Abstract
Background
This study assesses the impact of preoperative chemoradiation on recurrence, surgical morbidity, histopathological data and survival in resectable adenocarcinoma of the pancreatic head.
Methods
We carried out a retrospective study with an intention-to-treat analysis. From 1997 to 2006, 173 patients with resectable pancreas head carcinoma were treated in two reference centres in France using different treatment strategies.
Results
Sixty-seven of 85 (79%) patients in the surgery-first (SF) group and 38 of 88 (43%) patients in the chemoradiation (CR) group underwent surgical resection (P < 0.001). Overall morbidity was 40% (15/38) in the CR group and 43% (29/67) in the SF group (P = 0.837). In the CR group, median tumour size was smaller (1.5 cm vs. 3.0 cm; P < 0.001) and fewer patients were node-positive (29% vs. 64%; P = 0.001) than in the SF group. There was less perineural (43% vs. 93%; P < 0.001), lymphatic and vascular (21% vs. 92%; P < 0.001) invasion in the CR group than in the SF group. In both groups, 89% of patients had recurrence (31/35 in the CR group and 57/64 in the SF group; P = 1.000), predominantly involving metastasis and carcinomatosis in the CR group (30/31 vs. 35/57; P < 0.001) and locoregional recurrence in the SF group (24/57 vs. 3/31; P = 0.002). Median survival for all patients and for resected patients in the CR and SF groups was, respectively, 15 months vs. 17 months, and 21 months vs. 18 months (P = non-significant).
Conclusions
Preoperative chemoradiation allows for good local control of the disease but does not increase survival, mainly for reasons of metastatic spread. Other options should be developed to improve both local and distant control of the disease.
Keywords: adenocarcinoma < pancreatic neoplasia, resection < pancreatic neoplasia, radiotherapy < pancreatic neoplasia
Introduction
An estimated 43 140 patients are newly diagnosed with pancreatic cancer and 36 800 deaths occur from pancreatic cancer every year in the USA.1 The incidence of carcinoma of the pancreas has markedly increased over the past several decades and the mortality rate remains high despite a significant improvement in postoperative mortality and a slight improvement in longterm survival2 (the overall 5-year survival rate is 5.6%).1 Surgery is the standard reference treatment for resectable pancreatic head cancers, but curative intent surgery is only achieved in a small subgroup representing about 15% of the overall patient population.3 Several approaches have been proposed to improve survival, including: adjuvant chemotherapy;4,5 preoperative chemoradiation,6–8 and preoperative chemotherapy followed by chemoradiation.9,10 The foremost goal of neoadjuvant treatment is to allow patients to receive the most comprehensive treatment. Additionally, it decreases the proportion of lymph node involvement, limits positive resection margins and decreases tumour size, all of which are prognostic factors for recurrence.2,11 During the last two decades, neoadjuvant treatment has been developed in several specialized pancreatic cancer centres.6–8,10
This study describes the results of treatment of pancreatic surgery in terms of histopathology, morbidity, recurrence and survival at two reference centres in Marseille, France. One of these centres uses a neoadjuvant approach (chemoradiation [CR] group) and the other takes a surgery-first approach (surgery-first [SF] group).
Materials and methods
This was a retrospective study with an intention-to-treat analysis. From January 1997 to December 2006, 173 consecutive patients with resectable pancreatic head carcinoma were treated in two reference centres using different treatment strategies.
Patients
Preoperative staging included physical examination, chest radiography, thin-section contrast-enhanced helical dual-phase computed tomography (CT) scanning and endoscopic ultrasound if required. In the CR group, histological proof was obtained by endoscopic ultrasound (fine needle aspiration with Wilson–Cook 22-gauge, 8-cm needles). Patients with adenocarcinoma of the tail or neck of the pancreas, intraductal papillary mucinous adenocarcinoma, neuroendocrine tumours and carcinoma of the duodenum, distal common bile duct or ampulla of Vater were excluded from the study. Patients with locally advanced cancers (tumour extension to either the superior mesenteric artery [SMA] or coeliac axis, tumour surrounding >180 degrees of the circumference of the portal vein [PV] or superior mesenteric vein [SMV], occlusion of the SMV or PV confluence) or with evidence of hepatic or extrahepatic disease were considered as non-resectable and were excluded. Patients with resectable disease and high serum CA19-9 levels (>350 UI/ml) were included. Biliary stenting using a 10-French plastic stent was performed in the CR group if any sign of cholangitis or complete biliary obstruction occurred. Patients were reassessed after the completion of chemoradiation and surgery was planned for within 4–6 weeks.
Chemoradiation
The prescribed total dose was 45 Gy administered in fractions of 1.8 Gy five times weekly in association with concurrent chemotherapy, which included continuous infusion of 5-fluorouracil (5-FU) (650 mg/m2 on days 1–5 and days 21–25) with a cisplatine bolus (80 mg/day/m2 on days 2 and 22).
Surgery
At laparotomy, inspection of the liver and peritoneal cavity was routinely performed to exclude metastatic disease. In the SF group, a Whipple procedure was performed (without conservation of the pylorus) and reconstruction was achieved using one jejunal loop with an end-to-side pancreaticojejunostomy, an end-to-side hepaticojejunostomy and a gastrojejunostomy, according to the Child procedure.12 In the CR group, dissection of the SMA and eventual biopsy of its wall was first performed to assess local invasion, after which either pancreatojejunal or pancreatogastric anastomosis was performed.
Postoperative course
The definitions of complications were the same in both institutions. A biliary fistula corresponded to bile flow in the draining tubes or to a biliary leak seen during re-intervention. A pancreatic fistula corresponded to the presence of pancreatic juice in the draining tubes (assessed by an amylase level in the drain more than three times as high as that in the plasma) or to a collection in contact with the pancreatic anastomosis with clinical manifestations such as fever or high white blood cell count, or to a pancreatic anastomosis disunion seen perioperatively. Haemorrhage was defined by the loss of haemoglobin associated with either the presence of blood in the draining tubes or with bleeding in the abdominal cavity in cases of re-intervention. The other complications were surgical (e.g. abscess, wound dehiscence, urinary retention, etc.) or medical (e.g. pulmonary or urinary infections, diabetes, postoperative mental confusion, etc.).
Histopathological analysis
Prior to 2002, specimens were orientated, but not stained. From 2002, specimens were routinely stained on the PV bed, the pancreatic transection margin and the retroportal area. A margin was considered positive if tumour cells were present at <1 mm from the resection.13 Tumours were staged according to the TNM (tumour, node, metastasis) classification. Sterilized specimens after chemoradiation were considered to demonstrate a complete pathological response (pT0).
Postoperative treatment
No patient received postoperative chemotherapy in the CR group as medical treatment was considered to have been completed prior to surgery. In the SF group, adjuvant chemotherapy5 was proposed; this consisted of 5-FU-leucovorin (5-FU: 400 mg/m2 in bolus, then 600 mg/m2 in 22-h infusions on days 1 and 2, administered every 2 weeks) from 2004 or gemcitabine (1000 mg/m2 on days 1, 8, 15 and 22) from 2006.
If resection could not be performed, patients in the CR group received additional chemotherapy if there was no metastatic spread (such as in cases in which surgery was contraindicated). In the SF group, non-resectable patients were given chemotherapy (mainly 5-FU-leucovorin) or radiochemotherapy.
Data collection and patient follow-up
Patients were evaluated at 1, 4 and 6 months after surgery and then every year. Evaluation consisted of physical examination, tumour markers and CT scan. The presence and type of recurrence were noted.
Patient data were collected from clinical files and entered retrospectively into databases approved by the review boards of the Mediterranean University, La Conception Hospital and Paoli-Calmettes Institute. Where necessary, information on the patient's current condition was obtained from the patient's oncologist or general practitioner by telephone.
Statistical analysis
Survival was measured from the date of diagnosis to the date of death or census date (1 July 2008). Analysis was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). P < 0.05 was considered statistically significant. Qualitative variables were compared with the Fisher's exact test and quantitative variables with the unpaired t-test or Mann–Whitney U-test; results are given as median (range). Survival was examined using the Kaplan–Meier method and statistical comparisons were conducted using the log-rank (Mantel–Cox) test. Three- and 5-year survival rates are given with 95% confidence intervals (CIs) and medians with ranges.
Results
A total of 173 patients were treated for resectable carcinoma of the head of the pancreas from January 1997 to December 2006; 88 (51%) patients belonged to the CR group and 85 (49%) to the SF group. Preoperative patient characteristics are summarized in Table 1. In the CR group, 37 of 88 (42%) patients did not proceed to surgery because of metastatic (n = 16) or locoregional (n = 16) progression or contra-indications to surgery (n = 4), and one patient died of septic shock during chemoradiation caused by biliary obstruction. Thirty-eight (43%) patients in the CR group and 67 (79%) in the SF group underwent resection (P < 0.001). One patient in the CR group was found perioperatively to have liver cirrhosis. Figure 1 represents the subgrouping of patients within the two groups.
Table 1.
Characteristics of patients in the chemoradiation (CR) and surgery-first (SF) groups
| CR group | SF group | P-value | |
|---|---|---|---|
| Number of patients | 88 | 85 | – |
| Median age, years | 65 (39–81) | 64 (37–79) | 0.195 |
| CA19-9 > 350 UI/ml | 14 (16%) | 13 (15%) | 1.000 |
| Preoperative venous involvementa | 21 (24%) | 19 (22%) | 0.858 |
| Number of resected patients | 38 (43%) | 67 (79%) | <0.001 |
Preoperative venous involvement is defined by the tumour surrounding <180 degrees of the portal vein or superior mesenteric vein, regardless of length
Figure 1.
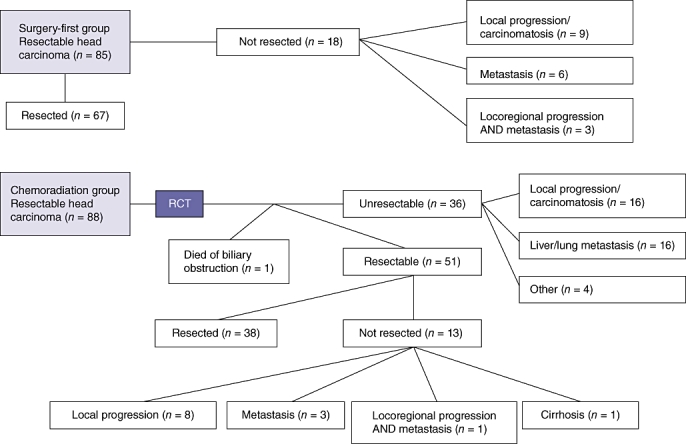
Flow chart showing patient treatment subgroups in the surgery-first and chemoradiation groups, RCT, radiochemotherapy
Surgical outcomes are shown in Table 2. In the CR group, one patient underwent a total pancreatectomy, two underwent a resection extended to organs other than the pancreas or spleen, and six underwent a venous resection (three lateral and three truncal). In the SF group, four patients underwent a resection extended to the pancreas and/or spleen, four patients underwent a resection extended to other organs, and 20 (30%) patients had a venous resection (four lateral and 16 truncal; en bloc resection in eight patients). The difference in the number of venous resections (6/38 [16%] in the CR group vs. 20/67 [30%] in the SF group) was not statistically significant (P = 0.158).
Table 2.
Surgical outcomes in the chemoradiation (CR) and surgery-first (SF) groups
| CR group | SF group | P-value | |
|---|---|---|---|
| Number of patients | 38/88 (43%) | 67/85 (79%) | – |
| Overall morbidity | 15 (40%) | 29 (43%) | 0.837 |
| Complications with re-intervention | 5 (13%) | 11 (16%) | 1.000 |
| Blood transfusion, yes | 2 (5%) | 16 (24%) | 0.016 |
| Median number of units (range) | 2.5 (2–3) | 2 (1–8) | 0.921 |
| Median length of stay, days (range) | 23 (12–72) | 15 (0–80) | 0.001 |
| In-hospital mortality | 3 (8%) | 3 (4%) | 0.665 |
Postoperative complications (biliary fistula, pancreatic fistula, haemorrhage, other complications) occurred in 15 patients in the CR group and 29 patients in the SF group (P = 0.837). Five patients in the CR group and 11 in the SF group required a re-intervention (P = 1.000). In the SF group, 37 of 64 (58%) patients (three of the 67 patients resected died postoperatively) received postoperative chemotherapy.
The results of histopathological examination are shown in Table 3. In the CR group, three patients had no residual cancer in the resected specimen, and nine patients had tumour remnants measuring <1 mm. In the three patients with positive margins, margins were located at the retroportal lamina (identified from 2002 by specific staining on the resected specimen) in two patients and at the posterior surface of the pancreas in one. In the SF group, 22 of the 67 (33%) patients had positive margin(s). Margins involved the portomesenteric vein in eight patients, the SMA in seven, the pancreas section in seven, the retroportal lamina in four and the biliary transection in one. Three patients had two or more positive margins. These differences are statistically significant (P = 0.004).
Table 3.
Histopathological examination outcomes in the chemoradiation (CR) and surgery-first (SF) groups
| CR group | SF group | P-value | |
|---|---|---|---|
| Number of patients | 38 | 67 | – |
| Median tumour size, cm | 1.5 (0–8.0) | 3.0 (0.8–8.0) | <0.001 |
| Total lymph nodes | 9 (1–26) | 12.5 (1–38) | 0.004 |
| Number of node-positive patients | 11/38 (29%) | 42/66 (64%) | 0.001 |
| Perineural invasion | 10/23 (43%) | 56/60 (93%) | <0.001 |
| Lymphatic and vascular invasion | 4/19 (21%) | 47/51 (92%) | <0.001 |
| Positive margins | 3/38 (8%) | 22/67 (33%) | 0.004 |
| Positive vein (if resected) | 3/3 (100%) | 13/18 (72%) | 0.549 |
Data do not always correspond to the total number of patients because some data are missing
No patient was lost to follow-up through to the census date. The median length of follow-up of survivors was 27 months (range: 21–109 months). The median time from diagnosis to surgery in the CR group was 4 months (range: 2–7 months). Recurrence in both groups was 89% (31/35 patients in the CR group, 57/64 patients in the SF group; P = 1.000). Recurrences predominantly involved metastasis and carcinomatosis in the CR group (30/31 [97%] patients vs. 35/57 [61%] in the SF group; P < 0.001), and locoregional disease in the SF group (24/57 [42%] patients vs. 3/31 [10%] in the CR group; P = 0.002). Median recurrence-free survival was 15 months (range: 5–51 months) in the CR group and 11 months (range: 0–162 months) in the SF group (ratio 1.35, 95% CI 0.7–2.0). There was no significant difference in survival between the two groups or among the subgroups (log-rank = 0.75) (Fig. 2A–C), except between patients in whom R0 and R1 resections were achieved (Fig. 2D), irrespective of treatment arm.
Figure 2.
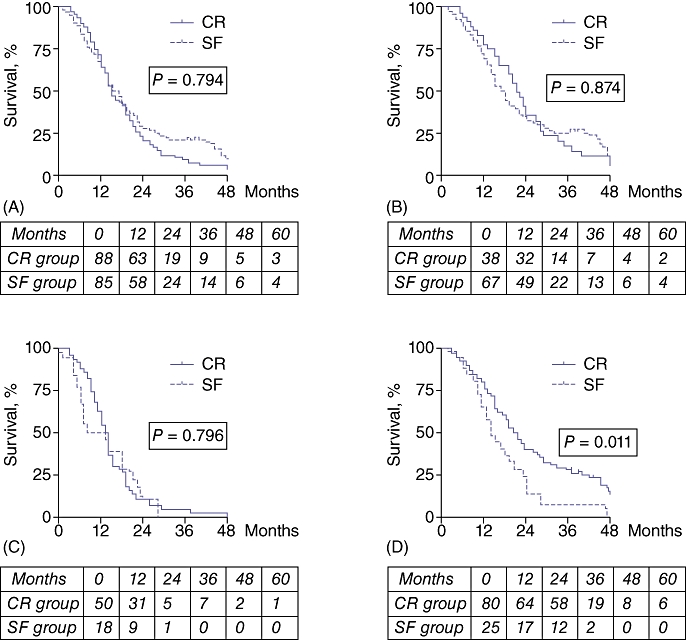
Survival curves for all patients and for resected and non-resected patients. The tables under each graph give the number at risk. (A) Overall survival. Median survival is 15 months (range: 3–72 months) in the chemoradiation (CR) group and 17 months (range: 1–109 months) in the surgery-first (SF) group. Three- and 5-year survival rates are, respectively, 10% (95% confidence interval [CI] 5.3–7.6) and 2% (95% CI 2.0–5.3) in the CR group, and 21% (95% CI 8.4–9.8) and 8% (95% CI 5.5–9.6) in the SF group. (B) Survival of resected patients. Median survival is 21.5 months (range: 5–72 months) in the CR group and 18 months (range: 2–109 months) in the SF group (non-significant). Three- and 5-year survival rates are, respectively, 15% (95% CI 9.4–13.7) and 3% (95% CI 2.8–10.2) in the CR group, and 26% (95% CI 10.2–11.4) and 10% (95% CI 6.9–11.6) in the SF group. (C) Survival of non-resected patients. Median survival is 13.5 months (range: 3–69 months) in the CR group and 10.5 months (range: 1–28 months) in the SF group (non-significant). Three- and 5-year survival rates are 2% (95% CI 1.8–7.2) in the CR group and 0% in the SF group. (D) Survival of patients with R0 vs. R1 margins. Median survival is 20 months (range: 2–109 months) in R0 patients and 14 months (range: 4–47 months) in R1 patients
Discussion
The current study found that fewer patients in the chemoradiation group subsequently underwent resection compared with patients whose treatment followed a surgery-first approach. In addition, histopathological examination revealed smaller tumours, less perineural and lymphatic invasion, fewer positive lymph nodes and fewer positive margins after chemoradiation. Yet despite these findings, recurrence rates and recurrence-free survival rates were identical in the two groups, whether the analyses referred to the overall population, or to the resected or non-resected subsets. However, patterns of recurrence differed; dissemination represented a more common presentation of recurrence than local disease in the CR group. Thus, chemoradiation may have substantial impact on local development of the tumour, but the type of molecules as well as the dosage and delivery pattern may not be efficient enough to sterilize disseminated metastatic cells. This observation has led some authors9 to propose preoperative gemcitabine and cisplatine chemotherapy followed by gemcitabine-based chemoradiation.
Surgery does not appear to be more difficult after chemoradiation, as evidenced by the absence of any significant difference in morbidity and mortality rates; although significant differences were seen in both the proportions of patients receiving blood transfusions and length of stay, these may well be explained by differences in treatment protocols between the two centres.
One potential problem raised by preoperative chemoradiation is reassessment before surgery. In the current study, 32 patients developed contra-indications to surgery because of distant or local spread. However, both CT scans and endoscopic ultrasound are influenced by radiation-induced pancreatic changes, and endoscopic ultrasound does not enable reliable definitive selection for surgery.14 Therefore, dissection of the SMA was performed as the first step in patients who had undergone chemoradiation. In cases of doubt about involvement, frozen sections were analysed and a positive result was considered to rule out a curative resection.
Although the findings of the current study, which show that patients who respond to chemoradiotherapy have more favourable histology and fewer positive margins, are consistent with findings reported from previous studies analysing neoadjuvant chemoradiation,6,11 currently no randomized data analysing longterm outcome on an intention-to-treat basis are available.
The positivity of the margins should be consensually defined. The definition of margin positivity as tumour clearance of ≤1 mm recommended by the Royal College of Pathologists (http://www.rcpath.org), used in our study, can increase the percentage of R1 resections,13 especially in the SF group in the present population. However, the impact of resection status on survival has not yet been proven in other studies and remains controversial.13,15,16 Hernandez et al.17 showed that survival is not improved by extending pancreatic resections to achieve negative margins and that other tumour-specific factors may be involved.
There were several biostatistical limits to this study, the first of which concerns its retrospective and non-randomized nature. The fact that each treatment arm was carried out in a different centre introduces biases related to the general management of the patient (length of stay), the senior operator and the anaesthetic team (surgical technique, blood transfusions, etc.), and to histopathological examination, which was conducted in two different laboratories. However, morbidity and mortality rates did not differ between the centres and the pathology was more accurate because specimens were stained in both centres. The lengthy time period (10 years) to which the study refers also implies changes at different levels: imaging techniques have improved and are likely to have affected preoperative assessment and diagnosis of recurrence; the specimen staining and the standardization of histopathological reports from 2002 results in more precise indications and may have increased the rate of R1 resections (especially in the SF group), and finally, patients in the SF group received systematic postoperative chemotherapy from 2004. For a type 1 error of 0.05, the power of this study (in an intention-to-treat analysis) to test that the difference between the two survival means is not 0 would be 91.5%, which implies a type 2 error risk for failing to identify a difference that truly exists of 8.5%. Despite the statistical issues, the current study attempted to evaluate the outcomes of two different approaches to the treatment of resectable adenocarcinoma of the pancreatic head over a 10-year period. This study shows that neoadjuvant chemoradiation has a positive impact on local control of the disease, but fails to highlight any significant difference in survival rates.
Conflicts of interest
None declared.
References
- 1.National Cancer Institute, US National Institutes of Health. Pancreatic Cancer Treatment. 2010. http://www.cancer.gov. [Accessed 10 October 2010]
- 2.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 3.Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231–241. doi: 10.1016/j.critrevonc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. European Study Group for Pancreatic Cancer A randomized trial of chemotherapy after resection of pancreatic cancer. New Egypt J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 5.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 6.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;21:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 7.Golcher H, Brunner T, Grabenbauer G, Merkel S, Papadopoulos T, Hohenberger W, et al. Preoperative chemoradiation in adenocarcinoma of the pancreas. A single-centre experience advocating a new treatment strategy. Eur J Surg Oncol. 2008;34:756–764. doi: 10.1016/j.ejso.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Mornex F, Girard N, Delpero JR, Partensky C. Radiochemotherapy in the management of pancreatic cancer. Part I: neoadjuvant treatment. Semin Radiat Oncol. 2005;15:226–234. doi: 10.1016/j.semradonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et al. Preoperative gemcitabine and cisplatine followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;21:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 10.Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, et al. Neoadjuvant 5-fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: a 10-year single-institution experience. Oncology. 2009;76:413–419. doi: 10.1159/000215928. [DOI] [PubMed] [Google Scholar]
- 11.Le Scodan R, Mornex F, Partensky C, Mercier C, Valette PJ, Ychou M, et al. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: the French phase II FFCD 9704-SFRO trial. Am J Clin Oncol. 2008;31:545–552. doi: 10.1097/COC.0b013e318172d5c5. [DOI] [PubMed] [Google Scholar]
- 12.Child CG. Pancreaticojejunostomy and other problems associated with the surgical management of carcinoma involving the head of the pancreas: report of five additional cases of radical pancreaticoduodenectomy. Ann Surg. 1944;119:845–855. doi: 10.1097/00000658-194406000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito I, Kleeff J, Bergmann F, Reiser C, Herpel E, Friess H, et al. Most pancreatic resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 14.Bettini N, Moutardier V, Turrini O, Bories E, Monges G, Giovannini M, et al. Preoperative locoregional re-evaluation by endoscopic ultrasound in pancreatic ductal adenocarcinoma after neoadjuvant chemoradiation. Gastroenterol Clin Biol. 2005;29:659–663. doi: 10.1016/s0399-8320(05)82153-0. [DOI] [PubMed] [Google Scholar]
- 15.Raut RP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. doi: 10.1016/j.ejso.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez J, Mullinax J, Clark W, Toomey P, Villadolid D, Morton C, et al. Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg. 2009;250:76–78. doi: 10.1097/SLA.0b013e3181ad655e. [DOI] [PubMed] [Google Scholar]


