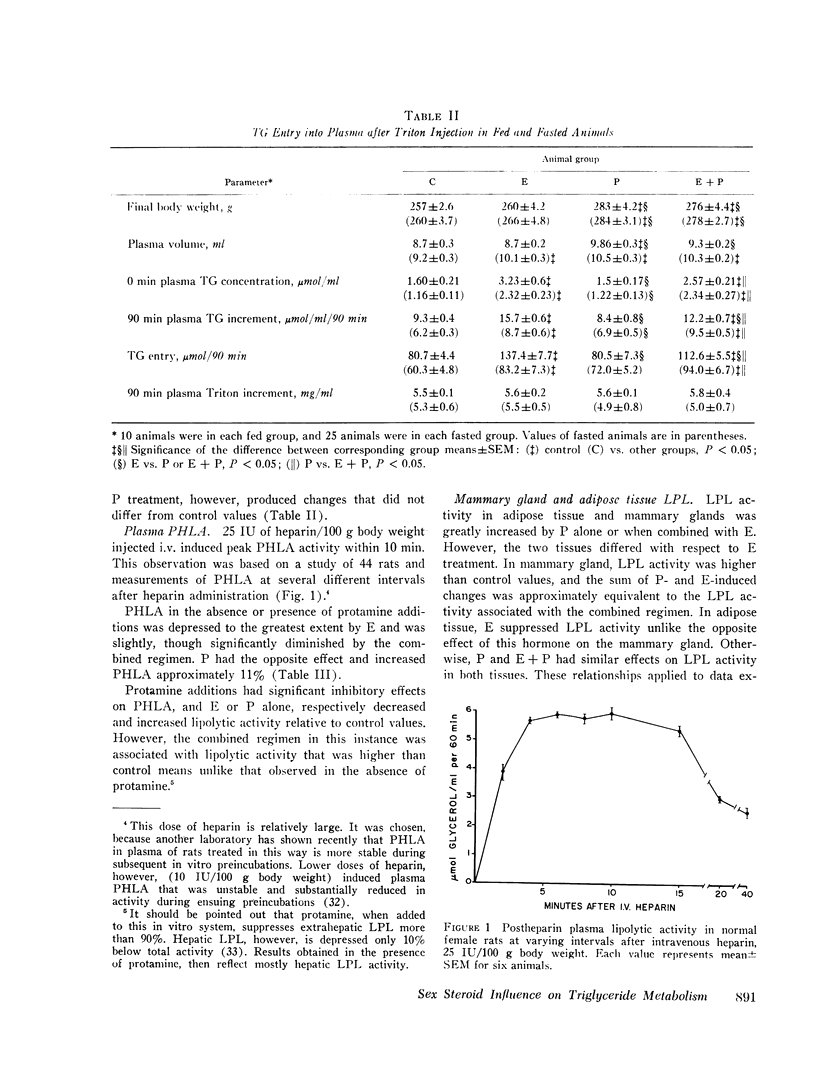
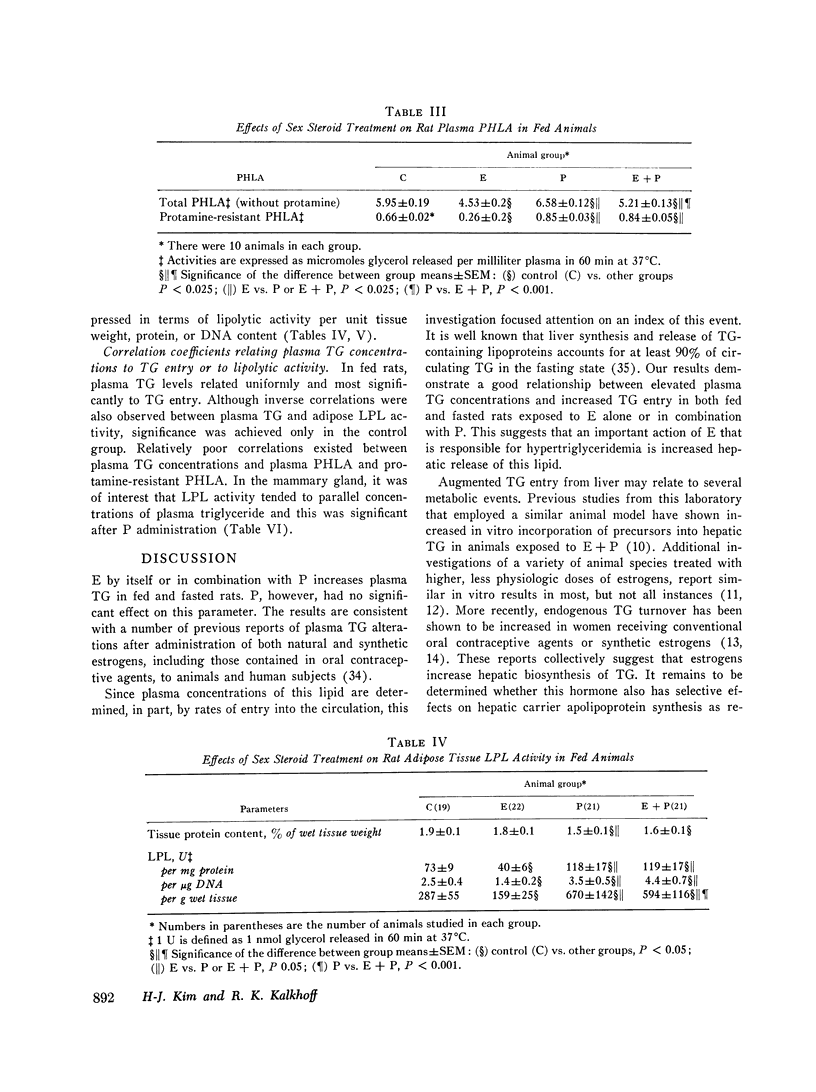
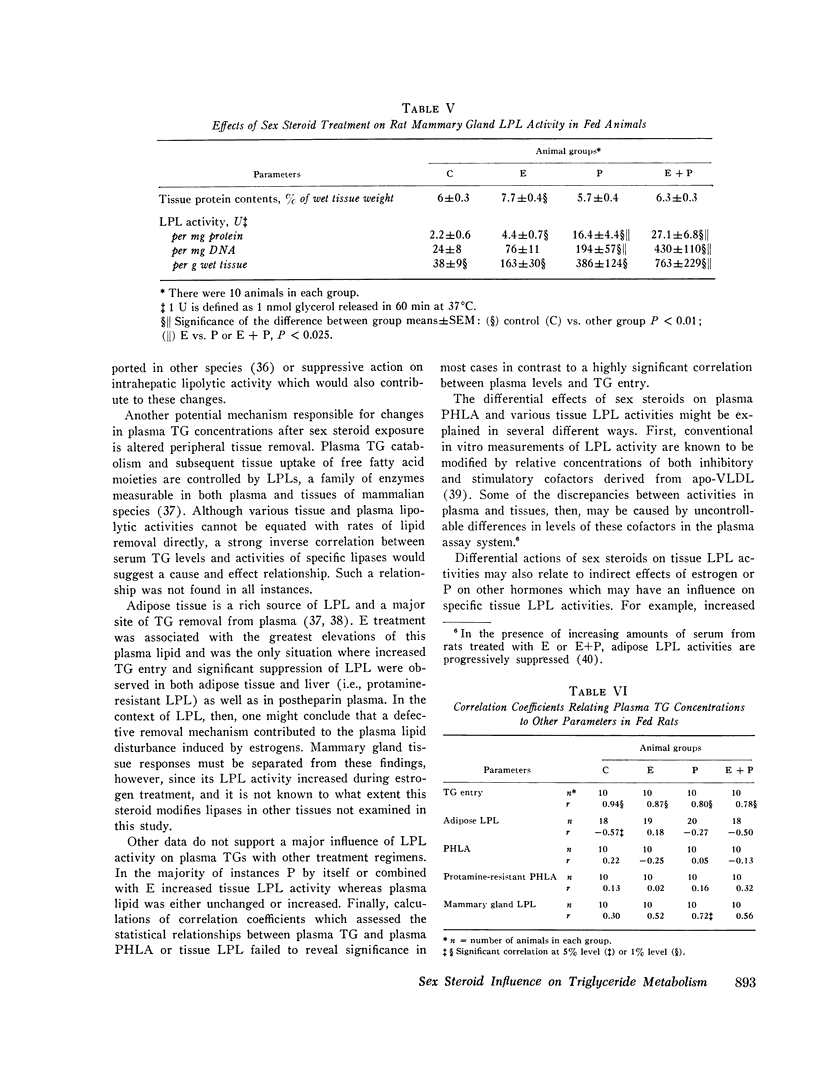
Triglyceride metabolism was investigated in groups of fed and fasted rats after 21 days of parenteral estradiol (5 mug daily), progesterone (5 mg daily), or the two steroids in combination. Results were compared with control groups receiving an oil solvent alone. In rats given estradiol separately or combined with progesterone, hypertriglyceridemia was uniformly associated with increased plasma triglyceride entry, estimated with the i.v. Triton WR1339 technique. Progesterone alone had no effect on these parameters. Plasma postheparin lipolytic activity (PHLA), adipose, mammary gland, and protamine-resistant liporotein lipases (LPL) were significantly increased in progesterone-treated rats and significantly decreased in rats receiving estradiol with the exception of mammary gland LPL, which was also increased to a slight extent. The combined regimen reduced plasma PHLA and increased protamine-resistant adipose, and mammary gland LPL activity. Sex steroid treatments had minimal effects on plasma glucose and free fatty acid concentrations, but all increased plasma insulin significantly. Hyperinsulinemia did not parallel changes in body weight or other measured parameters. Linear regression analyses revealed that plasma triglyceride concentrations in all fed, treated rats correlated significantly with triglyceride entry but not very uniformly with plasma or tissue LPL activity. We conclude that estradiol, unlike progesterone, has substantial lipemic effects in the rat which relate best to triglyceride entry. Hyperinsulinemia, changes in body weight, plasma PHLA, and tissue LPL activities did not consistently predict the influence of sex steroid treatment on plasma triglyceride concentrations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S. K., Moore D., Kalkhoff R. K. Progesterone. Suppression of the plasma growth hormone response. J Clin Endocrinol Metab. 1972 Sep;35(3):364–369. doi: 10.1210/jcem-35-3-364. [DOI] [PubMed] [Google Scholar]
- Biale Y., Gorin E., Shafrir E. Characterization of tissue lipolytic and esterolytic activities cleaving full and partial glycerides. Biochim Biophys Acta. 1968 Jan 10;152(1):28–32. doi: 10.1016/0005-2760(68)90005-2. [DOI] [PubMed] [Google Scholar]
- Biale Y., Shafrir E. Lipolytic activity toward tri- and monoglycerides in postheparin plasma. Clin Chim Acta. 1969 Mar;23(3):413–419. doi: 10.1016/0009-8981(69)90341-6. [DOI] [PubMed] [Google Scholar]
- Boyd E. M. THE LIPEMIA OF PREGNANCY. J Clin Invest. 1934 Mar;13(2):347–363. doi: 10.1172/JCI100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIKOFF I. L., ENTENMAN C., HILLYARD L. A. Concentration and composition of serum lipoproteins of cholesterol-fed and stilbestrol-injected birds. J Biol Chem. 1956 Nov;223(1):359–368. [PubMed] [Google Scholar]
- Costrini N. V., Kalkhoff R. K. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest. 1971 May;50(5):992–999. doi: 10.1172/JCI106593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Saperstein R. The involvement of RNA synthesis and cyclic AMP in the activation of fat cell lipolysis by growth hormone and glucocorticoids. Horm Metab Res. 1970;2(Suppl):20–27. [PubMed] [Google Scholar]
- Fewster M. E., Pirrie R. E., Turner D. A. Effect of estradiol benzoate on lipid metabolism in the rat. Endocrinology. 1967 Feb;80(2):263–271. doi: 10.1210/endo-80-2-263. [DOI] [PubMed] [Google Scholar]
- Ford S., Jr, Bozian R. C., Knowles H. C., Jr Interactions of obesity, and glucose and insulin levels in hypertriglyceridemia. Am J Clin Nutr. 1968 Sep;21(9):904–910. doi: 10.1093/ajcn/21.9.904. [DOI] [PubMed] [Google Scholar]
- GALLETTI F., KLOPPER A. THE EFFECT OF PROGESTERONE ON THE QUANTITY AND DISTRIBUTION OF BODY FAT IN THE FEMALE RAT. Acta Endocrinol (Copenh) 1964 Jul;46:379–386. doi: 10.1530/acta.0.0460379. [DOI] [PubMed] [Google Scholar]
- Glueck C. J., Brown W. V., Levy R. I., Greten H., Fredrickson D. S. Amelioration of hypertriglyceridaemia by progestational drugs in familial type-V hyperlipoproteinaemia. Lancet. 1969 Jun 28;1(7609):1290–1291. doi: 10.1016/s0140-6736(69)92225-9. [DOI] [PubMed] [Google Scholar]
- Greten H. Enzymatic degradation of plasma lipoproteins. Expos Annu Biochim Med. 1972;31:160–168. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Fielding C. J., Olivecrona T., Shore V. G., Fielding P. E., Egelrud T. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoproteins lipase from different sources. Biochemistry. 1973 Apr 24;12(9):1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- Hazzard W. R., Notter D. T., Spiger M. J., Bierman E. L. Oral contraceptives and triglyceride transport: acquired heparin resistance as the mechanism for impaired post-heparin lipolytic activity. J Clin Endocrinol Metab. 1972 Sep;35(3):425–437. doi: 10.1210/jcem-35-3-425. [DOI] [PubMed] [Google Scholar]
- Hazzard W. R., Spiger M. J., Bagdade J. D., Bierman E. L. Studies on the mechanism of increased plasma triglyceride levels induced by oral contraceptives. N Engl J Med. 1969 Feb 27;280(9):471–474. doi: 10.1056/NEJM196902272800904. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- JUNGAS R. L., BALL E. G. Studies on the metabolism of adipose tissue. XII. The effects of insulin and epinephrine on free fatty acid and glycerol production in the presence and absence of glucose. Biochemistry. 1963 Mar-Apr;2:383–388. doi: 10.1021/bi00902a035. [DOI] [PubMed] [Google Scholar]
- Kekki M., Nikkilä E. A. Plasma triglyceride turnover during use of oral contraceptives. Metabolism. 1971 Sep;20(9):878–889. doi: 10.1016/0026-0495(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Kissebah A. H., Harrigan P., Wynn V. Mechanism of hypertriglyceridaemia associated with contraceptive steroids. Horm Metab Res. 1973 May;5(3):184–190. doi: 10.1055/s-0028-1093969. [DOI] [PubMed] [Google Scholar]
- Krauss R. M., Windmueller H. G., Levy R. I., Fredrickson D. S. Selective measurement of two different triglyceride lipase activities in rat postheparin plasma. J Lipid Res. 1973 May;14(3):286–295. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCBRIDE O. W., KORN E. D. THE LIPOPROTEIN LIPASE OF MAMMARY GLAND AND THE CORRELATION OF ITS ACTIVITY TO LACTATION. J Lipid Res. 1963 Jan;4:17–20. [PubMed] [Google Scholar]
- Meites J., Lu K. H., Wuttke W., Welsch C. W., Nagasawa H., Quadri S. K. Recent studies on functions and control of prolactin secretion in rats. Recent Prog Horm Res. 1972;28:471–526. [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON D. S. CHANGES IN THE LIPOLYTIC ACTIVITY OF THE GUINEA PIG MAMMARY GLAND AT PARTURITION. J Lipid Res. 1963 Jan;4:21–23. [PubMed] [Google Scholar]
- Reaven G. M., Lerner R. L., Stern M. P., Farquhar J. W. Role of insulin in endogenous hypertriglyceridemia. J Clin Invest. 1967 Nov;46(11):1756–1767. doi: 10.1172/JCI105666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Gavareski D. J., Henderson J. D., Porte D., Jr, Bierman E. L. Accelerated triglyceride secretion. A metabolic consequence of obesity. J Clin Invest. 1973 Jul;52(7):1620–1626. doi: 10.1172/JCI107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössner S., Larsson-Cohn U., Carlson L. A., Boberg J. Effects of an oral contraceptive agent on plasma lipids, plasma lipoproteins, the intravenous fat tolerance and the post-heparin lipoprotein lipase activity. Acta Med Scand. 1971 Oct;190(4):301–305. doi: 10.1111/j.0954-6820.1971.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Schotz M. C., Garfinkel A. S. A simple lipase assay using trichloroacetic acid. J Lipid Res. 1972 Nov;13(6):824–826. [PubMed] [Google Scholar]
- Schurr P. E., Schultz J. R., Parkinson T. M. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids. 1972 Jan;7(1):68–74. doi: 10.1007/BF02531272. [DOI] [PubMed] [Google Scholar]
- Spellacy W. N., Carlson K. L., Schade S. L. Human growth hormone levels in normal subjects receiving an oral contraceptive. JAMA. 1967 Nov 6;202(6):451–454. [PubMed] [Google Scholar]
- Svanborg A., Vikrot O. The effect of estradiol and progesterone on plasma lipids in oophorectomized women. Acta Med Scand. 1966 May;179(5):615–622. doi: 10.1111/j.0954-6820.1966.tb07979.x. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Majumder G. C., Kadoama N., MacIndoe J. H., Frantz W. L. Hormonal regulation of gene expression in mammary cells. Recent Prog Horm Res. 1973;29:417–455. doi: 10.1016/b978-0-12-571129-6.50015-4. [DOI] [PubMed] [Google Scholar]
- VAN HANDEL E., ZILVERSMIT D. B. Micromethod for the direct determination of serum triglycerides. J Lab Clin Med. 1957 Jul;50(1):152–157. [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]



