Abstract
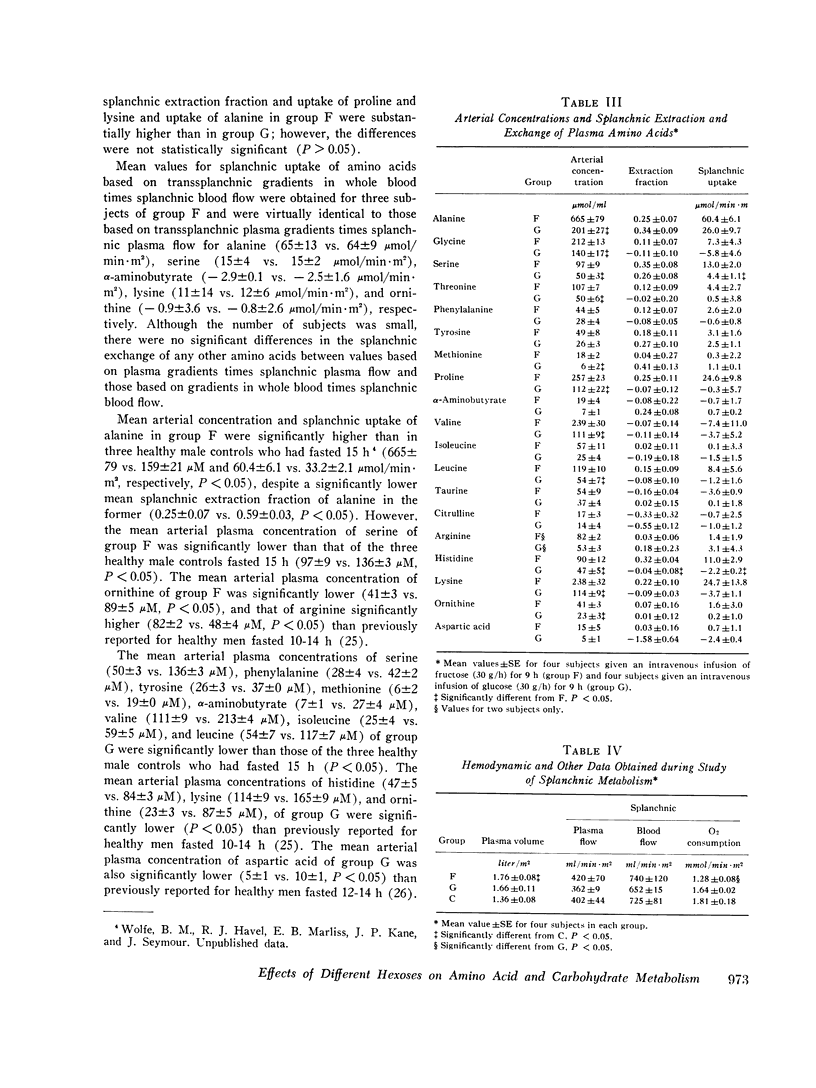
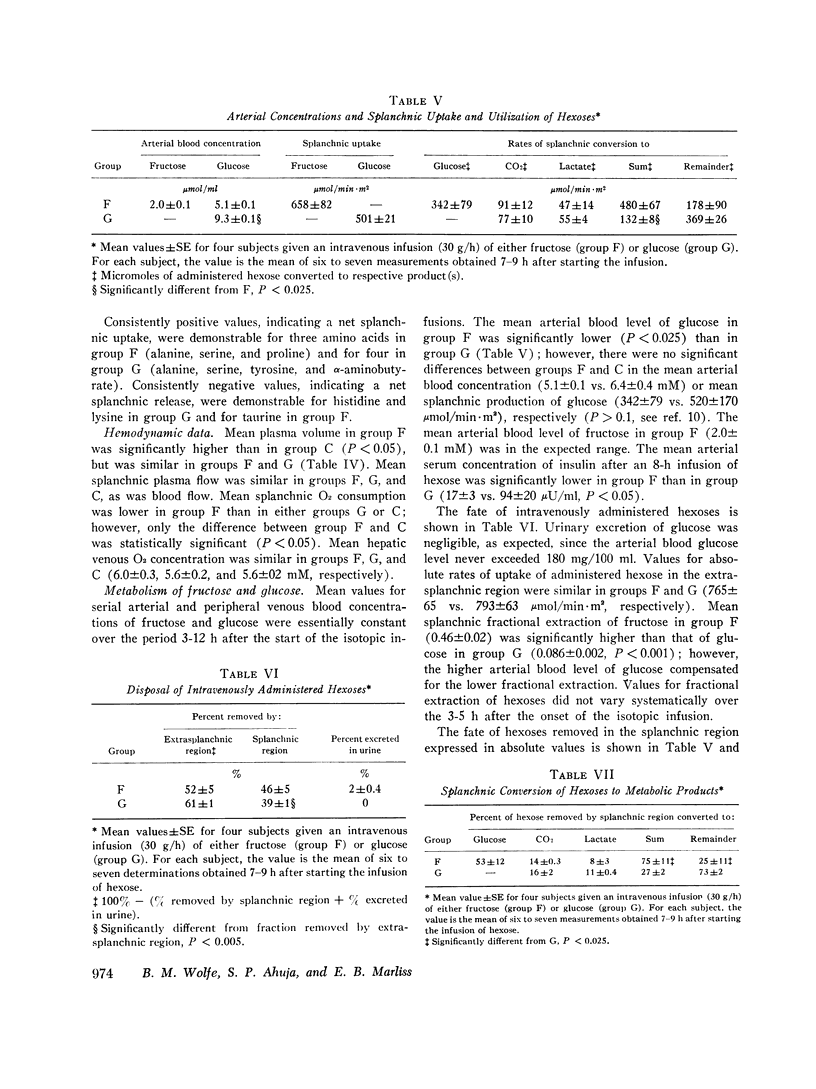
Splanchnic metabolism was studied in the fed state during prolonged intravenous administration (30 g/h) of either fructose or glucose to hypertriglyceridemic men who had been maintained on a high-carbohydrate diet for 2 wk. Splanchnic exchange of amino acids and carbohydrates was quantified by measurement of splanchnic flow and of blood or plasma arteriohepatic venous concentration gradients. Results obtained in subjects receiving fructose were compared with those obtained in (a) similar subjects receiving glucose and (b) postabsorptive controls maintained on isocaloric, balanced diets. Mean arterial plasma levels of alanine, glycine, serine, threonine, methionine, proline, valine, leucine, histidine, lysine, and ornithine were significantly higher in subjects given fructose than in those give glucose (P less than 0.05). The mean arterial concentration and splanchnic uptake of alanine were significantly higher in subjects given fructose than in postabsorptive controls, despite a significantly lower fractional extraction of alanine in the former (P less than 0.05). The mean arterial plasma levels of serine and ornithine were significantly lower in subjects receiving fructose than in postabsorptive controls (P less than 0.05). About half of the administered fructose or glucose was taken up in the splanchnic region, where approximately 15% was converted to CO2 and 10% to lactate. Half of the fructose taken up in the splanchnic region was converted to glucose released from the liver. The amount of hexose carbon remaining for hepatic synthesis of liquids in subjects given fructose was less than half of that of subjects given glucose. These studies demonstrate that fructose and glucose have divergent effects on amino acid metabolism and that during hypercaloric infusion of glucose (as with fructose), the human liver is a major site of lactate production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- BLAIR A., SEGAL S. The isolation of blood glucose as potassium gluconate. J Lab Clin Med. 1960 Jun;55:959–964. [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- Bergström J., Hultman E. Synthesis of muscle glycogen in man after glucose and fructose infusion. Acta Med Scand. 1967 Jul;182(1):93–107. doi: 10.1111/j.0954-6820.1967.tb11503.x. [DOI] [PubMed] [Google Scholar]
- CROFFORD O. B., FELTS P. W., LACY W. W. EFFECT OF GLUCOSE INFUSION ON THE INDIVIDUAL PLASMA FREE AMINO ACIDS IN MAN. Proc Soc Exp Biol Med. 1964 Oct;117:11–14. doi: 10.3181/00379727-117-29483. [DOI] [PubMed] [Google Scholar]
- Cook G. C. Absorption products of D(-) fructose in man. Clin Sci. 1969 Dec;37(3):675–687. [PubMed] [Google Scholar]
- Ellis D. A., Strickland J. M., Eccleston J. F. The direct interconversion of glucose and fructose in human skeletal muscle with special reference to childhood muscular dystrophy. Clin Sci. 1973 Apr;44(4):321–334. doi: 10.1042/cs0440321. [DOI] [PubMed] [Google Scholar]
- FARQUHAR J. W., GROSS R. C., WAGNER R. M., REAVEN G. M. VALIDATION OF AN INCOMPLETELY COUPLED TWO-COMPARTMENT NONRECYCLING CATENARY MODEL FOR TURNOVER OF LIVER AND PLASMA TRIGLYCERIDE IN MAN. J Lipid Res. 1965 Jan;6:119–134. [PubMed] [Google Scholar]
- FINE M., MICHAELS G., SHAH S., CHAI B., FUKAYAMA G., KINSELL L. The incorporation of C14 from uniformly labeled glucose into plasma triglycerides in normals and hyperglyceridemics. Metabolism. 1962 Aug;11:893–911. [PubMed] [Google Scholar]
- FROESCH E. R., GINSBERG J. L. Fructose metabolism of adipose tissue. I. Comparison of fructose and glucose metabolism in epididymal adipose tissue of normal rats. J Biol Chem. 1962 Nov;237:3317–3324. [PubMed] [Google Scholar]
- Felig P., Marliss E., Cahill G. F., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969 Oct 9;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Brundin T. Splanchnic glucose and amino acid metabolism in obesity. J Clin Invest. 1974 Feb;53(2):582–590. doi: 10.1172/JCI107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON E. E., CRAIGIE A. Effect of intravenous glucose on splanchnic and peripheral metabolism of endogenous pyruvate and citrate in patients with cirrhosis and in subjects without liver disease. J Lab Clin Med. 1960 Jun;55:841–848. [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Perry G., Rogers J., Peters N., Day S., Pilkington T. R. Dietary diabetes. The influence of a low carbohydrate diet on forearm metabolism in man. Diabetes. 1973 Mar;22(3):145–159. doi: 10.2337/diab.22.3.145. [DOI] [PubMed] [Google Scholar]
- Jandl J. H., Aster R. H. Increased splenic pooling and the pathogenesis of hypersplenism. Am J Med Sci. 1967 Apr;253(4):383–398. doi: 10.1097/00000441-196704000-00001. [DOI] [PubMed] [Google Scholar]
- KULKA R. G. Colorimetric estimation of ketopentoses and ketohexoses. Biochem J. 1956 Aug;63(4):542–548. doi: 10.1042/bj0630542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedenburg C. P. A lithium buffer system for accelerated single-column amino acid analysis in physiological fluids. Anal Biochem. 1971 Mar;40(1):35–42. doi: 10.1016/0003-2697(71)90081-9. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Owen W. C., Siegal A. M. Ethanol-induced hyperlacticacidemia: inhibition of lactate utilization. J Clin Invest. 1971 Jan;50(1):166–174. doi: 10.1172/JCI106470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDELOFF A. I., WEICHSELBAUM T. E. Role of the human liver in the assimilation of intravenously administered fructose. Metabolism. 1953 Sep;2(5):450–458. [PubMed] [Google Scholar]
- MILLER M., CRAIG J. W., DRUCKER W. R., WOODWARD H., Jr The metabolism of fructose in man. Yale J Biol Med. 1956 Dec;29(3):335–360. [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. H., Hultman E. Liver and muscle glycogen in man after glucose and fructose infusion. Scand J Clin Lab Invest. 1974 Feb;33(1):5–10. doi: 10.3109/00365517409114190. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Kari I. E., De Vivo D. C., Feigin R. D., Kipnis D. M. Hypoalaninemia: a concomitant of ketotic hypoglycemia. J Clin Invest. 1972 Jun;51(6):1440–1449. doi: 10.1172/JCI106940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS M., VINUELA E., SOLS A. INSULIN-DEPENDENT SYNTHESIS OF LIVER GLUCOKINASE IN THE RAT. J Biol Chem. 1963 Nov;238:3535–3538. [PubMed] [Google Scholar]
- SMITH L. H., Jr, ETTINGER R. H., SELIGSON D. A comparison of the metabolism of fructose and glucose in hepatic disease and diabetes mellitus. J Clin Invest. 1953 Apr;32(4):273–282. doi: 10.1172/JCI102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley R. A., Chudzik E. B., Gibbons A. P. Rate of disposal of glucose carbon to CO2, fat, protein, and glycogen in the diabetic rat. Am J Physiol. 1970 Aug;219(2):364–373. doi: 10.1152/ajplegacy.1970.219.2.364. [DOI] [PubMed] [Google Scholar]
- Sizonenko P. C., Paunier L., Vallotton B., Cuendet G. S., Zahnd G., Marliss E. B. Response to 2-deoxy-D-glucose and to glucagon in "ketotic hypoglycemia" of childhood: evidence for epinephrine deficiency and altered alanine availability. Pediatr Res. 1973 Dec;7(12):983–993. doi: 10.1203/00006450-197312000-00007. [DOI] [PubMed] [Google Scholar]
- Swendseid M. E., Tuttle S. G., Drenick E. J., Joven C. B., Massey F. J. Plasma amino acid response to glucose administration in various nutritive states. Am J Clin Nutr. 1967 Mar;20(3):243–249. doi: 10.1093/ajcn/20.3.243. [DOI] [PubMed] [Google Scholar]
- TYGSTRUP N., WINKLER K., LUNDQUIST F. THE MECHANISM OF THE FRUCTOSE EFFECT ON THE ETHANOL METABOLISM OF THE HUMAN LIVER. J Clin Invest. 1965 May;44:817–830. doi: 10.1172/JCI105194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITALLIE T. B., MORGAN M. C., CATHCART R. T., LEDUC G. G., DOTTI L. B. Peripheral assimilation of fructose in man. Proc Soc Exp Biol Med. 1953 Dec;84(3):713–715. doi: 10.3181/00379727-84-20762. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L. W. Regulation of hepatic cholesterol biosynthesis by clofibrate administration. J Pharmacol Exp Ther. 1971 Aug;178(2):361–370. [PubMed] [Google Scholar]
- Wolfe B. M., Belbeck L. W. Splanchnic and hepatic triglyceride secretion during hypercaloric intravenous glucose infusion in conscious swine. J Lipid Res. 1975 Jan;16(1):19–27. [PubMed] [Google Scholar]
- Wolfe B. M., Kane J. P., Havel R. J., Brewster H. P. Mechanism of the hypolipemic effect of clofibrate in postabsorptive man. J Clin Invest. 1973 Sep;52(9):2146–2159. doi: 10.1172/JCI107399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinneman H. H., Nuttall F. Q., Goetz F. C. Effect of endogenous insulin on human amino acid metabolism. Diabetes. 1966 Jan;15(1):5–8. doi: 10.2337/diab.15.1.5. [DOI] [PubMed] [Google Scholar]



