Abstract
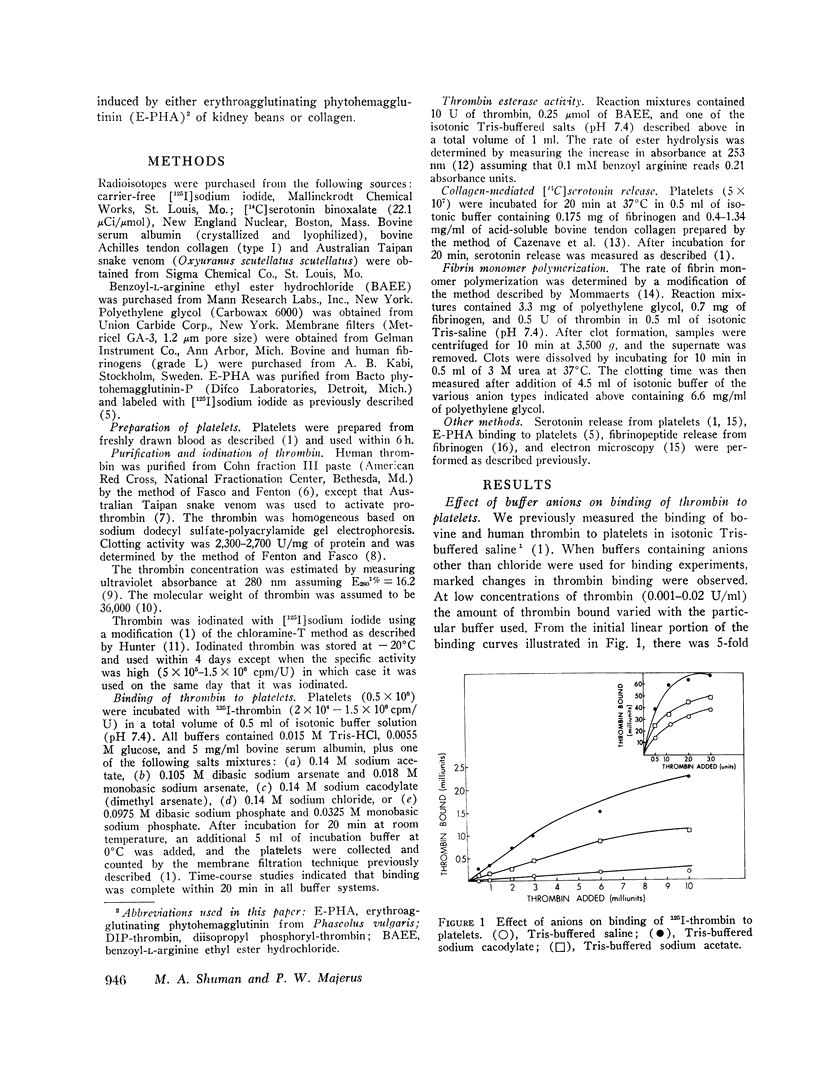
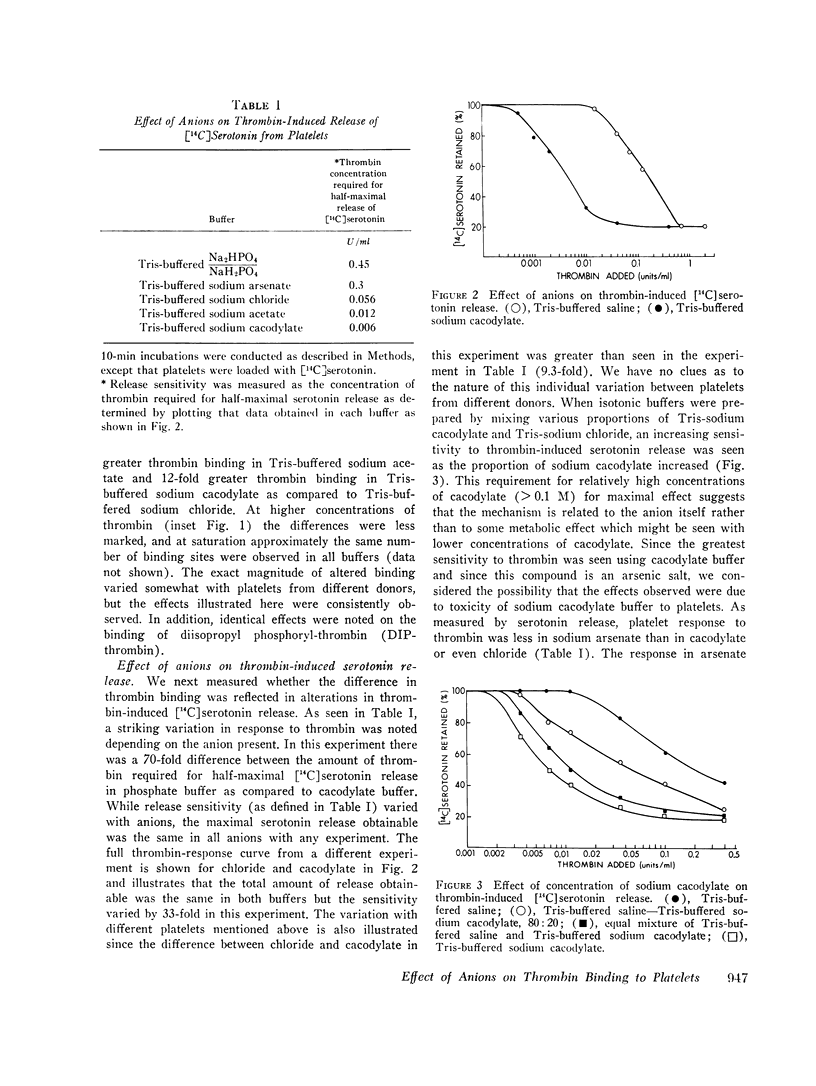
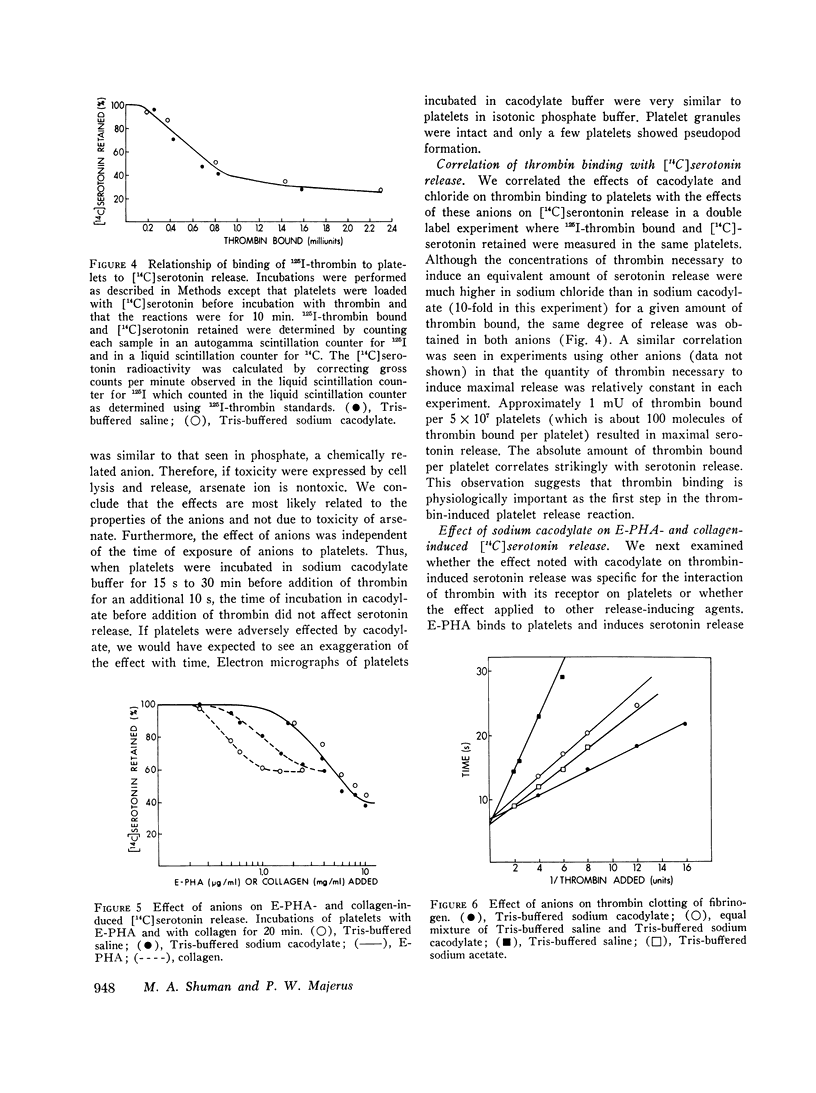
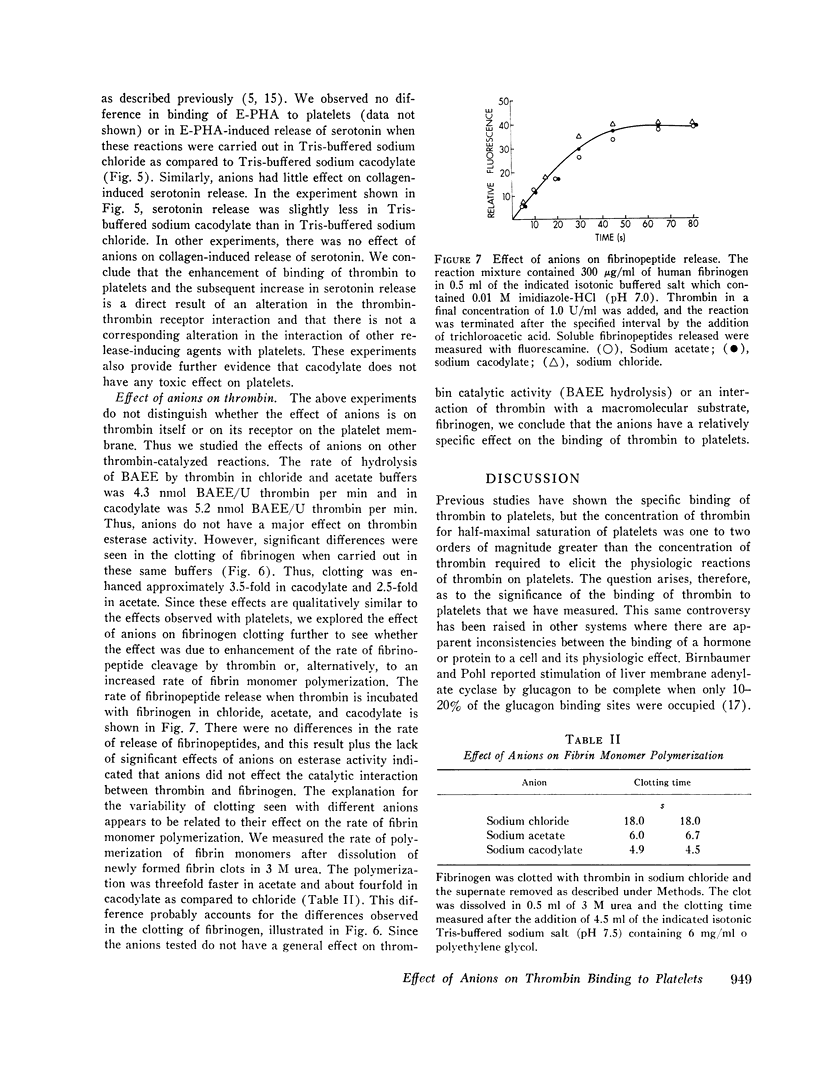
Thrombin binds with high affinity to specific cell-surface receptors on washed human platelets. We present experiments indicating that thrombin binding correlates withe the release reaction when binding is perturbed by anions. Marked differences in the affinity of human 125I-thrombin for platelets wer observed in various isotonic buffers at pH 7.4. At low concentrations of thrombin (0.001-0.01 U/ml), binding was 5-fold greater in Tris-sodium acetate and 12-fold greater in Tris-sodium cacodylate than in Tris-sodium chloride. These anion-induced changes in 125I-thrombin binding paralleled changes in [14C] serotonin release when both parameters were measured in the same platelets. Thus, equivalent release occurred for equal amounts of thrombin bound in all buffers, even though the thrombin concentration varied by up to 30-fold. After approximately 100 molecules of thrombin bound per platelet, complete release occurred in all buffers in 2 min. The effect of anions was specific for the thrombin-receptor interaction as there was no corresponding effect on the binding of erythroagglutinating phytohemagglutinin (E-PHA) to platelets nor on E-PHA or collagen-induced serotonin release. The various anions did not alter platelet morphology as judged by electron microscopy. The anions had no effect on thrombin esterase catalytic activity. In addition, the total number of thrombin receptors per platelet was approximately the same in all buffers. Thus anions alter the affinity between platelet thrombin receptors and a site on thrombin distinct from the catalytic site. We conclude that the thrombin receptor is essential for thrombin-induced platelet reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Brodie G. N., Majerus P. W. Isolation and properties of a thrombin-sensitive protein of human platelets. J Biol Chem. 1972 May 10;247(9):2723–2731. [PubMed] [Google Scholar]
- Birnbaumer L., Pohl S. L. Relation of glucagon-specific binding sites to glucagon-dependent stimulation of adenylyl cyclase activity in plasma membranes of rat liver. J Biol Chem. 1973 Mar 25;248(6):2056–2061. [PubMed] [Google Scholar]
- Brodie G. N., Baenziger N. L., Chase L. R., Majerus P. W. The effects of thrombin on adenyl cyclase activity and a membrane protein from human platelets. J Clin Invest. 1972 Jan;51(1):81–88. doi: 10.1172/JCI106800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Packham M. A., Mustard J. F. Adherence of platelets to a collagen-coated surface: development of a quantitative method. J Lab Clin Med. 1973 Dec;82(6):978–990. [PubMed] [Google Scholar]
- Cheng S., Michuda-Kozak C., Martinez-Carrion M. Effects of anions on the substrate affinities of the pyridoxal and pyridoxamine forms of mitochondrial and supernatant aspartate transaminases. J Biol Chem. 1971 Jun 10;246(11):3623–3630. [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Binding of insulin and other hormones to non-receptor materials: saturability, specificity and apparent "negative cooperativity". Biochem Biophys Res Commun. 1975 Jan 6;62(1):31–41. doi: 10.1016/s0006-291x(75)80401-3. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J. Polyethylene glycol 6,000 enhancement of the clotting of fibrinogen solutions in visual and mechanical assays. Thromb Res. 1974 Jun;4(6):809–817. doi: 10.1016/0049-3848(74)90024-3. [DOI] [PubMed] [Google Scholar]
- Lyons R. M., Stanford N., Majerus P. W. Thrombin-induced protein phosphorylation in human platelets. J Clin Invest. 1975 Oct;56(4):924–936. doi: 10.1172/JCI108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus P. W., Brodie G. N. The binding of phytohemagglutinins to human platelet plasma membranes. J Biol Chem. 1972 Jul 10;247(13):4253–4257. [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. Induction of the platelet release reaction by phytohemagglutinin. J Clin Invest. 1974 Jan;53(1):211–218. doi: 10.1172/JCI107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Tollefsen D. M., Jackson C. M., Majerus P. W. Binding of the products of prothrombin activation to human platelets. J Clin Invest. 1975 Jul;56(1):241–245. doi: 10.1172/JCI108075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Inhibition of human platelet aggregation by monovalent antifibrinogen antibody fragments. J Clin Invest. 1975 Jun;55(6):1259–1268. doi: 10.1172/JCI108045. [DOI] [PMC free article] [PubMed] [Google Scholar]



