Abstract
Background
Intrahepatic cholangiocarcinoma (ICC) remains a rare tumour, although its incidence is increasing. Surgical resection is the mainstay of treatment. Published data regarding prognostic factors and optimal patient selection for resection are scant. We sought to determine the clinicopathologic characteristics of resectable ICC and outcomes following surgical treatment.
Methods
We reviewed prospectively collected clinical data including patient, pathologic and operative details. Survival and recurrence outcomes were analysed using Cox hazard models and the Kaplan–Meier method.
Results
We identified 31 surgically treated patients. Their 3-year overall survival rate (OS) was 40.1%; median follow-up was 16.2 months (range: 0.2–86.9 months). R0 resection was associated with significantly improved OS compared with R1/R2 resection (3-year OS was 68.6% in R0 vs. 24.0% in R1/R2; P = 0.042). The postoperative complication rate was 58.1%. Two patients died of postoperative liver failure within 30 days. Preoperative hypoalbuminaemia was significantly associated with worse survival.
Conclusions
Surgical therapy for ICC is associated with longterm survival in the subset of nutritionally replete patients in whom an R0 resection can be achieved. Surgical mortality is significant in patients undergoing extended resection. The margin involvement rate is high and surgeons should consider the infiltrative nature of the disease in operative planning.
Keywords: cholangiocarcinoma < liver, resection < cholangiocarcinoma, outcomes < cholangiocarcinoma
Introduction
Intrahepatic cholangiocarcinoma (ICC) is second only to hepatocellular carcinoma (HCC) in terms of incidence of primary hepatic tumours. It accounts for approximately 15% of primary liver cancers and is associated with a median survival of 4.5 months from the time of diagnosis.1,2 The poor overall outcome associated with the disease is likely to be related to a pattern of clinical presentation in which patients remain asymptomatic until the tumour has reached a relatively advanced stage.3 Recent studies have clearly demonstrated an unexplained rise in the incidence of ICC in the Western hemisphere.3,4 By contrast, rates of extrahepatic cholangiocarcinoma (ECC) have either stabilized or, in some populations, declined.1
The increasing incidence of ICC has resulted in a number of reports describing surgical treatment and outcomes.3,5–8 However, the majority of these reports come from a relatively few high-volume centres and there are few data from regional treatment centres or community hospitals involved in the treatment of this group of patients.3,6,9
This study aimed to use pooled clinical data from hepatobiliary programmes at an academic and a community-based hospital to assess short- and longterm outcomes after surgical treatment of ICC. Our analysis focuses on the identification of prognostic factors to assist in patient selection, as well as on the identification of predictors of postoperative adverse events.
Materials and methods
Outcome data for patients diagnosed with ICC who underwent surgical resection at either Oregon Health and Science University (OHSU) or Providence Portland Medical Center (PPMC) between January 1998 and July 2009 were prospectively collected and retrospectively reviewed.
Study patients were identified either through the OHSU tumour registry (January 1998 to July 2009) or the PPMC Health Plan database (January 2002 to July 2009). Patients who were treated non-operatively, had hilar cholangiocarcinoma or ECC were excluded. The final study group comprised a total of 31 patients (23 from OHSU, eight from PPMC) and the study protocol was approved by the institutional review boards of both OHSU (eIRB #5257) and PPMC (IRB #09-043A).
Patient demographics, tumour characteristics, operative data, tumour pathology and disease recurrence were obtained from hospital medical records. Demographic details included age, gender, body mass index (BMI) and American Society of Anesthesiologists (ASA) class, as well as data on co-morbid conditions and preoperative symptoms. Co-morbid conditions were organized into four major categories based on diagnoses listed in a patient's medical record as follows: cardiac (coronary artery disease, myocardial infarction, hypertension); pulmonary (congestive heart failure, chronic obstructive pulmonary disease, asthma, chronic bronchitis, tracheal stenosis); diabetes/obesity (either), and previous cancer (any solid organ, cutaneous or haematologic malignancy). Similarly, symptoms present preoperatively were organized into three main categories: obstructive symptoms (jaundice, pruritis, elevated liver function tests); pain, and weight loss. Postoperative complications were organized into the following categories: cardiac (myocardial infarction, atrial fibrillation); pulmonary (pneumonia, pulmonary effusion, respiratory failure requiring mechanical ventilation); infectious (intra-abdominal abscess, sepsis); biliary leak (biloma on imaging, bilious drainage from drains), and liver insufficiency (hepatic encephalopathy, ascites, elevated INR [international normalized ratio]). Data on tumour characteristics, such as size, solitary vs. multiple tumours and T stage, were also collected. Survival status was obtained from either the hospital medical record or the social security death index.
Survival and recurrence rates were analysed by univariate Cox proportional hazard models. Development of a postoperative complication was analysed by univariate logistic regression models. Cox or logistic models were created for each predictor variable and outcome combination because an insufficient sample size limited the utility of more traditional multivariate modelling. The natural log of length of stay (LOS) was analysed with t-tests for binary predictors, and Pearson correlations for continuous predictors. P-values of <0.05 were considered significant. All data were analysed using pasw Version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Demographics and clinical presentation
Of the 31 surgically treated patients (10 male, 21 female), 93.5% were symptomatic at the time of diagnosis. The most common symptoms at presentation were unintentional weight loss (45.2%, 14/31), biliary obstruction (38.7%, 12/31) and preoperative pain (35.5%, 11/31). The median patient age, BMI, ASA classification and major co-morbid classes of the study population are listed in Table 1.
Table 1.
Patient demographics
| Variable | n (%) |
|---|---|
| Mean age, years | 62.3 |
| Male gender | 10 (32%) |
| Mean body mass index | 29.4 |
| American Society of Anesthesiologists class | |
| Grade 1 | 1 (3%) |
| Grade 2 | 11 (35%) |
| Grade 3 | 12 (39%) |
| Grade 4 | 5 (16%) |
| Unknown | 2 (6%) |
| Mean preoperative albumin, g/dl (range) | 3.4 (1.8–4.7) |
| Presence of hypoalbuminaemia | 11 (35%) |
| Co-morbid conditions | |
| Cardiac | 14 (45%) |
| Pulmonary | 12 (39%) |
| Diabetes mellitus/obesity | 4 (13%) |
| Prior history of cancer | 7 (23%) |
| Preoperative symptoms | |
| Obstruction | 12 (39%) |
| Pain | 11 (36%) |
| Unintentional weight loss | 7 (23%) |
Treatment
At the time of diagnosis, 93.5% (n = 29) of patients presented with disease that appeared to be confined to either the left or right lobe of the liver, whereas 6.5% (n = 2) of patients presented with bilobar disease (Table 2). The mean tumour size was 6.0 cm (range: 1.2–16.0 cm) and the majority of tumours were solitary (93.5%, n = 29). All patients had mass-forming tumours, although pathologic reporting of the presence or absence of periductal and intraductal involvement was inconsistent. None of the patients had documented capsular invasion. The presence or absence of microvascular invasion was not consistently documented in pathologic reports. The majority of the study patients had solitary tumours and therefore multifocality analysis on survival was not performed. Liver resections were classified according to the International Hepato-Pancreato-Biliary Association Brisbane 2000 Terminology of Liver Anatomy and Resections guidelines.10 The majority of patients underwent major resection (n = 29, 93.5%); 11 (35.5%) underwent extended resection.
Table 2.
Treatment details
| Treatment variable | n (%) |
|---|---|
| Tumour characteristics | |
| Mean tumour size, cm | 6.0 |
| Lobar | 29 (94%) |
| Bilobar | 2 (6%) |
| Multifocal | 2 (6%) |
| Operative details | |
| Resection type | |
| Extended hepatectomy | 11 (35%) |
| Major hepatectomy | 17 (55%) |
| Minor hepatectomy | 3 (10%) |
| Degree of resection | |
| R0 | 16 (52%) |
| R1 | 13 (42%) |
| R2 | 2 (6%) |
| Mean length of stay, days | 16 (median 10, range 2–125) |
| Postoperative complications | |
| Cardiac | 1 (3%) |
| Pulmonary | 4 (13%) |
| Infectious | 11 (36%) |
| Bile leak | 8 (26%) |
| Liver insufficiency | 8 (26%) |
Outcomes
Patient demographic variables, presenting symptoms, co-morbidities, preoperative laboratory values and tumour characteristics were analysed to determine predictors of specific outcome measures, including margin status (microscopically negative margins [R0], vs. microscopically positive [R1] or macroscopically positive [R2] margins), LOS, development of postoperative complications, and time to recurrence and mortality.
Overall survival (OS) is summarized in Fig. 1, which demonstrates a 3-year OS of 40.1%. The median OS was 30.9 months; median follow-up was 16.2 months (range: 0.2–86.9 months). Median OS periods for microscopically negative margin (R0, n = 16), microscopically positive margin (R1, n = 13) and macroscopically positive margin (R2, n = 2) resections were 54.4, 19.4 and 0.6 months, respectively (P = 0.055). The short OS for R2 resections derives from two patients, the first of whom was jaundiced at presentation, had an LOS of 13 days and survived approximately 20 months post-resection. The second R2 patient was anicteric at presentation and underwent an extended resection but was unable to be rendered disease-free. This patient died on postoperative day (POD) 19 from liver insufficiency complicated by a urinary tract infection, which progressed to sepsis and acute renal failure. When R0 resections were compared with R1/R2 resections, curative resection significantly improved 3-year OS (68.6% vs. 24.0%; P = 0.042) (Fig. 2).
Figure 1.
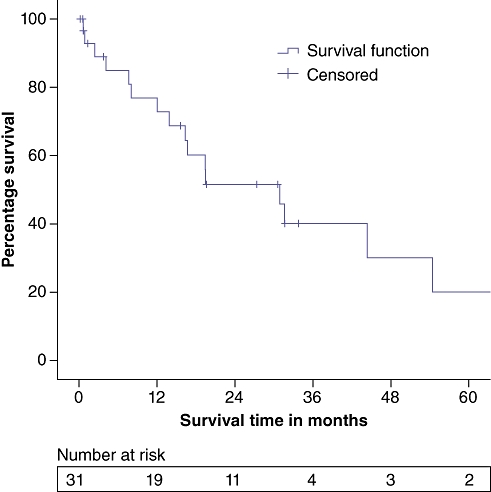
Overall survival, entire study group (n = 31)
Figure 2.
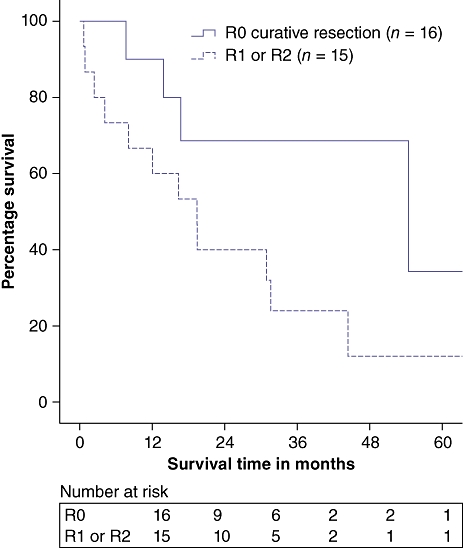
Survival in R0 vs. R1/R2 resection (P = 0.042)
When patient and tumour factors were analysed, preoperative weight loss was found to be a significant predictor for increased mortality (P = 0.01) and elevated preoperative total bilirubin demonstrated a trend towards increased mortality (P = 0.05, hazard ratio [HR] 1.102, 95% confidence interval [CI] 1.000–1.214). Increasing tumour size did not correlate with decreasing time to death. In patients who were rendered disease-free (R0, n = 16), 1-, 2- and 3-year recurrence-free survival rates were 77.9%, 64.9% and 48.7%, respectively. Analysis of patient and tumour factors in this relatively small group of patients did not demonstrate any predictors for recurrence, although the majority of recurrences occurred in the liver.
Regional lymph node sampling and formal lymphadenectomy were performed at the discretion of the surgeon. Patients with pathologically confirmed node-negative disease (N0) had a 3-year OS of 60.0% (P = 0.076), although lymph nodes were submitted for analysis in only a third of patients (Fig. 3). When node-negative vs. node-positive disease was analysed to determine impact on other outcomes such as LOS or postoperative complication rate, no correlations were identified. However, there were no recurrences in the subset of patients who underwent R0 resection with pathologically confirmed node-negative disease.
Figure 3.
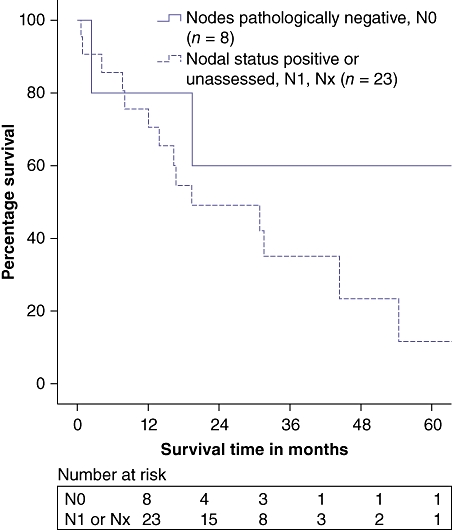
Survival in node-negative (N0) vs. node-positive (N1) or unassessed (Nx) cases (P = 0.18)
Major complications occurred in 58.1% of patients (n = 18) and most patients suffered more than one complication. Complications are listed in Table 2. When patient, tumour and treatment variables were assessed, none were found to correlate with the development of a postoperative complication, although the presence of at least one co-morbidity demonstrated a trend towards an increased complication rate, but this was not significant (P = 0.072). Two patients died from liver failure on POD 19 and POD 25, respectively. Both had undergone extended resection and had other co-morbidities, and resection was considered to be their only viable option. No patient in this series underwent preoperative portal vein embolization.
The mean LOS for all patients was 16 days (range: 2–125 days); when a single outlier (LOS 125 days) was removed, the mean LOS dropped to 12 days. Mean LOS was 9 days (range: 2–15 days) for uncomplicated procedures and 20 days (range: 7–125 days) for complicated procedures. Analysis to identify predictors for increased LOS showed that only an ASA level ≥3 was predictive for increased LOS (P = 0.004).
Preoperative hypoalbuminaemia was associated with a median OS of 11.0 months compared with 17.0 months in patients with normal albumin levels (P = 0.01). When gender, albumin (normal vs. low), co-morbidities (yes vs. no) and nodal status (positive vs. negative/unassessed) were assessed, hypoalbuminaemia was the only significant predictor for survival (P = 0.045, HR = 3.6). Preoperative elevated INR was significantly associated with preoperative hypoalbuminaemia (P = 0.017), conferring an odds ratio (OR) of 4.6 for every 0.1 increase in INR (95% CI 1.308–16.150), whereas preoperative weight loss and tumour size were not (P = 0.758 and P = 0.335, respectively).
Discussion
In this review of the clinicopathologic features of resectable ICC tumours and patient outcomes at two regional cancer centres, longterm survival was seen in patients in whom an R0 resection was achieved. Few meaningful predictors for survival and morbidity were identified and it is uncertain whether the association of preoperative hypoalbuminaemia with worse outcomes is reflective of nutritional status, extent of disease or a combination of both. Extended resections in our series were associated with morbidity and mortality, and liver failure represented a life-threatening problem.
One of our principle findings was a high rate of R1 resections (n = 13). The infiltrative nature of ICC is well known and can make the achievement of negative margins technically challenging, yet this is a known important prognostic factor.3,11,12 Jonas et al. recommend extended resections to increase the rate of curative resections.6 In their series, 60% of patients underwent extended liver resections and R0 resections were achieved in 71% of the total study group, conferring overall 1- and 5-year survival rates of 72.4% and 30.4%, respectively. This was supported by Jiang et al., who reported longterm survival in patients who underwent extensive major hepatectomy and did not have nodal involvement.12 In the current study, R0 resection was associated with significantly improved OS compared with R1/R2 resection (3-year OS 68.6% in R0 vs. 24.0% in R1/R2; P = 0.042) (Fig. 2). Extended resection carried a relatively high morbidity rate, specifically for the development of postoperative liver insufficiency. Recently, preoperative portal vein embolization has been used more frequently at both our institutions to improve the margin of safety when subjecting patients to extended resections.
Another finding of the current study was that preoperative hypoalbuminaemia was associated with worse survival. Preoperative albumin levels are a known predictor for surgical complications and outcomes in population-level studies of non-cardiac surgery13,14 and liver resection patients.15 Gibbs et al. demonstrated that serum albumin was the strongest predictor of postoperative mortality and morbidity in non-cardiac cases,14 with an exponential increase in both morbidity and mortality rates when albumin levels dropped below 21 g/l. Similarly, in a review of liver resection patients, Virani et al. demonstrated that higher serum albumin levels were associated with decreased 30-day morbidity.15 The current study demonstrated that preoperative hypoalbuminaemia was also associated with a significantly worse median OS with an HR of 3.6 in the ICC patient population. There are several possible mechanisms by which hypoalbuminaemia might negatively impact survival. It may be a surrogate marker of disease progression, although no correlation between tumour size and albumin level was identified. Preoperative albumin levels are often used as a marker of nutritional status, although in our study no correlation between preoperative weight loss and hypoalbuminaemia was seen. Finally, albumin level may be a surrogate for synthetic function of the liver. Preoperative elevated INR was significantly associated with preoperative hypoalbuminaemia (P = 0.017), conferring an OR of 4.6 for every 0.1 increase in INR (95% CI 1.308–16.150), which suggests that preoperative hypoalbuminaemia may reflect poor synthetic function. Regardless of the mechanism, surgeons should be aware that marked hypoalbuminaemia is a marker for poor prognosis. In patients with significant hypoalbuminaemia, consideration should be given to a brief programme of intensive nutritional therapy. If nutritional status cannot be optimized, patients may be best served with non-operative treatment (involving chemotherapy, radiation or both) rather than radical resection. These patients could be re-evaluated for surgical candidacy at a later date if they respond to treatment.
Conclusions
Our review of regional cancer centres' experience with ICC corroborates the findings of studies carried out in national centres.3,6,9 Despite the infiltrative and aggressive nature of ICC, surgical therapy is associated with longterm survival in the subset of patients who are nutritionally replete and, based on our small sample size, have node-negative disease, and in whom preoperative studies suggest that an R0 resection is possible. Preoperative portal vein embolization can be considered in order to reduce the morbidity associated with extended liver resections.
Acknowledgments
The authors would like to thank Jun Ma for her assistance with data collection, and Roshanthi Weerasinghe and Brian Diggs for their help with statistical analysis.
Conflicts of interest
None declared.
References
- 1.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 2.Key C, Meisner A. National Cancer Institute, SEER Survival Monograph. Bethesda, MD: NCI, National Institutes of Health; 2008. Surveillance, epidemiology, and end results. 6. Cancers of the liver and biliary tract. [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 4.Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 5.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594–603. doi: 10.1016/j.jamcollsurg.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Jonas S, Thelen A, Benckert C, Biskup W, Neumann U, Rudolph B, et al. Extended liver resection for intrahepatic cholangiocarcinoma: a comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Ann Surg. 2009;249:303–309. doi: 10.1097/SLA.0b013e318195e164. [DOI] [PubMed] [Google Scholar]
- 7.Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma – a population-based study. Ann Surg Oncol. 2008;15:600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 8.Hong SM, Pawlik TM, Cho H, Aggarwal B, Goggins M, Hruban RH, et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery. 2009;146:250–257. doi: 10.1016/j.surg.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, et al. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 11.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 12.Jiang BG, Sun LL, Yu WL, Tang ZH, Zong M, Zhang YJ. Retrospective analysis of histopathologic prognostic factors after hepatectomy for intrahepatic cholangiocarcinoma. Cancer J. 2009;15:257–261. doi: 10.1097/PPO.0b013e31819e3312. [DOI] [PubMed] [Google Scholar]
- 13.Khuri SF, Daley J, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 14.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 15.Virani S, Michaelson JS, Hutter MM, Lancaster RT, Warshaw AL, Henderson WG, et al. Morbidity and mortality after liver resection: results of the Patient Safety in Surgery Study. J Am Coll Surg. 2007;204:1284–1292. doi: 10.1016/j.jamcollsurg.2007.02.067. [DOI] [PubMed] [Google Scholar]


