Abstract
Background
Intraoperative radiofrequency ablation (IRFA) of liver metastases can be used to treat patients with complex tumours that are unsuitable for parenchymal resection alone. This systematic review assesses the frequency, patterns and severity of complications associated with this procedure.
Methods
We carried out a bibliographic search on MEDLINE focused on IRFA for liver metastases, excluding hepatocarcinomas, and on intraoperative use, excluding percutaneous application.
Results
Thirty papers published between 1999 and 2007 were analysed. They covered a total of 2822 patients and 1755 IRFA procedures. The indications and techniques for IRFA differ from those for percutaneous treatment, as do associated results and complications. Specific complications associated with IRFA, such as liver abscesses, biliary stenoses and vascular thromboses, are directly correlated with the indications and associated procedures. Published results should be interpreted with caution as IRFA can be used alone or combined with parenchymal resection.
Conclusions
Specific complications related to IRFA are rare, especially for lesions of <35 mm in size located far from a main biliary duct, when additional septic procedures are not used. A lesion-by-lesion approach based on the benefit : risk ratio should therefore be used in the process of making surgical decisions. Combining resection with IRFA leads to higher morbidity, especially in difficult patients with numerous bilateral lesions, but may be necessary to achieve R0 (microscopically negative margins) outcomes.
Keywords: colorectal metastases < liver, non-colorectal metastases < liver, liver, resection < liver, radiological intervention/treatment < liver
Introduction
Intraoperative radiofrequency ablation (IRFA) of liver metastases was first reported by Curley et al.1 in 1999 and Elias et al.2 in 2000. Promising initial results appeared to herald a new era in modern liver surgery.3 However, unexpectedly, most of the teams who launched this technique in the late 1990s have since decided to forego IRFA4–7 because of disagreement between hepatopancreatobiliary surgeons and radiologists. Radiologists have challenged the treatment of liver metastases by parenchymal resection only and proposed using percutaneous RFA (PRFA) as a minimally invasive alternative. In response, surgeons have simply decided to sacrifice both IRFA and PRFA at the same time. The results of PRFA are inferior to those of IRFA, especially in terms of local recurrence rates, and the presentation of combined outcomes for both techniques resulted in the exclusion of both.8 In the same manner, Abdalla et al.4 compared RFA with surgery, but allocated the easiest-to-treat lesions with wide margins to resection and the difficult-to-treat lesions to ablation, with unsurprising results: RFA, even via the intraoperative route, offered inferior results and hence its abandonment was recommended. Subsequently, and surprisingly, some published material9 declared the absence of need for multicentre prospective studies, which, with less biased methodology, might have produced different results. Curiously enough, in this same paper, Curley acknowledged that he continued to treat almost 10% of his patients with IRFA, indicating that perhaps the technique should not be discarded just yet.
In the last decade, efforts have focused mostly on learning about this treatment and most reports have been retrospective and have originated from expert centres. Data from two prospective studies, the CLOCC (Chemotherapy + Local Ablation Versus Chemotherapy for unresectable liver metastasis) Study10 and the ARF2003 Study,11 are to be published in 2010. Preliminary results from these are positive, despite a 22% lack of enrolment in the CLOCC Study. As a consequence, IRFA may enter an era of rejuvenation, in which its place in the surgical armamentarium is reserved on the strength of scientific evidence. Before we can embrace this new era, it is necessary to analyse the complications associated with IRFA by examining the available literature, mainly to explain how the best benefit : risk ratio can be determined for an individual patient. This review of the available IRFA literature covers 30 articles and identifies the frequencies, patterns and severity of complications associated with this procedure.
Materials and methods
Search strategy and selection criteria
A bibliographic search was conducted on MEDLINE. Mesh requests were: ‘liver neoplasms’‘catheter ablation’, ‘laparotomy’ and ‘hepatectomy’. Additional keywords were added: ‘intraoperative’, ‘coeliotomy’ and ‘open’. The exact request was: (‘catheter ablation’[Mesh] AND ‘liver neoplasms’[Mesh]) AND (‘hepatectomy’[Mesh] OR ‘laparotomy’[Mesh] OR ‘intraoperative’ OR ‘open’ OR ‘coeliotomy’).
The search was run in December 2008. Articles dealing exclusively with PRFA or with hepatocellular carcinoma (HCC) were excluded. Articles in all languages were accepted. Papers reporting complications of IRFA were selected and data were collected in a database (Microsoft Access 2003©). The following variables were recorded for each article.
Article characteristics
Title.
First author.
Year of publication.
Country of origin.
Population to be studied
Number of patients.
Number of IRFA procedures.
Average age of patients.
Frequency of cirrhotic patients.
Description of the diseases to be treated
Ratio of HCCs : metastases treated.
Size and number of lesions.
Description of complications
Mortality.
Liver failure.
-
Infections:
intra- and perihepatic abscesses;
peritonitis, and
abdominal wall abscesses.
-
Vascular complications:
haemorrhages and vascular injuries;
haematomas, and
portal thromboses.
-
Biliary complications:
bile collections;
bile leakages;
biliary stenoses, and
cholangitis.
-
Collateral lesions (or visceral lesions):
burns (biliary structures, digestive tract, diaphragm …);
injuries, and
pneumothorax.
Skin burns.
Pleural collections.
The terminology used was that recommended by the Interventional Radiology Technology Assessment Committee and the International Working Group on Image-Guided Tumour Ablation12 updated by the Society of Interventional Radiology.13
Results
Our search retrieved 493 articles (Fig. 1). After exclusions for percutaneous-only treatment and HCCs, we selected 30 papers published between 1999 and 2007 (Table 1).1,2,14–41 These papers covered a total of 2822 patients and 1755 IRFA procedures. In 961 of these procedures (54.8%), the lesions were treated by resection combined with IRFA. Complications were reported for series varying from five to 382 procedures. Only one series was a prospective multicentre study.25 Eighteen articles were based on fewer than 50 patients.
Figure 1.
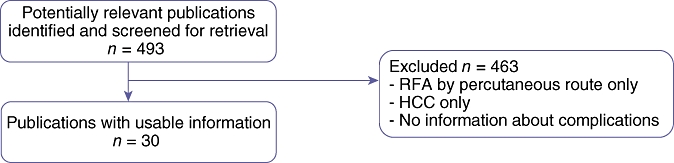
Details of methods used to select articles for the review of complications after intraoperative radiofrequency ablation of liver metastases. RFA, radiofrequency ablation; HCC, hepatocellular carcinoma
Table 1.
Publications retrieved after a literature search for articles on intraoperative radiofrequency ablation of liver metastases
| Author(s) | IRFA, n | HCC | Combined resection + IRFA | Mortality | Morbidity |
|---|---|---|---|---|---|
| Pearson et al. (1999)32 | 92 | 36.9% | 19.5% | 0% | 3.3% |
| Curley et al. (1999)1 | 92 | 23.9% | 19.5% | 0% | 3.2% |
| Jiao et al. (1999)26 | 30 | 20% | 43.3% | 3.3% | 10% |
| Wood et al. (2000)41 | 39 | – | 51.2% | 5% | 5% |
| Elias et al. (2000)2 | 21 | 0% | 80.9% | 0% | 47.6% |
| Wong et al. (2001)40 | 39 | 5% | 48.7% | 0% | 20.5% |
| Risse et al. (2001)34 | 19 | 31.5% | 52.6% | 5.2% | 26.3% |
| Stippel et al. (2002)37 | 23 | 0% | 100% | 0% | 17.3% |
| Stella et al. (2002)36 | 21 | 19% | 61.9% | 0% | 14.3% |
| Choy et al. (2002)17 | 12 | – | 75% | 0% | 33.3% |
| Pawlik et al. (2003)31 | 172 | 2.9% | 100% | 2.3% | 19.8% |
| de Baere et al. (2003)19 | 124 | – | 95.1% | 2.4% | 9.6% |
| Bleicher et al. (2003)15 | 66 | – | 100% | 0% | 12.1% |
| Oshowo et al. (2003)30 | 16 | 0% | 62.5% | 0% | 12.5% |
| Curley et al. (2004)18 | 382 | 14.6% | 48.1% | 0.52% | 8.6% |
| Poon et al. (2004)33 | 56 | – | 0% | 1.8% | 16% |
| Tepel et al. (2004)38 | 26 | – | 42.3% | 0% | 27% |
| Basdanis et al. (2004)14 | 18 | 0% | 11.1% | 0% | 0% |
| Jansen et al. (2005)25 | 108 | 10.6% | 34.2% | 1.8% | 20.3% |
| Elias et al. (2005)20 | 63 | 0% | 100% | 0% | 27% |
| Navarra et al. (2005)29 | 53 | 0% | 22.6% | 1.8% | 14% |
| Ritz et al. (2006)35 | 6 | 0% | 0% | 0% | 0% |
| Hildebrand et al. (2006)22 | 68 | 5.8% | 52.9% | 2.9% | 7.3% |
| Hildebrand et al. (2006)23 | 64 | – | 56.3% | 0% | 12.5% |
| Chhabra et al. (2006)16 | 27 | 44.4% | 0% | 0% | 3.7% |
| Low et al. (2006)28 | 13 | – | 0% | 0% | 0% |
| Gomez et al. (2006)21 | 5 | – | 0% | 0% | 0% |
| Topal et al. (2007)39 | 49 | 20.4% | 24.5% | 2% | 34.7% |
| Hubert et al. (2007)24 | 32 | 12.5% | 72% | 0% | 18% |
| Kornprat et al. (2007)27 | 19 | 0% | 100% | 5.2% | 31.5% |
IRFA, intraoperative radiofrequency ablation; HCC, hepatocellular carcinoma
In five papers15,20,27,31,37 involving 450 patients and 343 IRFA procedures, IRFA was always combined with resection. In five other papers16,21,28,33,35 (226 patients, 107 IRFA), no resections were performed in combination with IRFA. Only four papers reported frequencies of patients with cirrhosis (varying from 13.1% to 33.3%) and 22 articles reported frequencies of HCC, varying from none to 44.4%. The average lesion size (reported in 13 articles) ranged from 13 mm to 56 mm. Thirteen articles specified the mean number of lesions to be treated, which ranged from one to 6.2 lesions per patient.
Frequency of complications
Table 2 reports mortality and morbidity rates, as well as frequencies and types of complications, depending on whether or not IRFA was associated with resection. Table 3 presents the same results according to the size of the series.
Table 2.
Morbidity rates in intraoperative radiofrequency ablation with or without resection
| All series, mean (min–max) | IRFA with resections, mean (min–max) | IRFA alone, mean (min–max) | |
|---|---|---|---|
| Number of procedures, n | 1755 | 343 | 107 |
| Mortality | 1.2% (0–5.2%) | 1.5% (0–5.2%) | 0.9% (0–1.8%) |
| Morbidity | 13.6% (0–47.6%) | 20.1% (12.1–31.5%) | 9.3% (0–16%) |
| Wound infection | 2.8% (0–13.4%) | 3.5% (1.7–13.4%) | 0.9% (0–1.8%) |
| Biliary complications | 1.7% (0–9.5%) | 3.8% (0–7.9%) | 0.9% (0–1.8%) |
| Pleural effusion | 1.4% (0–6.2%) | 0.3% (0–0.6%) | 1.9% (0–3.6%) |
| Liver failure | 1.4% (0–14.2%) | 2.6% (0–7.9%) | 0.9% (0–1.8%) |
| Vascular complications | 1.3% (0–5.3%) | 0.6% (0–1.6%) | 0.0% (0–0%) |
| Skin burns | 0.5% (0–6.2%) | 0.0% (0%) | 0.9% (0–1.8%) |
| Collateral lesions | 0.2% (0–1.5%) | 0.6% (0–1.5%) | 0.0% (0%) |
Table 3.
Morbidity rates in intraoperative radiofrequency ablation (IRFA) by size of series
| All series, mean (min–max) | Series with > 50 patients, mean (min–max) | Series with ≤ 50 patients, mean (min–max) | |
|---|---|---|---|
| Number of procedures, n | 1755 | 1340 | 415 |
| Mortality | 1.2% (0–5.2%) | 1.1% (0–2.9%) | 1.4% (0–5.2%) |
| Morbidity | 13.6% (0–47.6%) | 12.0% (2–27%) | 18.8% (0–47.6%) |
| Wound infection | 2.8% (0–13.4%) | 2.7% (0–6.5%) | 3.1% (0–13.4%) |
| Biliary complications | 1.7% (0–9.5%) | 1.3% (0–7.9%) | 2.7% (0–9.5%) |
| Pleural effusion | 1.4% (0–6.2%) | 1.6% (0–6.2%) | 0.7% (0–5.1%) |
| Liver failure | 1.4% (0–14.2%) | 1.3% (0–7.9%) | 1.4% (0–14.2%) |
| Vascular complications | 1.3% (0–5.3%) | 1.4% (0–4.6%) | 0.7% (0–5.3%) |
| Skin burns | 0.5% (0–6.2%) | 0.4% (0–4%) | 0.7% (0–6.2%) |
| Collateral injuries | 0.2% (0–1.5%) | 0.3% (0–1.5%) | 0.0% (0–0%) |
Mortality
A total of 21 deaths were reported in 11 series,18,19,23,25–27,29,31,33,39,41 with overall mortality varying from 0% to 5.2%. Four deaths were related to cirrhosis. Eleven occurred in patients undergoing resections, eight of which were major hepatectomies.
Causes of death are summarized in Fig. 2. Eight deaths were related to liver failure,18,19,25,31,34,41 four of which were subsequent to major hepatectomy with IRFA on the remnant liver for bilobar disease.18,19,31,41 Five deaths were caused by myocardial infarct;18,23,39,41 one of these related to a carcinoid crisis41 and another to a haemorrhage.18 Four deaths resulted from portal thrombosis,19,25,34 three of which occurred in cirrhotic patients.19,34 One of these patients had been treated by IRFA alone.34 Four deaths were related to septic complications; two of these referred to pulmonary infections,26,31 one to infection of the ascites25 and one to multiple deep abscesses.25 Lastly, three deaths were reported after postoperative haemorrhaging;18,25,31 one was caused by liver failure after an intrahepatic haematoma in a cirrhotic setting,25 one resulted from myocardial infarction following a haemorrhage in a large metastasis treated by IRFA,18 and one patient was treated by major hepatectomy and two IRFA sessions and died of cardiac arrest after postoperative bleeding.31
Figure 2.
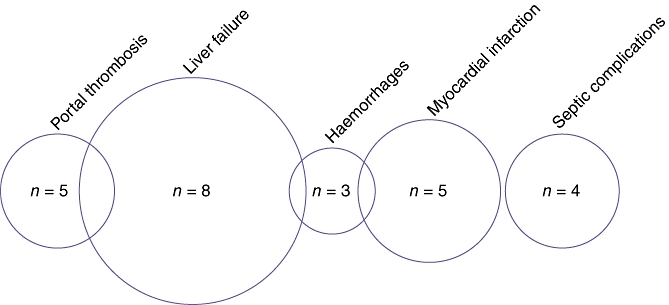
Causes of death reported in the literature after intraoperative radiofrequency ablation of liver metastases
Infections
Abdominal infections were reported in 49 patients in 21 series.9,15,17,18,20,22–27,29,31–34,36–38,40,41 Diagnosis of infection was delayed by up to 5 months.25,41 Seventeen liver abscesses were reported, of which one was fatal25 and 11 were related to IRFA. Only one case of biliary digestive anastomosis was observed.22 Ten cases of perihepatic abscesses at resection sites were reported. Twelve were following digestive system-associated procedures. These abscesses were treated by percutaneous drainage and antibiotics. One patient needed re-operation and died from septic shock.25 Seven cases25,33,34,38,40 of wound infection were reported; two were re-operated.25 Lastly, one case of peritonitis after infection of the ascites was reported and was fatal.25
Biliary complications
Twenty-five early (≤30 postoperative days) and 14 delayed (sometimes for >4 months18) biliary complications were reported in 10 series.15,17,18,20,25,31,33,36,38,40 Twelve biliary leakages occurred, 10 of which were early. Six occurred in resection combined with IRFA. One early leakage was caused by a prophylactic cholecystectomy, but two delayed leakages were associated with a biliary stenosis.18 Fifteen intrahepatic bile collections were described, one of which induced duodenal compression. One article18 gave details of the treatment of eight biliomas: all eight were drained percutaneously and two recurred after drain clamping. Two were related to biliary stenoses and were treated by intrahepatic stenting; the other six patients underwent endoscopic sphincterotomy. Eleven biliary stenoses associated with jaundice and biliary dilatation were reported, of which five were early. These were complicated by biliomas,18,25 biliary leakage25 and cholangitis.37 In their prospective study, Jansen et al.25 did not observe a correlation between central or peripheric localization of the tumour and the frequency of biliary complications, by contrast with Curley et al.18 Elias et al.20 reported the efficiency of biliary cooling in five patients, which prevented biliary destruction in paraportal lesions.
Liver failure
Liver failure was reported in 24 patients in 11 articles2,15,18–20,25,29,31,33,34,41 and was fatal in eight patients. Fourteen liver failures occurred after IRFA combined with major resection. Six liver failures occurred in cirrhotic patients; three of these failures occurred after IRFA alone.18,34 Two liver failures were subsequent to portal thrombosis.34
Vascular complications
Different types of vascular complication were described in 11 articles in a total of 22 patients.9,18,19,22,23,25,26,31,32,34,38 Associated procedures such as cholecystectomy or colectomy induced six haemorrhages, two of which were fatal31,25 and one required re-operation after prophylactic cholecystectomy.38 Three haemorrhages from the needle track were treated during surgery18 by compression. In three cirrhotic patients, haemorrhage occurred in the necrosis induced by the IRFA; one patient died as a result.25 Treatment of two juxta-portal lesions induced haemorrhages from arterial injuries.25,26 In one patient, an arterio-portal fistula appeared in an area of necrosis 6 weeks later and was treated by a transfemoral embolization.18 Similarly, a false aneurysm occurred in one patient 6 months after IRFA and led to a haemorrhage.25 Five portal thromboses were reported,19,25,34 four of which were complete and fatal. Three of these occurred in cirrhotic patients treated with Pringle vascular occlusion.19,34
Skin burns
Eight dispersive pad skin burns were reported in four articles.16,19,30,38 Skin burns occurred when RFA ran for >30 min on high power and within large and multiple skin pads. One skin burn occurred in a patient with bilateral hip prostheses.30 One third-degree skin burn required surgical treatment.38
Visceral damage
Two instances of thermal gastric damage15,18 and one of acute cholecystitis near the gallbladder25 were observed after IRFA during surgery and were treated immediately.
Discussion
On the whole, the methodologies used in the articles retrieved by the MEDLINE search were of a low scientific standard in terms of evidence-based research. All but one reported retrospective experiences and most involved only a single centre. The methodology used to report and describe complications was not standardized and mixed low and high grades of complication severity at different durations of follow-up. Consequently, the data obtained cannot be compared with those reported in studies that define complications according to classifications such as that described by Dindo et al.43 In addition, the populations studied were heterogeneous and it is thus impossible to calculate a confidence interval for the means. Nevertheless, the different types of complication reported are relatively similar across studies, which allows us to assume a common general profile of the morbidity associated with this technique.
Comparison with PRFA
Percutaneous RFA is a recommended treatment for HCC in cirrhotic patients. This procedure differs from IRFA in terms of indications, techniques, results and morbidity. Table 4 summarizes the different morbidities associated with IRFA and PRFA. Results for PRFA were extracted from the literature review conducted by Mulier et al., which reported 3670 RFA procedures, 2898 (78.9%) of which were PRFAs.44 Mortality and morbidity rates after PRFA were lower than after IRFA (0.5% and 8.9% vs. 1.2% and 13.6%, respectively) when all patients receiving IRFA were considered. However, when mortality and morbidity rates in patients who received PRFA were compared with those in patients who received only IRFA, thus excluding the effects of surgery, mortality and morbidity rates were similar (0.5% and 8.9% vs. 0.9% and 9.3%, respectively).
Table 4.
Comparison of morbidity rates in intraoperative radiofrequency ablation (IRFA) and percutaneous radiofrequency ablation (PRFA)
| IRFA, mean (min–max) | IRFA without resection, mean (min–max) | PRFA, meana | |
|---|---|---|---|
| Number of procedures, n | 1755 | 107 | 3670 |
| Mortality | 1.2% (0–5.2%) | 0.9% (0–1.8%) | 0.5% |
| Morbidity | 13.6% (0–47.6%) | 9.3% (0–16%) | 8.9% |
| Wound infection | 2.8% (0–13.4%) | 0.9% (0–1.8%) | 1.1% |
| Biliary complications | 1.7% (0–9.5%) | 0.9% (0–1.8%) | 1.0% |
| Liver failure | 1.4% (0–14.2%) | 0.9% (0–1.8%) | 0.8% |
| Vascular complications | 1.3% (0–5.3%) | 0.0% (0–0%) | 2.2% |
| Skin burns | 0.5% (0–6.2%) | 0.9% (0–1.8%) | 0.6% |
| Visceral damage | 0.2% (0–1.5%) | 0.0% (0–0%) | 0.5% |
Results of PRFA were extracted from the review by Mulier et al.44 Minimum and maximum data were not available for PRFA
The open procedure not only allows complete abdominal exploration, but also enables visceral protection or immediate reparation of visceral damage. However, Mulier et al. reported two cases of gastric wall burns after IRFA.44 These complications were diagnosed and treated successfully during the procedures. Only one case of cholecystitis occurring after IRFA near the gallbladder was reported. Prophylactic cholecystectomy may prevent this complication, but specific vascular and biliary complications may occur. As a result, systematic prophylactic cholecystectomy cannot be recommended, but should be performed only for IRFA near the gallbladder. Finally, specific complications, such as wound infections, evisceration and abdominal dehiscence, are associated with laparotomy.
Comparison with hepatectomy
The morbidity of hepatectomy depends on the extent and complexity of the hepatic resection. Intraoperative RFA as a stand-alone treatment is indicated for unresectable tumours in patients in whom major hepatectomy would leave a low level of functional hepatic reserve. Mortality and morbidity rates in major hepatic resection are 0–5% and 20–50%, respectively.45 Rates of liver failure after major hepatectomy preceded by portal embolization are 4–10%46,47 vs. 2.6% after IRFA combined with hepatic resection. Pawlik et al. reported mortality of 2.3% and morbidity of 19.8% in patients treated by resection and combined IRFA, and estimate their results to be comparable with those of resection alone.31 Morbidity rates after major hepatic resection and IRFA combined with hepatic resection are comparable, even if IRFA is indicated in tumours unresectable by hepatectomy alone.48Table 5 summarizes differences in morbidity in patients undergoing hepatectomy alone and hepatectomy combined with IRFA, illustrating the great similarity between the techniques.
Table 5.
Comparison of morbidity rates after intraoperative radiofrequency ablation (IRFA) with those after hepatectomy
| All series, mean (min–max) | IRFA combined with resection, mean (min–max) | Hepatectomies, min–maxa | |
|---|---|---|---|
| Number of procedures, n | 1755 | 343 | NA |
| Mortality | 1.2% (0–5.2%) | 1.5% (0–5.2%) | 0–5% |
| Morbidity | 13.6% (0–47.6%) | 20.1% (12.1–31.5%) | 20–50% |
| Wound infection | 2.8% (0–13.4%) | 3.5% (1.7–13.4%) | 1–9% |
| Biliary complications | 1.7% (0–9.5%) | 3.8% (0–7.9%) | 3–4% |
| Pleural effusion | 1.4% (0–6.2%) | 0.3% (0–0.6%) | 5–10% |
| Liver failure | 1.4% (0–14.2%) | 2.6% (0–7.9%) | 1–5% |
| Vascular complications | 1.3% (0–5.3%) | 0.6% (0–1.6%) | 1–3% |
| Skin burns | 0.5% (0–6.2%) | 0.0% (0–0%) | |
| Visceral damage | 0.2% (0–1.5%) | 0.6% (0–1.5%) | |
Results on hepatectomies were extracted from a review by Fong,45 in which only extreme values are given
NA, not applicable
Specific complications of IRFA
Some complications are specific to RFA and thus can also be induced by IRFA. Hepatic abscesses are caused by infection of the necrotic tissue in the RFA site. They appear after an asymptomatic period ranging from 8 days to 5 months.19,41,49 Imaging examinations detect a lot of gas in the necrotic zone, which indicates an abscess. However, the presence of a low quantity of gas is usual in the necrotic zone during the month after RFA.50 Despite the fact that, in our experience, an additional septic procedure such as colectomy has a major role in the occurrence of such a complication, this has never been reported in the literature. It may be that special prophylaxis antibiotics are indicated in such cases. Diabetes mellitus and bilioenteric anastomosis are recognized risk factors for hepatic abscess after liver ablation procedures and the risk for abscess is estimated to be 40–50% in the presence of bilioenteric anastomosis.19,51 Biliary colonizations (antecedents of sphincterotomy, biliary stent, biliary stenoses, etc.) are other possible risk factors.44 Such abscesses should be treated by antibiotics and percutaneous drainage, and sometimes by re-operation. Only symptomatic biliary leakage such as infection or bowel compression should be treated by percutaneous drainage. In cases of associated biliary stenosis, intrahepatic stenting or endoscopic sphincterotomy should be considered.
Biliary stenoses appear after the fibrous healing of biliary tract thermal damage. They are diagnosed by imaging survey. Many of these biliary stenoses are asymptomatic and do not require treatment.52 Only biliary stenoses that occur near the major biliary tract after RFA are symptomatic18,44 and can be treated by intrahepatic stenting or endoscopic sphincterotomy. Vascular occlusions do not affect the frequency of biliary complications, although experimental studies have suggested that vessels protect the biliary tract by a ‘heat sink’ effect.53 Biliary refrigeration may protect against biliary complications, but the oncological impact remains to be evaluated.54
Intraoperative bleeding from the needle track can be treated during surgery by manual compression. Postoperative haemorrhages sometimes require emergency re-operation or transfemoral embolization, and may lead to death if care is not immediately possible.
Vascular thromboses after IRFA are caused by thermal endothelial damage. Experimental studies suggest that vascular occlusion increases the risk for vascular thrombosis, especially when the distance is <5 mm.55 Mulier et al. confirmed this hypothesis in their literature review.44 Portal thromboses are more frequent in cirrhotic patients and may be complicated by liver failure and fatal issue.19 Thrombosis of veins of <3 mm in diameter is common after IRFA. It is asymptomatic and spontaneous regression is often observed after 2 months.19
Skin burns occur when the dispersion surface is inadequate for the radiofrequency power. Goldberg et al. recommend that multiple (e.g. four) large dispersive pads should be placed at equal distances of 50 cm from the RFA site to prevent skin burns.56
Conclusions
The past 10 years have represented a period of learning for surgeons who deal with liver metastases with the aim of treating more patients by combining IRFA with resection. The benefit : risk ratio is now well known and surgeons have access to the knowledge they need to make more informed choices about whether to resect, ablate or renounce treatment on a lesion-by-lesion basis. Surgeons who are skilled in intraoperative ultrasound diagnosis and guidance are now not only able to choose whether or not to perform surgery, but are also able to perform IRFA and do not need to involve a radiologist.
Specific complications related to IRFA are rare, especially if the lesion is <35 mm in diameter and is located far from a main biliary duct and no additional septic procedures are used. The surgeon can decide to ablate a lesion in a more difficult situation, but this carries greater risk. Combining resection with IRFA leads to higher morbidity, especially in difficult patients with numerous bilateral lesions, but this may be necessary to achieve R0 (microscopically negative) resection margins. The final results of the CLOCC Trial and the ARF2003 Study will shortly become available and will help to define the proper place of IRFA in the surgical armamentarium.
Acknowledgments
We gratefully acknowledge Pippa McKelvie-Sebileau for her help with the English-language manuscript. This study was funded by the Institut Bergonié, Bordeaux, France.
Conflicts of interest
None declared.
References
- 1.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias D, Goharin A, El OA, Taieb J, Duvillard P, Lasser P, et al. Usefulness of intraoperative radiofrequency thermoablation of liver tumours associated or not with hepatectomy. Eur J Surg Oncol. 2000;26:763–769. doi: 10.1053/ejso.2000.1000. [DOI] [PubMed] [Google Scholar]
- 3.Elias D. [Radiofrequency: storm looming over hepatic surgery.] Ann Chir. 2000;125:815–817. doi: 10.1016/s0003-3944(00)00013-4. [DOI] [PubMed] [Google Scholar]
- 4.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Data validation in an economic evaluation of surgery for colon cancer. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdalla EK, Vauthey JN. Colorectal metastases: resect or ablate? Ann Surg Oncol. 2006;13:602–603. doi: 10.1245/ASO.2006.09.920. [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–466. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evrard S, Mathoulin-Pélissier S. Controversies between surgical and percutaneous radiofrequency ablation. Eur J Surg Oncol. 2006;32:3–5. doi: 10.1016/j.ejso.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Curley SA. Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol. 2008;15:11–13. doi: 10.1245/s10434-007-9668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruers T, van Coevorden F, Pierie J, Borel Rinkes I, Punt C, Ledermann J, et al. Radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM): interim results of a randomized phase II study of the EORTC-NCRI CCSG-ALM Intergroup 40004 (CLOCC) J Clin Oncol. 2008;26(Suppl 20):4012. [Google Scholar]
- 11.Bécouarn Y, Mathoulin-Pélissier S, Rivoire M, Ayav A, Arnaud JP, Sa Cunha A, et al. Efficacy of intraoperative radiofrequency ablation (IRFA) combined or not with resection to treat unresectable colorectal metastases, with or without preoperative chemotherapy. The ARF2003 Study (NTC 00210106): preliminary results. J Clin Oncol. 2009;27(Suppl 15):4095. [Google Scholar]
- 12.Goldberg SN, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumour ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, et al. Image-guided tumour ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 14.Basdanis G, Michalopoulos A, Papadopoulos V, Tzeveleki I, Efthimiadis C, Kosmidis C, et al. Clinical short-term results of radiofrequency ablation in patients with liver metastases from colorectal cancer. Tech Coloproctol. 2004;8(Suppl 1):187–189. doi: 10.1007/s10151-004-0152-7. [DOI] [PubMed] [Google Scholar]
- 15.Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumours: lessons learned. Ann Surg Oncol. 2003;10:52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Chhabra DG, Shah RC, Parikh V, Jagannath P. Radiofrequency ablation of liver tumours: experience with open and percutaneous approach. Indian J Gastroenterol. 2006;25:66–70. [PubMed] [Google Scholar]
- 17.Choy PY, Koea J, McCall J, Holden A, Osbourne M. The role of radiofrequency ablation in the treatment of primary and metastatic tumours of the liver: initial lessons learned. N Z Med J. 2002;115:U128. [PubMed] [Google Scholar]
- 18.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumours in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Baere T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumours. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 20.Elias D, Baton O, Sideris L, Boige V, Malka D, Liberale G, et al. Hepatectomy plus intraoperative radiofrequency ablation and chemotherapy to treat technically unresectable multiple colorectal liver metastases. J Surg Oncol. 2005;90:36–42. doi: 10.1002/jso.20237. [DOI] [PubMed] [Google Scholar]
- 21.Gomez SS, Gomez RC, Mancenido MN, Martin CS, Carrion AG, Olveira MA, et al. Radiofrequency ablation for hepatocellular carcinoma and liver metastases: experience in Hospital La Paz. Clin Transl Oncol. 2006;8:688–691. doi: 10.1007/s12094-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch HP, et al. Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome. Eur J Surg Oncol. 2006;32:430–434. doi: 10.1016/j.ejso.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand P, Kleemann M, Roblick UJ, Mirow L, Birth M, Leibecke T, et al. Radiofrequency ablation of unresectable primary and secondary liver tumours: results in 88 patients. Langenbecks Arch Surg. 2006;391:118–123. doi: 10.1007/s00423-006-0024-x. [DOI] [PubMed] [Google Scholar]
- 24.Hubert C, Gras J, Goffette P, Grajeda JM, Van Beers BE, Laurence A, et al. Percutaneous and surgical radiofrequency ablation of liver malignancies: a single institutional experience. Acta Gastroenterol Belg. 2007;70:188–194. [PubMed] [Google Scholar]
- 25.Jansen MC, van Duijnhoven FH, van Hillegersberg R, Rijken A, van Coevorden F, van der Sijp J, et al. Adverse effects of radiofrequency ablation of liver tumours in the Netherlands. Br J Surg. 2005;92:1248–1254. doi: 10.1002/bjs.5059. [DOI] [PubMed] [Google Scholar]
- 26.Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumours. Am J Surg. 1999;177:303–306. doi: 10.1016/s0002-9610(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 27.Kornprat P, Jarnagin WR, DeMatteo RP, Fong Y, Blumgart LH, D’Angelica M. Role of intraoperative thermoablation combined with resection in the treatment of hepatic metastasis from colorectal cancer. Arch Surg. 2007;142:1087–1092. doi: 10.1001/archsurg.142.11.1087. [DOI] [PubMed] [Google Scholar]
- 28.Low SC, Lo RH, Lau TN, Ooi LL, Ho CK, Tan BS, et al. Image-guided radiofrequency ablation of liver malignancies: experience at Singapore General Hospital. Ann Acad Med Singapore. 2006;35:851–857. [PubMed] [Google Scholar]
- 29.Navarra G, Ayav A, Weber JC, Jensen SL, Smadga C, Nicholls JP, et al. Short- and longterm results of intraoperative radiofrequency ablation of liver metastases. Int J Colorectal Dis. 2005;20:521–528. doi: 10.1007/s00384-005-0743-4. [DOI] [PubMed] [Google Scholar]
- 30.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 31.Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10:1059–1069. doi: 10.1245/ASO.2003.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–599. doi: 10.1016/s0002-9610(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation of liver tumours: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Risse O, Sengel C, Penillon S, Arvieux C, Voirin D, Letoublon C. [Radiofrequency ablation of malignant hepatic tumours. Preliminary experience a propos of 25 cases.] Ann Chir. 2001;126:118–126. doi: 10.1016/s0003-3944(00)00474-0. [DOI] [PubMed] [Google Scholar]
- 35.Ritz JP, Lehmann KS, Reissfelder C, Albrecht T, Frericks B, Zurbuchen U, et al. Bipolar radiofrequency ablation of liver metastases during laparotomy. First clinical experiences with a new multipolar ablation concept. Int J Colorectal Dis. 2006;21:25–32. doi: 10.1007/s00384-005-0781-y. [DOI] [PubMed] [Google Scholar]
- 36.Stella M, Minuto MN, Pasqualini M, Percivale A, Profeti A, Serafini G, et al. [Intraoperative use of radiofrequency thermoablation of liver tumours: considerations on indications and related therapeutic aspects.] Ann Ital Chir. 2002;73:511–516. [PubMed] [Google Scholar]
- 37.Stippel DL, Bohm S, Beckurts KT, Brochhagen HG, Holscher AH. Intraoperative radiofrequency ablation using a 3D navigation tool for treatment of colorectal liver metastases. Onkologie. 2002;25:346–350. doi: 10.1159/000066052. [DOI] [PubMed] [Google Scholar]
- 38.Tepel J, Hinz S, Klomp HJ, Kapischke M, Kremer B. Intraoperative radiofrequency ablation (RFA) for irresectable liver malignancies. Eur J Surg Oncol. 2004;30:551–555. doi: 10.1016/j.ejso.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Topal B, Hompes D, Aerts R, Fieuws S, Thijs M, Penninckx F. Morbidity and mortality of laparoscopic vs. open radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol. 2007;33:603–607. doi: 10.1016/j.ejso.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Wong SL, Edwards MJ, Chao C, Simpson D, McMasters KM. Radiofrequency ablation for unresectable hepatic tumours. Am J Surg. 2001;182:552–557. doi: 10.1016/s0002-9610(01)00813-3. [DOI] [PubMed] [Google Scholar]
- 41.Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumours: indications, limitations, and complications. Ann Surg Oncol. 2000;7:593–600. doi: 10.1007/BF02725339. [DOI] [PubMed] [Google Scholar]
- 42.Jansen MC, van Wanrooy S, van Hillegersberg R, Rijken AM, van Coevorden F, Prevoo W, et al. Assessment of systemic inflammatory response (SIR) in patients undergoing radiofrequency ablation or partial liver resection for liver tumours. Eur J Surg Oncol. 2007;34:662–667. doi: 10.1016/j.ejso.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 45.Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin. 1999;49:231–255. doi: 10.3322/canjclin.49.4.231. [DOI] [PubMed] [Google Scholar]
- 46.Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single-institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Hemming AW, Reed AI, Howard RJ, Fujita S, Hochwald SN, Caridi JG, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691. doi: 10.1097/01.SLA.0000065265.16728.C0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schumacher G, Eisele R, Spinelli A, Neuhaus P. The surgical approach for radiofrequency ablation of liver tumours. Recent Results Cancer Res. 2006;167:53–68. doi: 10.1007/3-540-28137-1_4. [DOI] [PubMed] [Google Scholar]
- 49.Zagoria RJ, Chen MY, Shen P, Levine EA. Complications from radiofrequency ablation of liver metastases. Am Surg. 2002;68:204–209. [PubMed] [Google Scholar]
- 50.Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–454. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- 51.Elias D, Di PD, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumours in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823–827. doi: 10.1016/s0399-8320(06)73327-9. [DOI] [PubMed] [Google Scholar]
- 52.Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, et al. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: frequency and clinical significance. AJR Am J Roentgenol. 2004;183:1611–1617. doi: 10.2214/ajr.183.6.01831611. [DOI] [PubMed] [Google Scholar]
- 53.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the ‘heat sink’ effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 54.Elias D, Sideris L, Pocard M, Dromain C, De Baere T. Intraductal cooling of the main bile ducts during radiofrequency ablation prevents biliary stenosis. J Am Coll Surg. 2004;198:717–721. doi: 10.1016/j.jamcollsurg.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 55.Frich L, Hol PK, Roy S, Mala T, Edwin B, Clausen OP, et al. Experimental hepatic radiofrequency ablation using wet electrodes: electrode-to-vessel distance is a significant predictor for delayed portal vein thrombosis. Eur Radiol. 2006;16:1990–1999. doi: 10.1007/s00330-006-0177-6. [DOI] [PubMed] [Google Scholar]
- 56.Goldberg SN, Solbiati L, Halpern EF, Gazelle GS. Variables affecting proper system grounding for radiofrequency ablation in an animal model. J Vasc Interv Radiol. 2000;11:1069–1075. doi: 10.1016/s1051-0443(07)61341-4. [DOI] [PubMed] [Google Scholar]


