Abstract
Background
Radiofrequency ablation (RFA) of hepatocellular carcinoma (HCC) is widely utilized as a bridge to liver transplant with limited evidence to support efficacy. The purpose of the present study was to measure the effect of RFA on time to drop-off in HCC-listed patients.
Methods
Patients with Milan criteria tumours listed between January 1999 and June 2007 were stratified into RFA (n = 77) and No Treatment groups (n = 93).
Results
The primary effectiveness of RFA was 83% (complete radiographic response). RFA was associated with a longer median wait time to transplant (9.5 vs. 5 months). Tumour-specific drop-off events were equivalent between RFA (21%) and No Treatment (12%) groups (P = 0.11). Controlling for wait time, there was no difference in overall (P = 0.56) or tumour-specific drop-off (P = 0.94). Furthermore, there were no differences in 5-year overall or tumour-free survivals from list date or transplant. Using multivariate analysis, the likelihood of receiving a transplant and patient survivals were associated with tumour characteristics (AFP, tumour number and size) and not with bridge therapy or waiting time.
Discussion
RFA allows patients to be maintained longer on the waiting list without negative consequences on drop-off or survival compared with no treatment. Post-transplant outcomes are affected more by tumour characteristics than RFA or wait time.
Keywords: radiofrequency ablation, hepatocellular carcinoma, waiting list drop-off, Milan criteria, surgical treatment
Introduction
Liver transplantation is the most effective treatment for patients with hepatocellular carcinoma (HCC) arising in the setting of cirrhosis.1 Liver transplant for HCC is only effective if the majority of patients awaiting transplantation survive and remain eligible to receive this treatment.2–4 The primary goal of bridging therapy is to prevent waiting list drop-off by reducing tumour progression while secondary goals include the prevention of HCC recurrence and improving survival after liver transplantation.5–9 In 2006, more than 50% of all patients listed for liver transplant in the United States with a diagnosis of HCC received bridging therapy.6 Although widely offered and applied, recent reviews have uniformly concluded that there is no evidence in the literature proving the efficacy of bridging therapies in maintaining patients on the list or improving post transplant survival.5,8–12
Tumour ablation is a process whereby known or suspected HCC tumour nodules are destroyed by thermal or chemical injury via either a percutaneous or a surgical approach. Tumour destruction is then monitored by radiological measures. There are a variety of ablation techniques although radiofrequency ablation (RFA) has become the most common technique used in North America.6 RFA has been shown to be safe; with minimal morbidity and mortality both in transplant and non-transplant populations.13–16 There are several single centre reports of using RFA as a bridge to transplant, documenting the safety of this intervention.17–20 Although conceptually attractive, there are no controlled studies to suggest that RFA truly impacts relevant HCC transplant patient outcomes.
The present study was undertaken to determine the efficacy of RFA as a bridge to transplant in patients with Milan criteria HCC. The primary aim was to determine whether RFA increased the time taken to drop-off the waiting list. The secondary aims were to determine whether RFA improved the chances of being transplanted, overall and disease-free survival (DFS). We hypothesized that RFA as a bridge to transplant would decrease waiting list drop-off in a time to event model.
Methods
Ethics approval for this study was obtained from the Research Ethics Board of University Health Network (Protocol no. 06-0181-CE).
Patients
During the period between January 1999 and June 2007, all patients with the diagnosis of HCC who were placed on the liver transplant waiting list or listed patients who developed an HCC while waiting for liver transplant were evaluated for this study. Only patients whose HCC tumour pattern fulfilled the Milan criteria were included in this study. These patients were identified using the Organ Transplant Tracking Registry (OTTR, HKS Medical Information Systems, Omaha, NE), which is an internal web-based electronic medical record that encompasses all patients evaluated for a solid organ transplant at the University Health Network, University of Toronto. For the purpose of the present study, the diagnosis of HCC was verified by review of the pre-transplant radiological imaging, pre-transplant tumour histology (when performed) and the liver explant pathology.
A total of 343 patients were listed and/or transplanted with a diagnosis of HCC. The following patients were excluded from this study: 105 patients with a tumour pattern that exceeded the Milan criteria, 24 patients with incidental tumours identified by explant pathology that were not suspected or recognized before transplant, 4 patients who received their RFA procedures at a different institution and 3 patients who received a liver transplant at a different institution. An additional 18 patients with a history of a partial hepatectomy for HCC were excluded as these patients already had ‘recurrent HCC’ and likely exhibited more aggressive tumour biology. To specifically analyse the effect of RFA as bridging therapy vs. no treatment, Milan criteria patients who received bridging therapies other than RFA were also excluded: 14 patients that received a percutaneous ethanol injection (PEI), 3 patients who received transarterial chemoembolization (TACE) therapy and 2 patients who received external beam radiotherapy.
There were a total of 170 patients with the diagnosis of HCC recognized prior to transplant with a tumour pattern that radiographically fulfilled the Milan criteria: 77 patients received RFA as a sole bridging therapy and 93 patients received no bridging therapy.
Bridging therapy
RFA interventions were offered to patients at the University of Toronto beginning in 1999 and became our institution's recommended treatment (over TACE or PEI) for patients with small (≤4 cm), few (≤3 lesions) unresectable HCCs that were remote from major biliary structures. Given the equipoise between RFA and TACE as effective bridge therapy, RFA evolved as our institutional standard for reasons of local expertise, resource availability and overall lower morbidity in favour of RFA. Bridging therapies were offered to patients after review at a multidisciplinary HCC Tumour Review Board. RFA as bridging therapy was recommended to patients with ablatable lesions who were expected to have a transplant wait time more than 3 months. Wait time was estimated based upon the patient's position on the transplant waiting list and availability of living liver donor. Specifically, if on the waiting list at the time of listing, the waiting time (current date minus list date) of the top tumour patient with the same ABO blood type was ≤3 months, or if the patent had a potential live donor volunteer to be assessed, then the patient was counselled by a Transplant Surgeon regarding the relative risks and benefits of bridge therapy, and ‘no bridging therapy’ was recommended. Through surveillance with CT scans every 3 months, a Transplant Surgeon reassessed RFA bridging recommendations if the live donor did not materialize, or if the waiting time was estimated to be an additional 2–3 months, the patient was counselled again, and bridge therapy was recommended. The use of RFA as a bridging therapy over time has increased in frequency in parallel with the increased numbers of patients being listed for transplant and the prolongation of waiting times.
Radiofrequency ablation therapy was delivered via a percutaneous approach in 97% of patients by one of three Interventional Radiologists. RFA was provided to the remaining patients via open operations (3%) jointly by the interventional radiologist and transplant surgeon. Percutaneous RFA procedures were performed on an outpatient basis with conscious sedation using titrated doses of intravenous midazolam and fentanyl, and utilizing ultrasound and/or CT guidance. A multi-tined LeVeen electrode (Boston Scientific Corporation, Natick, MA, USA) was used for most (79%) ablations. Technical details of the ablative therapies were prospectively entered into an Interventional Radiology database along with pre-intervention imaging characteristics and post-treatment radiological response.
Pre-transplant clinical care
Patients on the liver transplant waiting list with HCC were given a priority listing of 1T. The Canadian Liver Transplant allocation agreement assigns the following Medical Status to patients on the waiting list: (i) patients at home, (ii) patients hospitalized for a liver failure-related diagnosis, (iii) patients hospitalized with a creatinine >200 µm/l or grade 3 encephalopathy, or (iv) patients intubated in the intensive care unit. Organs are allocated according to the patient priority listing on a national and provincial level; the priority 1T lies between priorities 1 and 2.21 As all tumour patients are eligible for a priority of at least 1T, waiting time is the dominant factor in the likelihood of being offered an organ.
Patients were monitored by a transplant surgeon while on the liver transplant waiting list. Computed tomography (CT) or magnetic resonance imaging (MRI) was performed every 3 months. Indications for de-listing (i.e. drop-off or transplantation) were identified for all patients. Drop-off events were further classified into medical (cardiovascular, sepsis, upper gastrointestinal bleed and medically unfit) and tumour specific (lymph node metastases, peritoneal disease, tumour progression, pulmonary metastases, tumour rupture and vascular invasion).
Study design
This cohort study was a retrospective analysis. Patients were categorized into Radiofrequency Ablation or No Treatment groups. Primary outcomes were time to event analysis (listing to drop-off). Analyses were performed for both drop-off as a result of all causes (HCC-specific and medical aetiologies) as well as tumour-specific drop-off. Wait time was calculated from the date of listing until either transplant or delisting. For patients who developed HCC while on the list, wait time was adjusted to begin at the date of the diagnosis of HCC. Patients were censored at the time of transplant or at the time they refused transplant. Survival analysis was based upon the intention to treat.
Statistical analysis
Characteristics of the patients in each group were compared using t-tests and Mann–Whitney U-tests for continuous outcomes and χ2-tests of association for categorical outcomes. Survival analysis was performed by the University of Toronto Statistical Consulting Service using SAS statistical software (version 9.1, SAS Institute, Cary, NC, USA). The objective of the present study was to assess how various predictors could affect the risk of a patient dropping off the list while waiting for a transplant. As the probability of such an event (dropping off the list) must increase the longer a patient is on the list, we used survival analysis models which allowed us to test various predictors while controlling for differences between subjects due to differences in time on the list. Patient characteristics that we screened were: age at the time of listing, gender, blood type (O or not), RFA treatment (yes or no), model for end-stage liver disease (MELD) score at the time of listing, AFP at the time of listing and aetiology. For categorical candidate predictor variables, we constructed Kaplan–Meier survival curves of time to drop-off for each level of the predictor (SAS: Proc Lifetest) and used a log-rank test to test for differences in the survival function between the levels. We used Cox Regression (SAS : Proc Phreg) to assess the relationship between continuous predictors such as age and the survival function. The following predictors were not significant and were not used in subsequent models: aetiology (P = 0.37), MELD at list (P = 0.47) and gender (P = 0.59). Age was not significant (P = 0.29) but was retained in models because of its potential clinical importance. All other predictors were significant and were retained in the final models. We used Cox proportional hazard and logistic regression models to conduct a multivariate analysis of the candidate predictors, which allowed us to test for the significance of individual predictors while controlling for all other predictors in the model. Interactions were removed from the models if they were not significant and only final models are reported.
After initial analysis of the whole cohort, further subgroup analysis was undertaken with 1:1 nearest neighbour propensity score matching. Waiting time, age, tumour number, tumour size, AFP and MELD score were incorporated into this analysis to ensure the relationship between predictor variables and outcome variables remained consistent across the RFA vs. no treatment group.
Results
Patients and demographics
Between January 1999 and June 2007, 170 patients who were listed for liver transplant with a diagnosis of HCC that fulfilled the Milan criteria by radiographic assessment either at the time of listing or while active on the waiting list were eligible for the primary analysis of this study. There were 77 patients that received RFA as a sole bridging therapy and 93 patients that received no bridging therapy (Table 1). No patients were lost to follow-up. All patients have either received a liver transplant or have experienced a drop-off event.
Table 1.
Patient demographics at the time of listing
| RFA | No treatment | Significance | |
|---|---|---|---|
| Total patients | 77 | 93 | |
| Age mean (range) | 5635–76 | 5528–71 | 0.68 |
| Gender | 0.25 | ||
| Male | 66 (86%) | 75 (81%) | |
| Female | 11 (14%) | 18 (19%) | |
| Blood group | 0.26 | ||
| O | 37 (48%) | 41 (44%) | |
| A | 31 (40%) | 33 (35%) | |
| B | 9 (12%) | 15 (16%) | |
| AB | 0 (0%) | 4 (4%) | |
| Aetiologya | |||
| Hepatitis B | 17 (22%) | 18 (19%) | 0.66 |
| Hepatitis C | 49 (64%) | 52 (56%) | 0.31 |
| Alcohol | 9 (12%) | 24 (26%) | 0.02 |
| NASH/ cryptogenic | 3 (4%) | 4 (4%) | 0.89 |
| Other | 1 (1%) | 5 (5%) | 0.15 |
| Tumour characteristics | |||
| Mean number | 1.33 | 1.35 | |
| Mean maximal size | 2.5 cm | 2.4 cm | |
| Median AFP (Range) | 280,000–65 | 200,000–119 | 0.60 |
| AFP > 200 | 16 (21%) | 21 (23%) | 0.78 |
| Mean MELD score (range)b | 147–26 | 156–25 | 0.33 |
| Time on list (months) | <0.001 | ||
| <2.5 | 10 (13%) | 30 (32%) | |
| 2.6–5.0 | 18 (23%) | 25 (27%) | |
| 5.1–12.7 | 22 (29%) | 21 (23%) | |
| >12.8 | 27 (35%) | 17 (18%) | |
| Time on list median (range) | 9.5 (0.8–51.1) | 5.0 (0.1–30.3) | |
Some patients had more than one aetiology of liver disease.
MELD scores reported are the medical MELD, without any exception points.
MELD, model for end-stage liver disease; NASH, non-alcoholic steato-hepatitis.
The only statistically significant difference in demographics (Table 1) between the groups was that fewer patients with a diagnosis of alcoholic liver disease received RFA as a bridge to transplant (12% RFA vs. 26% No Treatment, P = 0.02). Investigations could not identify any explanation for this observation. The mean listing medical MELD score at the time of listing was 14 in the RFA group compared with 15 in the No Treatment group (Tables 1, P = 0.33). There was no difference in the type of transplant (live vs. deceased donor) between the RFA and No Treatment group.
The median waiting time to transplantation was significantly greater in the RFA group compared with the No Treatment group (9.5 months vs. 5.0 months, P < 0.001). The differences in time on the list were most pronounced in the quartile of patients with the shortest waiting time (only 13% of the RFA group was transplanted within 2.5 months of listing compared with 32% in the No Treatment group) and the longest waiting time quartile (35% of the RFA group waited longer than 12.8 months compared with only 18% of the No Treatment group).
Tumour characteristics
The median alpha-fetoprotein levels at time of listing and the other tumour characteristics (including size and number) were similar between the RFA and No Treatment groups (Table 1).
Details of the RFA procedures
Details of the RFA procedures are listed in Table 2. The procedures were performed by interventional radiologists on an outpatient basis using conscious sedation and percutaneous electrode insertion in 97% of the patients. The ablation electrodes were inserted into the HCC tumours using ultrasound (US) (47%), CT (10%) or a combination of US and CT localization (43%). The mean number of separate RFA procedures was 1.4 per patient (range 1–4). The mean number of tumours treated per RFA procedure was 1.1 and the mean largest tumour diameter was 2.6 cm. The primary technique effectiveness was 83% (64/77) as measured by a complete radiological (ablative) response on the first post-procedure contrast-enhanced imaging study.
Table 2.
Technical details and complications of the RFA procedures
| Ablation access | Percutaneous | 75 (97%) |
| Open surgical | 2 (3%) | |
| Imaging guidance | US | 36 (47%) |
| CT | 8 (10%) | |
| CT & US | 33 (43%) | |
| Total RFA procedures | 1 RFA | 58 (75%) |
| 2 RFA | 12 (16%) | |
| 3 RFA | 5 (6%) | |
| 4 RFA | 2 (3%) | |
| Mean/ patient | 1.4 (range 1–4) | |
| Number of tumours ablateda | 1 tumour | 70 (91%) |
| 2 tumours | 6 (8%) | |
| 3 tumors | 0 (0%) | |
| 4 tumors | 1 (1%) | |
| Mean number ablated | 1.1 (range 1–4) | |
| Diameter of largest tumour nodulea | 2.6 cm (range 0.6–4.5 cm) | |
| Ablation electrodes utilized | LeVeen | 61 (79%) |
| Cool-tip | 7 (9%) | |
| Berchtold | 7 (9%) | |
| RITA StarBurst | 2 (3%) | |
| Primary technique effectivenessab | 83% (64/77) | |
| Complications | Major none | |
| Minor n = 2 | Left portal vein thrombus | |
| Vasovagal reaction | ||
| No deaths | ||
Data presented for the first RFA procedure only.
Defined as patients with a complete radiological response as measured on the first contrast enhanced imaging post-ablation.
LeVeen electrode (Boston Scientific, Natick, MA); Cool-tip electrode (Covidien/Valleylab, Boulder, CO); Berchtold electrode (Integra LifeSciences, Plainsboro, NJ); RITA StarBurst electrode (AngioDynamics, Queensbury, NY).
RFA, radiofrequency ablation; US, ultrasound; CT, computed tomography; cm, centimetres.
There were two minor complications of the RFA interventions including asymptomatic left portal vein thrombosis and a vaso-vagal reaction that occurred immediately after RFA application. Liver transplants have been performed in both of these patients with minor complications. There was one delayed tumour rupture three and a half months after ablation that was not attributed as a direct complication of the ablation. This patient did not receive a liver transplant.
Patient drop-off from the waiting list analysis
At the time of analysis, all patients in both groups had received a transplant, refused transplantation and were delisted, or dropped off the waiting list for medical or tumour-specific reasons (Table 3).
Table 3.
Aetiology of patient drop-off from the liver transplant waiting list
| RFA | No treatment | Significance | |
|---|---|---|---|
| Total patients | 77 | 93 | |
| Aetiology of drop-off | |||
| Refused transplant* | 7 (9%) | 1 (1%) | 0.01 |
| Medical (total) | 3 (4%) | 5 (7%) | 0.65 |
| Cardiovascular | 0 | 1 | |
| UGIB | 1 | 0 | |
| Unfit | 2 | 4 | |
| Tumor specific (total) | 16 (21%) | 11 (12%) | 0.11 |
| LN metastases | 0 | 1 | |
| Peritoneal disease | 1 | 0 | |
| Progression | 0 | 2 | |
| Pulmonary metastases | 5 | 0 | |
| Rupture | 1 | 0 | |
| Vascular invasion | 9 | 8 | |
| Transplant | 0.8 | ||
| Deceased donor organ | 45 (58%) | 65 (69%) | |
| Live donor right lobe | 6 (8%) | 11 (12%) | |
| Transplant + Refused transplant | 58 (75%) | 77 (83%) | 0.31 |
6/7 RFA patients who refused a transplant were interpreted to have a complete radiological response to tumour ablation.
UGIB, upper gastro-intestinal bleed; LN, lymph node; HCC, hepatocellular carcinoma; MELD, model for end-stage liver disease.
More patients in the RFA group requested delisting from the waiting list (9% RFA vs. 1% No Treatment, P = 0.01). Notably, six out of the seven requested delisting in the RFA group because they had a sustained radiographical response. Of these six patients, four remain free of disease, one patient developed an additional HCC that was completely ablated and one patient developed a peri-ablation recurrence that was resected. There were similar percentages of patients who dropped-off for medical reasons (4% RFA vs. 7% No Treatment, P = 0.65). For tumour-specific reasons, there was a trend towards a higher drop-off in the RFA group (21% RFA vs. 12% No Treatment, P = 0.11). The major differences in tumour events for the RFA group were the development of pulmonary metastases in five, peritoneal disease in one and tumour rupture in one, compared with the No-treatment group who dropped-off for local progression in two and lymph node metastases in one. The development of vascular invasion was equivalent between groups (12% RFA vs. 9% No Treatment, P = 0.43).
Although there were numerically more drop-offs events in the RFA group, the corresponding waiting time was also longer in the RFA group, which would increase the probability of experiencing a drop-off event. Waiting time was controlled for using survival analysis modelling. There was no significant difference in the time to drop-off between the RFA and No Treatment groups for all causes (medical + tumour; Fig. 1a) or for tumour-specific aetiology (Fig. 1b). The overall drop-off rate during the first 12 months after listing was approximately 2% per month for each group, and the rate of delisting for tumour specific drop-off events was approximately 1.7% per month.
Figure 1.
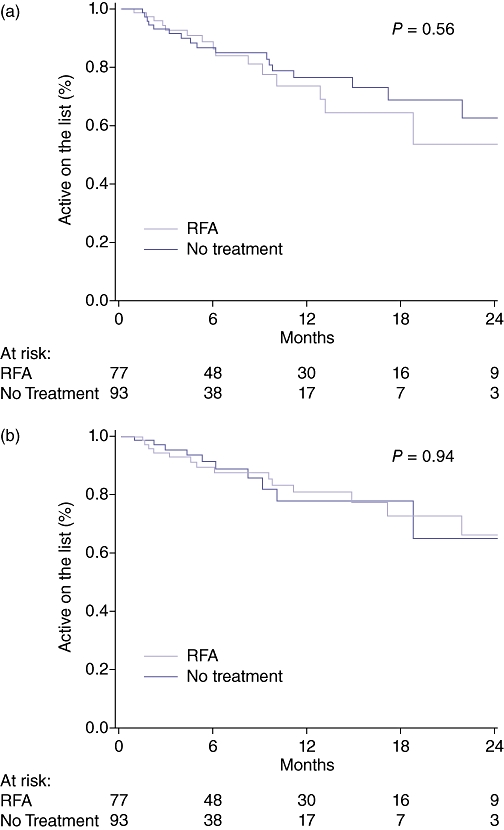
(a) Overall drop-off from the waiting list. (b) Tumour-specific drop-off from the waiting list
Transplantation
Transplantation was achieved in 75% of those patients treated with RFA as a bridge and 83% of those without treatment (P = 0.141). Tumour characteristics were most strongly associated with a reduction in the odds of achieving a transplant (Table 4). Patients with an AFP > 200 (OR 0.205, P < 0.001), greater number of tumours (OR 0.532, P = 0.037), bilobar disease (OR 0.344, P = 0.025), larger tumours (OR 0.665, P = 0.040) and a higher stage at listing (OR 0.155, P = 0.013) had a lower chance of being transplanted. This association was maintained on multivariate analysis for AFP > 200 (P < 0.001), higher tumour number (P = 0.013) and larger tumours (P = 0.014). The patients receiving RFA did not have a lower odds of undergoing transplant despite a lower overall percentage of patients transplanted.
Table 4.
Explant tumour characteristics
| RFA | No treatment | Significance | |
|---|---|---|---|
| Total patients | 77 | 93 | |
| Milan stage | |||
| Within Milan | 44 (76%) | 61 (80%) | 0.54 |
| Outside Milan | 14 (24%) | 15 (20%) | |
| Microvascular invasion | 11 (22%) | 15 (19.7%) | 0.76 |
| Macrovascular invasion | 2 (4%) | 4 (5%) | 0.75 |
| Number of tumours (median) | 2 | 1.5 | 0.80 |
| Size of largest tumour (median) | 2.5 | 2.7 | 0.90 |
| Bilobar disease | 18 (36%) | 22 (29%) | 0.405 |
| Pathological grade | |||
| Well differentiated | 1 (2%) | 8 (12%) | 0.11 |
| Moderately differentiated | 39 (89%) | 48 (74%) | |
| Poorly differentiated | 9 (4%) | 14 (8%) | |
After propensity matching of covariates the effect of RFA on drop-off and achieveing transplantation was reanalysed. Good matching of covariates was achieved, with no statistically significant differences for the included covariates. With matching there was no difference in the treatment effect of RFA on drop-off (t-statistic −2.26, P = 0.79) or achieving transplant (t-statistic −2.41, P = 0.79).
Non-transplanted patients (waiting list drop-off events)
The 1-, 3- and 5-year survival for those patients who were delisted was 87%, 76% and 55% for RFA group, which was significantly higher than the No treatment group where the survival was 71%, 39%, 30% (P = 0.009). On univariate analysis, the risk of death was lower in females (HR 0.307, P = 0.041), and those receiving RFA (HR 0.299, P = 0.014). On Multivariate analysis only RFA was associated with a lower risk of dying. Tumour characteristics did not influence risk of death in those patients delisted.
Explant pathology
Details of the explant pathology are listed in Table 4. There were no statistically significant differences in explant pathology between those patients who received RFA vs. No Treatment.
Survivals
There was no difference in the overall median follow-up time between the RFA and No Treatment groups (41 vs. 46 months, P = 0.44). After transplantation there was also no difference observed in the median follow-up time between the RFA and No Treatment groups (30 vs. 41 months, P = 0.075). There was no significant difference in the overall survival (OS) (P = 0.75) or DFS (P = 0.24) as calculated from the time of listing (intent to treat analysis) between the RFA and No Treatment groups. (Fig. 2a–b) After liver transplant, there were nine deaths (four HCC/ five non-HCC related) and one HCC recurrence out of 51 RFA patients transplanted (20% event rate). In comparison, there were 19 deaths (8 HCC/ 11 non-HCC) and 2 HCC recurrences in the 76 No Treatment group patients transplanted (25% event rate). The corresponding post-liver transplant OS (P = 0.76) and DFS (P = 0.86) curves did not demonstrate a statistical difference. (Fig. 3a–b).
Figure 2.
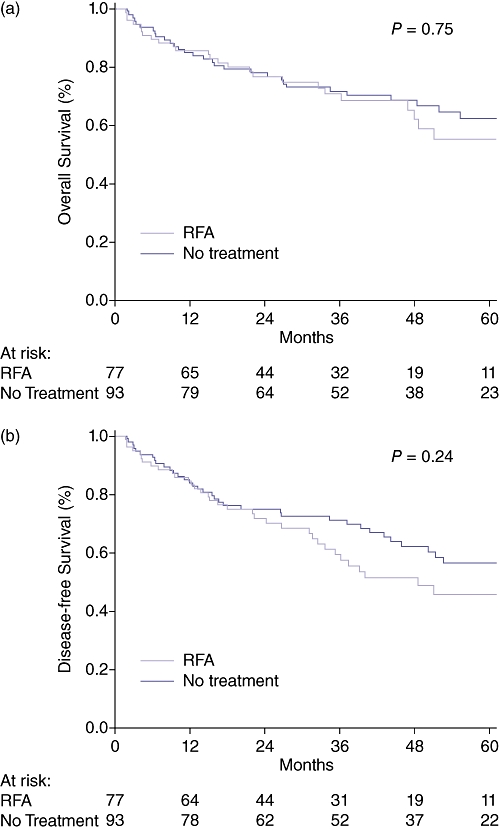
(a) Overall survival in all hepatocellular carcinoma (HCC) patients. Time is calculated from the date of listing. (b) Tumour-free survival in all HCC patients. Time is calculated from the date of listing
Figure 3.
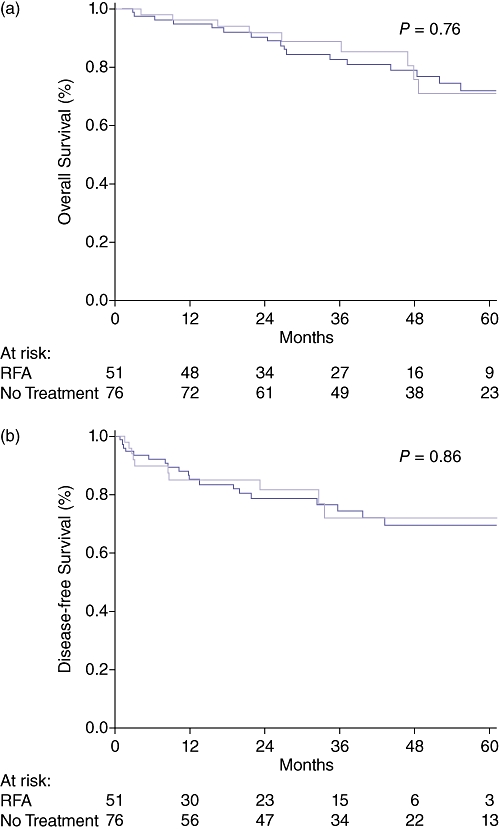
(a) Overall survival in hepatocellular carcinoma (HCC) patients that received a liver transplant. Time is calculated from the date of transplantation. (b) Tumour-free survival in HCC patients that received a liver transplant. Time is calculated from the date of transplantation
Details of the predictors of survival are listed in Table 5. Based upon the intention to treat on both univariate and multivariate analysis, survival was lower in those patients with an AFP > 200 (P = 0.01) and a higher stage at listing (P = 0.008). Other tumour characteristics and treatment with RFA were not significantly associated with OS.
Table 5.
Logistic regression model for factors predicting achieving transplantation
| Variable | Odds | 95% confidence | Significance | |
|---|---|---|---|---|
| Ratio | Interval | Univariate | Multivariate | |
| Gender | 1.05 | 0.40–2.83 | 0.92 | ns |
| Age | 0.97 | 0.92–1.02 | 0.20 | ns |
| AFP > 200 | 0.21 | 0.09–0.47 | <0.001 | <0.001 |
| Waiting time | 1.02 | 0.98–1.05 | 0.38 | ns |
| RFA | 0.68 | 0.32–1.45 | 0.32 | ns |
| Tumour numbera | 0.53 | 0.29–0.96 | 0.04 | 0.01 |
| Bilobar diseasea | 0.34 | 0.14–0.87 | 0.03 | ns |
| Size of largest tumoura | 0.67 | 0.45–0.98 | 0.04 | 0.01 |
| UNOS stagea | 0.16 | 0.04–0.68 | 0.01 | ns |
Data from time of listing.
AFP, alpha fetoprotein; RFA, radiofrequency ablation.
Discussion
The benefits of locoregional treatment for HCC patients prior to liver transplantation are unclear.5,8–12 In a situation where the waiting time for liver transplantation is long, there is a perception by both the physicians and patients that not treating patients with HCC is unacceptable, despite the absence of evidence supporting short- or long-term benefit. The present study has demonstrated that RFA can be safely performed for patients with tumours within the Milan Criteria. RFA also appeared to allow patients to be maintained for a longer period of time on the transplant waiting list without any adverse effect on drop off, achieving transplant or post-transplant survival, a significant benefit when waiting times are prolonged.
RFA was safely performed percutaneously 97% of the time, with a high primary technique effectiveness of 83% and a very low complication rate. Six of the 77 patients treated with RFA (intended as a bridge to transplant) had a sustained radiographical response such that these patients elected not to proceed with liver transplant. In the group of patients who were delisted, the survival in the RFA group was superior compared with the No treatment group (55% vs. 30%), and was equivalent to other treatments with curative intent such as surgery.22–24 RFA thus became an effective destination therapy for a small portion of patients and avoided the need for liver transplant – a clinically significant advantage to the use of RFA. In the setting of a long waiting time for transplant, the durability of the RFA response can be assessed and the need for transplantation for HCC be reconsidered.
There was no significant difference in tumour-specific drop-off, although the incidence of drop-off events tended to be higher in the RFA group. RFA was not observed to halt the development of vascular invasion while on the transplant waiting list (12% RFA vs. 9% No Treatment, P = NS). Moreover, the overall and tumour-free drop-off rates were equivalent when controlling for the longer waiting time in the RFA group. As the majority of the patients were transplanted within 12 months, there were too few patients remaining in the survival analysis after 12 months to determine the efficacy of RFA bridging therapy for those patients with a longer waiting time to transplantation, where the benefits of treatment may be most pronounced. Previous studies18,25 have suggested that RFA can reduce drop off on the waiting list compared with historical controls, however, these patients had longer waiting times than that those in our study and the cohorts used for comparison were not matched.
Perhaps the most clinically important impact of RFA for HCC patients that are typically transplanted within 1 year of listing is the ability of RFA to maintain patient eligibility on the transplant list. This is important because, while a clinician may estimate a short waiting time, this study demonstrates that the waiting time may be unpredictably prolonged, as 41% of the no-RFA group waited more than 5 months. The median time on the waiting list was significantly longer in the RFA group (9.5 vs. 5 months) and despite this, the rate of transplantation and drop offs in the RFA group were equivalent to those with much shorter waiting list times. Furthermore, the explant pathology was equivalent between these two groups suggesting that RFA may halt the progression of tumour growth. The importance of RFA being able to keep patients' tumour burden within the Milan criteria without increasing the rate of drop off or being detrimental to survival has implications for the priority given to these patients on the transplant waiting list.
Although the time-to-drop-off was similar between the RFA and No Treatment groups, there were some differences. The RFA group experienced a greater incidence of drop-off for tumour events, notably pulmonary metastases (n = 5), peritoneal metastases (n = 1) and tumour rupture (n = 1). These unexpected events may be a reflection of the tumour biology but given that none of these were seen in the No Treatment group, they may also have occurred as a consequence of treatment. Peritoneal metastases and tumour rupture are a known, albeit very rare, direct complication of the ablation technique. Although the lung is a common destination for HCC metastases in advanced disease, pulmonary metastases should be a relatively uncommon event in patients with a HCC tumour pattern that fulfils the Milan criteria. During the ablation process, it is common to see micro-air bubbles escaping the ablation zone via the hepatic veins to the systemic circulation. If these contents contain viable tumour emboli, the lung would be the first microcirculation filter. This proposed mechanism, however, is purely speculative. The overall procedure-related local complication rates were similar to those published in the literature.
In the present study, tumour characteristics were the best predictors of a failure to achieve transplantation and are probably reflective of the tumour biological behaviour. The main determinant of survival for non-resectable HCC within the Milan criteria is whether or not the patient is transplanted. Patients treated with RFA achieved the same overall and tumour-free survival, despite significantly longer waiting times. Given the excellent post-transplant survival for HCC this finding is not unexpected, and is consistent with several recently published studies that post-transplant survival is equivalent for patients treated with RFA.5,8–12 It is also notable that the 1997–2006 UNOS liver transplant review has suggested that patients who received bridging therapy on the waiting list had a slightly higher survival at 3 years (76% vs. 71%, P = 0.03).6 There are also single centre reports suggesting that multimodal bridging therapies may increase post-transplant survival.26–28 TACE is the predominant bridging therapy in the United States (60–70%)6 and thus it is possible that TACE provides an additional survival benefit above that of RFA.
This study has limitations inherent to all retrospective analyses. Probably the most important one is whether or not this is an appropriate comparison between RFA and No Treatment groups – specifically whether the groups were at the same risk of drop-off or survival? For uniformity and generalizability, only patients with a HCC that fulfilled the Milan criteria were considered and the demographics and tumour characteristics were similar. To reduce confounding variables, patients treated with other bridging therapies (TACE, ethanol ablation, radiation) or previous hepatectomy were excluded. The major systemic bias in this study is the criteria used to select patients for RFA: their tumours must have been safely ‘ablatable’ and their wait time was predicted to exceed 3 months based upon their position on the blood-type specific waiting list and availability of a living liver donor. Thus, the RFA patients may have been at a higher risk for drop-off as they were predictably on the wait list longer. The waitlist drop-off analysis, however, accounts for the difference in waiting times and demonstrated that the time to drop-off was similar between the groups for both tumour-specific and overall (medical and tumor-specific) indications. Furthermore, the nearest neighbour propensity score matching analysis demonstrated equivalent results between the RFA and No Treatment groups. On the other hand, many in the No-treatment group may have been at higher risk for drop-off or recurrence if their tumours were ‘not-ablatable’ for high-risk factors such as size, very exophytic nature or proximity to major biliary and vascular structures that might indicate having a worse or risky ‘biology’. These differences could only be normalized with a randomized prospective clinical trial.
The present study analyses the largest reported single centre cohort of patients treated with RFA as a bridge to liver transplant compared with a No-Treatment group treated during the same period. This study should be generalizable as it mirrors current clinical practice in many transplant centres with similar waiting times. Although no significant advantage of RFA in waitlist drop-off or HCC recurrence was identified, the RFA patients may have been at higher risk for these events because of the centre's criteria for selecting HCC patients for RFA bridging and despite this, the patients were maintained for longer on the waiting list without any negative consequences with respect to drop-off or survival.
Conflicts of interest
None declared.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 3.Shah SA, Cleary SP, Tan JC, Wei AC, Gallinger S, Grant DR, et al. An analysis of resection vs transplantation for early hepatocellular carcinoma: defining the optimal therapy at a single institution. Ann Surg Oncol. 2007;14:2608–2614. doi: 10.1245/s10434-007-9443-3. [DOI] [PubMed] [Google Scholar]
- 4.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416–1421. doi: 10.1111/j.1600-6143.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM. Pretransplant treatments for hepatocellular carcinoma: do they improve outcomes? Liver Transpl. 2005;11(Suppl 2):S10–S13. doi: 10.1002/lt.20598. [DOI] [PubMed] [Google Scholar]
- 6.Freeman RB, Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997–2006. Am J Transplant. 2008;8(Pt 2):958–976. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 7.Greig PD. Treatment before liver transplant – No treatment. 2008. Proceedings of the Fifth International Meeting, Hepatocellular Carcinoma: Eastern and Western Experiences.
- 8.Lubienski A. Hepatocellular carcinoma: interventional bridging to liver transplantation. Transplantation. 2005;80(Suppl):S113–S119. doi: 10.1097/01.tp.0000187109.69663.93. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz M, Roayaie S, Uva P. Treatment of HCC in patients awaiting liver transplantation. Am J Transplant. 2007;7:1875–1881. doi: 10.1111/j.1600-6143.2007.01863.x. [DOI] [PubMed] [Google Scholar]
- 10.Heckman JT, Devera MB, Marsh JW, Fontes P, Amesur NB, Holloway SE, et al. Bridging locoregional therapy for hepatocellular carcinoma prior to liver transplantation. Ann Surg Oncol. 2008;15:3169–3177. doi: 10.1245/s10434-008-0071-3. [DOI] [PubMed] [Google Scholar]
- 11.Belghiti J, Carr BI, Greig PD, Lencioni R, Poon RT. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- 12.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 13.Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381–391. doi: 10.1097/00000658-200009000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 16.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, et al. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165–1174. doi: 10.1053/jlts.2002.36394. [DOI] [PubMed] [Google Scholar]
- 18.Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 19.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala M, Llovet JM, Vilana R, Bianchi L, Sole M, Ayuso C, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 21.Bazarah SM, Peltekian KM, McAlister VC, Bitter-Suermann H, MacDonald AS. Utility of MELD and Child-Turcotte-Pugh scores and the Canadian waitlisting algorithm in predicting short-term survival after liver transplant. Clin Invest Med. 2004;27:162–167. [PubMed] [Google Scholar]
- 22.Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D'Onofrio M, et al. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192–198. doi: 10.1007/s11605-007-0392-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial] Zhonghua Yi Xue Za Zhi. 2006;86:801–805. [PubMed] [Google Scholar]
- 25.Brillet PY, Paradis V, Brancatelli G, Rangheard AS, Consigny Y, Plessier A, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186(Suppl):S296–S305. doi: 10.2214/AJR.04.1927. [DOI] [PubMed] [Google Scholar]
- 26.Yao FY, Kinkhabwala M, LaBerge JM, Bass NM, Brown R, Jr, Kerlan R, et al. The impact of pre-operative loco-regional therapy on outcome after liver transplantation for hepatocellular carcinoma. Am J Transplant. 2005;5(Pt 1):795–804. doi: 10.1111/j.1600-6143.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 27.Fisher RA, Maluf D, Cotterell AH, Stravitz T, Wolfe L, Luketic V, et al. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502–512. doi: 10.1111/j.1399-0012.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 28.Bharat A, Brown DB, Crippin JS, Gould JE, Lowell JA, Shenoy S, et al. Pre-liver transplantation locoregional adjuvant therapy for hepatocellular carcinoma as a strategy to improve longterm survival. J Am Coll Surg. 2006;203:411–420. doi: 10.1016/j.jamcollsurg.2006.06.016. [DOI] [PubMed] [Google Scholar]


