Abstract
Background
Patients undergoing cardiac surgery have a high frequency of preexisting cerebral ischemic lesions, the presence of which appears to predict cognitive sequelae. Patients undergoing aortic valve replacement for aortic stenosis (AS) incur an exceptionally high risk for perioperative cerebral ischemia. The extreme risk in this subgroup may arise from the preexisting burden of cerebral ischemic disease. We tested the hypotheses that increasing age, female sex, coronary artery disease, and the severity of AS are predictive of the severity of preexisting cerebral ischemic lesions.
Methods
A total of 95 subjects were included in this study. Subjects were imaged on 1.5 Tesla magnetic resonance imaging scanners to obtain multimodal image sets which were used for the automatic segmentation of cerebral lesion volume. The dependence of lesion volume upon age, sex, coronary artery disease, and the severity of AS were tested.
Results
The results demonstrate a strong correlation between aging, female sex, and white matter and ischemia-like lesion volume in patients with aortic stenosis.
Conclusions
Women and those of advanced age presenting for aortic valve replacement for AS may incur a particularly high risk for postoperative neurologic sequelae due to an exceptional preexisting burden of cerebral ischemic disease.
Postoperative cognitive dysfunction (POCD) occurs frequently (40% to 80%) [1] in patients after cardiac surgery. While the disorder resolves after weeks to months in most individuals [2], there may be subgroups especially vulnerable to long-term impairment [3]. Patients undergoing cardiac surgery have a high frequency of preexisting white matter lesions (WML) and nonwhite matter ischemia-like lesions (ILL) [4, 5], the presence of which appears to predict acute [6] and long-term POCD [7], as well as new ischemic events [4].
Cerebral white matter is supplied by penetrating arteries with little collateral circulation, and is therefore vulnerable to ischemic hypoxia [8]. Studies of periventricular hyperintensities (leukoaraiosis) in aging and dementia reveal arteriolar tortuosity and reduced vessel density [9-12]. Pathologic findings demonstrate a correlation between WML and arteriosclerosis, reduced vessel density, microglial activation, cystic microinfarctions, and demyelination [13, 14]. Immuohistochemical studies implicate hypoperfusion and hypoxia in the genesis of these lesions [15]. White matter lesions and ischemia-like lesions are also associated with hypoperfusion and increased oxygen extraction [5].
White matter ischemic lesion burden increases with age and female sex [16, 17]. Imaging biomarkers for systemic atherosclerosis, to include the severity of coronary artery calcification [18], internal carotid artery intimal-media thickness [19], and degree of carotid artery stenosis [20], have all been correlated with WML volume. Similarly, aortic stenosis is now recognized as related to the same inflammatory processes associated with atherosclerosis [21] and appears to be a biomarker of diffuse atherosclerosis in multiple organs, to include the brain [22].
White matter ischemic lesion volume at baseline is an important predictor of further ischemic lesion progression. The mean increase in total brain WML volume was approximately 2 mL/year over a three-year period in elderly subjects on a baseline of 16 mL in a study by Sachdev and colleagues [23]. When WMLs involve more than 25% of brain volume, severe changes in memory and attention occur, and have been associated with a progressive “sub-cortical vascular dementia” [24].
Patients undergoing aortic valve replacement (AVR) for aortic stenosis, especially when combined with coronary artery bypass grafting, appear to incur particularly high risk for perioperative cerebral ischemia, suffering stroke in 6.5% to 8.4% of cases [25-27], with an additional 30% suffering multiple “clinically silent” acute ischemic lesions [4, 28]. Even in studies where the frequency of reported stroke is lower, the presence of coronary artery disease (CAD) and need for revascularization during AVR doubles the stroke risk [29]. The coexistence of severe ascending aortic atherosclerosis with CAD also predisposes to stroke (11%) [30]. The high risk in this group may arise from the debridement of the calcific valve during surgery, clamping of the often calcified ascending aorta during cardiopulmonary bypass, or from the presence of preexisting large and small vessel cerebral ischemic disease. Due to the high risk of perioperative cerebral ischemia in the subgroup of cardiac surgery patients with AS, and the predictive value of WML and ILL volume in predicting both future ischemic events and cognitive impairment, we tested the hypotheses that increasing age, female sex, coronary artery disease, and the severity of AS are predictive of the severity of preexisting cerebral ischemic lesions as measured by WML and ILL volume.
Patients and Methods
Study Population and Magnetic Resonance Imaging (MRI) Datasets
This study was approved by the Institutional Review Board (IRB) of the University of Pennsylvania and all participants were appropriately consented prior to participation. Ninety-five (95) subjects were included who were recruited from two sites within the University of Pennsylvania Health System. Study participants included surgical and control subjects recruited for the National Institutes of Health funded R01HL084375, “Stroke and Cognition in Surgical Aortic Stenosis,” conducted by the Determining Neurologic Outcomes from Valve Operations (DENOVO) Investigator Group (see Appendix). Inclusion criteria were: age 65 years or greater; mild, moderate, or severe-critical AS by standard echocardiographic and (or) catheterization criteria, with or without concomitant coronary artery disease; male or female sex; fluency in English or Spanish; and completed the sixth grade. Subjects were excluded if they had suffered a stroke or transient ischemic attack within the preceding 6 months, undergone carotid stenting or carotid endarterectomy within the previous 6 weeks, had active major psychiatric disease, severe visual, auditory, or learning impairment, or any MRI incompatibility.
Subjects were imaged on 1.5 Tesla Siemens Magnetom Avanto (Siemens, Erlangen, Germany) or GE Signa Excite (General Electric Medical Systems, Milwaukee, WI) MRI scanners. The MR modalities of T1-weighted, T2-weighted, proton density-weighted (PD), and fluid attenuation inversion recovery (FLAIR) scans were acquired. Eighty-three subjects were imaged at baseline, prior to surgery, and in 12 subjects imaging was conducted postoperatively.
Image Processing
The discrimination of lesions primarily employs the multimodal MRI image sets of T1, T2, PD-weighted, and FLAIR. Integrating information from multiple MRI sequences can significantly increase the accuracy of the lesion segmentation [31]. When using postoperative datasets, the additional diffusion-weighted MRI data set, which is exquisitely temporally sensitive to the detection of new acute ischemic lesions, is compared with the much less temporally sensitive T1, T2, PD-weighted, and FLAIR image sets [32]. Regions with any new ischemic lesions can then be excluded or subtracted prior to subsequent lesion analysis.
A fully automatic, unbiased software approach was employed to conduct lesion segmentation. The technique, Bayesian automatic lesion segmentation in MRI (BALSAM) [33], utilizes the information from multimodal MRI sequences (ie, T1, T2, PD, FLAIR), and applies Bayesian statistical methods to detect significant differences in pixel signal-intensity or contrast, therefore assisting in the discrimination between normal and abnormal tissue in multimodal MRI image sets, each modality offering different contrast effects and sensitivity to ischemic pathology. The method first uses data from a training set of images to determine appropriate lesion classification statistics, and then it uses these statistics to classify the presence of lesion in new subjects, which is an example of “machine learning.” The BALSAM technique has demonstrated a high level of classification accuracy using an expert neuroradiologist as a standard [33].
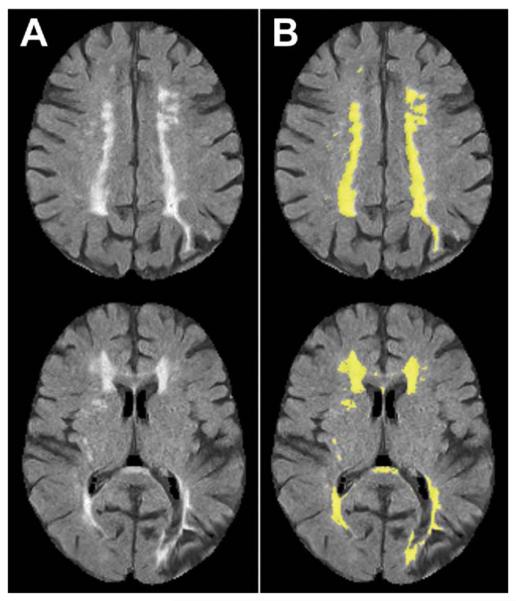
Briefly, the image processing included the following steps: first, a study specific brain template was created based on this specific population; then all MRI modalities were spatially normalized to this standard template so that spatial coordinates within the brain can be directly compared in terms of imaging characteristics; finally, BALSAM was employed to segment the lesions. The original codes-scripts of BALSAM are from Dr. Herskovits' Laboratory (https://www.rad.upenn.edu/sbia/braid/balsam_web/) at the University of Pennsylvania. Examples of the automatic segmented WML-ILL are shown in Figure 1; the segmented lesions are recognized as the binary lesion masks.
Fig 1.
Representative lesion segmentation result. The lesion typically appears brighter on the FLAIR scans. (A) FLAIR images, and (B) lesion masks (yellow regions) overlaid on FLAIR images. (FLAIR = fluid attenuation inversion recovery.)
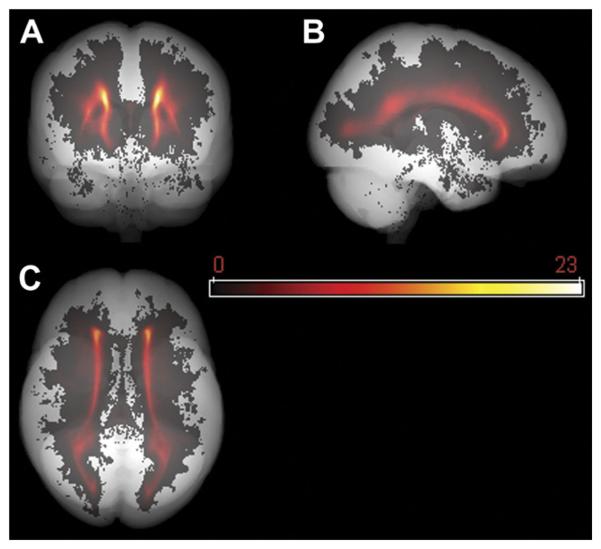
To demonstrate the lesion map of the study population, we defined the mean lesion mask by averaging all the lesion masks at each voxel (x,y,z); ie, . Here L̄ (x,y,z) and li (x,y,z) represent the mean lesion mask and the i th individual lesion mask respectively, N being the total number of subjects. Please note, li (x,y,z) is the binary lesion mask while L̄ (x,y,z) is in float value reflecting the probabilistic distribution of WML. The mean lesion mask L̄ (x,y,z) was further projected along axial, coronal, and sagittal orientations and overlaid onto the “glass brain” (obtained by projecting the template brain mask along the same three orientations) to form a global lesion map, as shown in Figure 2.
Fig 2.
Population lesion distribution map. Lesion distributions are mapped onto a grayscale “glass brain” (in template space), along coronal (A), sagittal (B), and axial (C) orientations. Most lesions are periventricular or deep, while few are subcortical.
Statistical Analysis
Statistical analysis was performed using JMP 8.0 (SAS Institute Inc, Cary, NC). The dependence of WML-ILL volume upon the variables age, sex, hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, and AS severity was tested with a univariate approach. Log transformation of the WML-ILL volume data was required to improve normality of distribution, and all the p values in the following sections are based on the log-transformed data.
Results
Summary of Demographics
The mean age of the population studied was 76 years, with women comprising 30% and men 70% of the total. The clinical characteristics of the population studied are summarized in Table 1. The frequency of the comorbidity of CAD is noted to increase markedly as AS severity increases to the severe-critical range. Additionally, WML-ILL volume is noted to increase by nearly 30% as AS severity progresses to the severe-critical range.
Table 1.
Clinical Characteristics by AS Severity
| AS Severity |
||||
|---|---|---|---|---|
| Variable | Mild (n = 18) |
Moderate (n = 35) |
Severe- Critical (n = 42) |
p Valuea |
| Age | 76 ± 5 | 75 ± 6 | 76 ± 6 | 0.53 |
| Sex (Male) | 12 (67) | 26 (74) | 26 (62) | 0.51 |
| CAD | 9 (50.0) | 16 (46) | 34 (81) | 0.0025b |
| HTN | 15 (83) | 29 (83) | 33 (79) | 0.86 |
| DM | 12 (67) | 13 (37) | 22 (52) | 0.11 |
| HL | 8 (44) | 20 (57) | 19 (45) | 0.52 |
| WML-ILL Volume (mL) | 8.7 ± 7.2 | 8.9 ± 8.9 | 11.3 ± 12.3 | 0.54 |
p value from one-way ANOVA or χ2 test.
p < 0.05.
Mean ± SD or n with percentage of total (%) shown for each group.
AS = aortic stenosis; CAD = coronary artery disease; DM = diabetes mellitus; HL = hyperlipidemia; HTN = hypertension; WML-ILL = white matter lesions and ischemia-like lesions.
Dependence of WML-ILL Volume Upon Clinical Variables
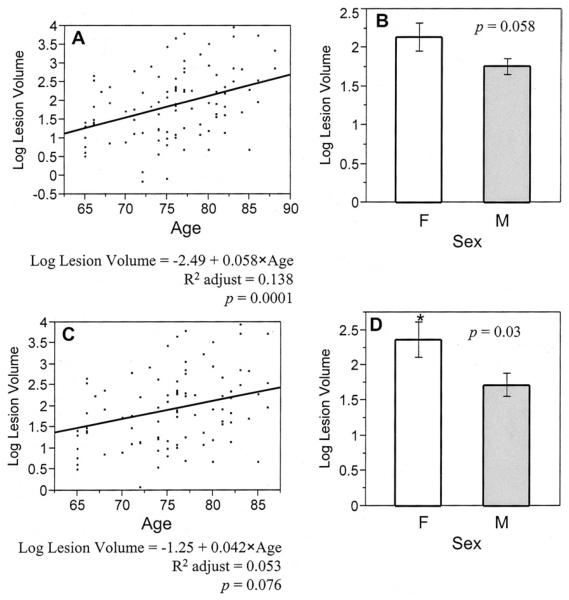
We first tested the dependence of WML-ILL volume upon the clinical variables of interest in the entire group of 95 subjects. Results are presented in Table 2 and Figures 3(A) and 3(B). In this analysis, only aging (p = 0.0001) was significantly associated with increasing WML-ILL volume, yet female sex (p = 0.058) also appeared to predict WML-ILL volume. Comparison of WML-ILL volume in those with either mild or moderate AS with those with severe-critical AS, revealed that more severe stenosis was associated with an increase in volume (mean ± SD) from 8.8 ± 8.3 mL to 11.3 ± 12.3 mL (30%), yet the difference did not reach statistical significance (p = 0.42).
Table 2.
Statistical Summary of Dependence of Ln (WML-ILL Volume) Upon Clinical Variable of Interest Using Univariate Analysis
|
p Value |
||
|---|---|---|
| Variable | Whole Group (n = 95) (Mild, Moderate, and Severe-Critical AS) |
Subgroup (n = 42) (Severe-Critical AS) |
| Age | 0.0001a | 0.076 |
| Sex | 0.058 | 0.030a |
| HL | 0.13 | 0.62 |
| DM | 0.28 | 0.38 |
| HTN | 0.75 | 0.27 |
| CAD | 0.61 | 0.38 |
| AS severity | 0.72–0.99b | — |
p < 0.05.
The range of p values, which are from the comparisons for all pairs of AS severity (mild, moderate, and severe-critical) using the Tukey-Kramer honestly significant difference test.
AS = aortic stenosis; CAD = coronary artery disease; DM = diabetes mellitus; HL = hyperlipidemia; HTN = hypertension; WML-ILL = white matter lesions and ischemia-like lesions.
Fig 3.
Univariate analyses of the dependence of Ln (WML-ILL volume) upon age and sex, for both the whole group (including all the subjects with mild, moderate, severe, or critical aortic stenosis), and the subgroup of severe or critical aortic stenosis subjects.(A) and (B) are the analyses of age and sex (F - female, M - male) for the whole group, (C) and (D) are the same analyses for the subgroup. The bars represent mean ± SE (standard error). (* Indicates a significant difference between groups [p < 0.05].)
Univariate analysis was then extended to the population most likely to present for surgery, those with severe-critical AS. Results are summarized in Table 2 and Figures 3(C) and 3(D). In this subgroup, while aging was slightly less strongly associated with increasing WML-ILL volume (p = 0.076), the importance of female sex became more important (p = 0.03). Among those with severe-critical AS, the mean WMLILL volume (mean ± SD) was 6.5 ± 3.7 mL in those without associated CAD, but increased to 12.4 ± 13.3 mL in those with coexisting CAD; this difference did not reach statistical significance (p = 0.38).
Comment
Calcific AS [34] is the leading valvular disease in the United States, occurring in 2% of those greater than 65 years and in 4% of those greater than 85 years of age [35]. The number of patients with this diagnosis requiring valve replacement is growing rapidly with the aging of our population. Patients undergoing AVR appear to be at considerably higher risk for postoperative ischemia [4, 28]. In those with coexisting CAD the risk may be even higher [26].
Aging in our AS population was associated with a greater burden of preexisting cerebral ischemic disease. Subjects in the current study ranged in age from 65 to 88 years and over this age range there was a dramatic 11-fold increase in WML-ILL lesion volume. Female sex accounted for a 50% increase in WML-ILL volume over male counterparts and may explain, to some degree, the increased frequency of POCD in women after cardiac surgery previously reported by Hogue and colleagues [36].
Severe-critical AS was associated with a statistically insignificant but potentially important 30% increase in WML-ILL volume compared with those with only mild-moderate AS. There is clearly a wide variation in WML-ILL volume from subject to subject, and this contributed to the lack of finding statistical significance between AS severity groups. Based upon the variance of our WML-ILL data, approximately 600 subjects would be needed in the groups in order to find these differences statistically significant. Our study was not powered to measure this outcome.
The presence of CAD in subjects with severe-critical AS was significantly greater than in those with mild-moderate AS. This is a known phenomenon, and was not sex specific. What is more noteworthy is that, among those with severe-critical AS, the coexistence of CAD was associated with a near doubling in WML-ILL volume. The marked increase in baseline cerebral ischemic disease in this group may predispose to both POCD and perioperative cerebral ischemia. Support for this comes from the documented association between the presence of baseline cerebral ischemic disease and postoperative cerebral ischemia [4] and POCD [6, 7]. Furthermore, there is evidence that patients undergoing AVR for AS with concomitant coronary artery bypass grafting represent a subgroup at “extraordinary” risk for postoperative stroke [26].
In conclusion, this is an attempt to study the impact of aging and cardiovascular comorbidities upon cerebral vascular ischemic disease in an aged population with aortic stenosis, a particularly high-risk group for the occurrence of postcardiac surgery neurologic sequelae. The MRI finding of increased preexisting white matter ischemic disease, previously associated with both POCD and postoperative cerebral ischemic events, was highly associated in this study with aging and female sex. Women and those of advanced age presenting for AVR for AS may, because of preexisting cerebrovascular ischemic disease, incur a particularly high risk for postoperative neurologic sequelae. The severity of AS may additionally prove in larger studies to be a marker of severe, diffuse atherosclerosis [37].
INVITED COMMENTARY.
Wang and colleagues [1] present interesting data that seeks to evaluate the frequency and volume of cerebral white matter lesions in patients with aortic stenosis. As the authors point out, these patients are at particularly high risk for adverse neurologic events after operations. Thus, understanding the basis for this high risk has important clinical implications.
The authors nicely summarize the pathologic vascular basis for white matter changes found with brain magnetic resonance imaging. These lesions are prevalent in the elderly, are known to represent a manifestation of chronic ischemic brain injury, and are linked to cognitive decline and stroke. These conclusions are based on data mostly from studies of community-dwelling adults. The results of the article by Wang and colleagues [1] are, thus, mostly confirmatory in describing a link between preexisting magnetic resonance imaging-detected white matter lesions with patient age and female sex.
A weakness of the investigation is the small sample size, which limits the data analysis to univariate statistics. Whether aortic stenosis per se is independently related to the risk for white matter lesions or whether this is simply a manifestation of patient age and gender is unanswered.
That said, one must acknowledge how difficult it is to perform studies involving brain imaging in cardiac surgical patients. Enrolling a large number of patients to answer these important questions would be difficult. Perhaps existing databases from large cohort studies that include results of brain imaging, such as the Framingham
Acknowledgments
We wish to acknowledge the assistance of Abigail Lyon, BS, Sara Heverly-Fitt, BS, and Scott Welden for their contributions to the study and data organization. This study was supported by National Institutes of Health Grant No. R01HL084375.
Abbreviation and Acronyms
- AS
aortic stenosis
- AVR
aortic valve replacement
- CAD
coronary artery disease
- DM
diabetes mellitus
- FLAIR
fluid attenuation inversion recovery
- HL
hyperlipidemia
- HTN
hypertension
- MRI
magnetic resonance imaging
- PD
proton density-weighted
- POCD
postoperative cognitive dysfunction
- T1
T1-weighed
- T2
T2-weighted
- WML-ILL
white matter lesions and ischemia-like lesions
Appendix
DENOVO Investigators
Michael A. Acker, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Joseph E. Bavaria, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Thomas F. Floyd, MD (Department of Anesthesiology & Critical Care and Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA); Tania Giovanetti, PhD (Department of Psychology, Temple University, Philadelphia, PA); W. Clark Hargrove III, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Scott E. Kasner, MD (Department of Neurology, University of Pennsylvania School of Medicine, Philadelphia, PA); William H. Matthai, Jr., MD (Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA); Elias R. Melhem, MD, PhD (Department of Radiology, University of Pennsylvania School of Medicine, Philadelphia, PA); Emile R. Mohler III, MD (Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA); Rohinton J. Morris, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Alberto A. Pochettino, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Catherine E. C. Price, PhD (Department of Clinical and Health Psychology, University of Florida, Gainesville, FL); Sarah J. Ratcliffe, PhD (Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, PA); Ola A. Selnes, PhD (Department of Neurology, Johns Hopkins University Hospital, Baltimore, MD); Wilson Y. Szeto, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Y. Joseph Woo, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); Susan E. Wiegers, MD (Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA); Nimesh D. Desai, MD (Department of Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA); and Martin G. Keane, MD (Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA).
References
- 1.Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Ann Neurol. 2005;57:615–21. doi: 10.1002/ana.20481. [DOI] [PubMed] [Google Scholar]
- 2.Selnes OA, Grega MA, Bailey MM, et al. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–90. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 3.Knipp SC, Matatko N, Wilhelm H, et al. Cognitive outcomes three years after coronary artery bypass surgery: Relation to diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2008;85:872–9. doi: 10.1016/j.athoracsur.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 4.Floyd TF, Shah PN, Price CC, et al. Clinically silent cerebral ischemic events after cardiac surgery: Their incidence, regional vascular occurrence, and procedural dependence. Ann Thorac Surg. 2006;81:2160–6. doi: 10.1016/j.athoracsur.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 5.Goto T, Yoshitake A, Baba T, Shibata Y, Sakata R, Uozumi H. Cerebral ischemic disorders and cerebral oxygen balance during cardiopulmonary bypass surgery: Preoperative evaluation using magnetic resonance imaging and angiography. Anesth Analg. 1997;84:5–11. doi: 10.1097/00000539-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Goto T, Baba T, Honma K, et al. Magnetic resonance imaging findings and postoperative neurologic dysfunction in elderly patients undergoing coronary artery bypass grafting. Ann Thorac Surg. 2001;72:137–42. doi: 10.1016/s0003-4975(01)02676-5. [DOI] [PubMed] [Google Scholar]
- 7.Lund C, Sundet K, Tennøe B, et al. Cerebral ischemic injury and cognitive impairment after off-pump and on-pump coronary artery bypass grafting surgery. Ann Thorac Surg. 2005;80:2126–31. doi: 10.1016/j.athoracsur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 9.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203-204:159–63. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 10.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–6. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–90. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 12.Thore CR, Anstrom JA, Moody DM, Challa VR, Marion MC, Brown WR. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. J Neuropathol Exp Neurol. 2007;66:337–45. doi: 10.1097/nen.0b013e3180537147. [DOI] [PubMed] [Google Scholar]
- 13.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental mri white matter signal hyperintensities. Neurology. 1993;43:1683–9. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 14.Black S, Gao F, Bilbao J. Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 15.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–8. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 16.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan Study. Stroke. 2008;39:2712–9. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 18.Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr, Newman AB. Coronary artery calcium: Associations with brain magnetic resonance imaging abnormalities and cognitive status. J Am Geriatr Soc. 2005;53:609–15. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- 19.Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, mri indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke. 2009;40:1590–6. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manolio TA, Burke GL, O'Leary DH, et al. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19:356–65. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 21.Kaden JJ, Dempfle CE, Grobholz R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol. 2005;14:80–7. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Kaden JJ, Eckert JP, Poerner T, et al. Prevalence of atherosclerosis of the coronary and extracranial cerebral arteries in patients undergoing aortic valve replacement for calcified stenosis. J Heart Valve Dis. 2006;15:165–8. [PubMed] [Google Scholar]
- 23.Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–22. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- 24.Price CC, Jefferson AL, Merino JG, Heilman KM, Libon DJ. Subcortical vascular dementia: integrating neuropsychological and neuroradiologic data. Neurology. 2005;65:376–82. doi: 10.1212/01.wnl.0000168877.06011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craver JM, Weintraub WS, Jones EL, Guyton RA, Hatcher CR., Jr Predictors of mortality, complications, and length of stay in aortic valve replacement for aortic stenosis. Circulation. 1988;78(3 Pt 2):I85–90. [PubMed] [Google Scholar]
- 26.Wolman RL, Nussmeier NA, Aggarwal A, et al. Cerebral injury after cardiac surgery: identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30:514–22. doi: 10.1161/01.str.30.3.514. [DOI] [PubMed] [Google Scholar]
- 27.Morell VO, Daggett WM, Pezzella AT, Moran JM, Bitran D. Aortic stenosis in the elderly: result of aortic valve replacement. J Cardiovasc Surg (Torino) 1996;37:33–5. [PubMed] [Google Scholar]
- 28.Stolz E, Gerriets T, Kluge A, Klövekorn WP, Kaps M, Bachmann G. Diffusion-weighted magnetic resonance imaging and neurobiochemical markers after aortic valve replacement: Implications for future neuroprotective trials? Stroke. 2004;35:888–92. doi: 10.1161/01.STR.0000120306.82787.5A. [DOI] [PubMed] [Google Scholar]
- 29.Chikwe J, Croft LB, Goldstone AB, et al. Comparison of the results of aortic valve replacement with or without concomitant coronary artery bypass grafting in patients with left ventricular ejection fraction < or =30% versus patients with ejection fraction >30% Am J Cardiol. 2009;104:1717–21. doi: 10.1016/j.amjcard.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 30.Goto T, Baba T, Matsuyama K, Honma K, Ura M, Koshiji T. Aortic atherosclerosis and postoperative neurological dys-function in elderly coronary surgical patients. Ann Thorac Surg. 2003;75:1912–8. doi: 10.1016/s0003-4975(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 31.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–13. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs MA, Mitsias P, Soltanian-Zadeh H, et al. Multiparametric mri tissue characterization in clinical stroke with correlation to clinical outcome: part 2. Stroke. 2001;32:950–7. doi: 10.1161/01.str.32.4.950. [DOI] [PubMed] [Google Scholar]
- 33.Herskovits EH, Bryan RN, Yang F. Automated Bayesian segmentation of microvascular white-matter lesions in the ACCORD-MIND study. Adv Med Sci. 2008;53:182–90. doi: 10.2478/v10039-008-0039-3. [DOI] [PubMed] [Google Scholar]
- 34.Mohler ER., 3rd Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–402. doi: 10.1016/j.amjcard.2004.08.013. A6. [DOI] [PubMed] [Google Scholar]
- 35.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–7. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 36.Hogue CW, Lillie R, Hershey T, et al. Gender influence on cognitive function after cardiac operation. Ann Thorac Surg. 2003;76:1119–25. doi: 10.1016/s0003-4975(03)00817-8. [DOI] [PubMed] [Google Scholar]
- 37.Alamanni F, Pompilio G, Polvani G, et al. Off-pump redo coronary artery bypass grafting: Technical aspects and early results. Heart Surg Forum. 2002;5(Suppl 4):S432–44. [PubMed] [Google Scholar]