Abstract
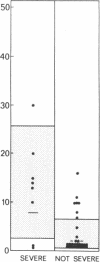
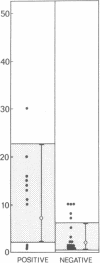
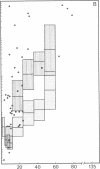
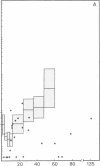
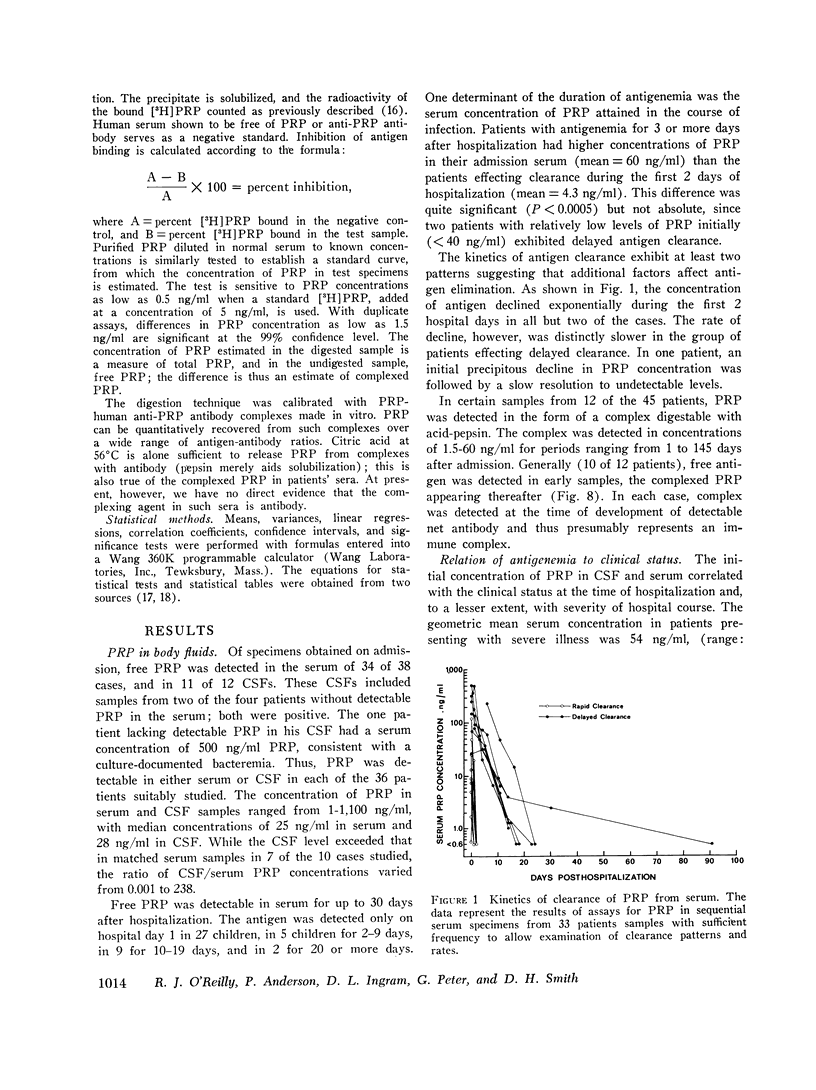
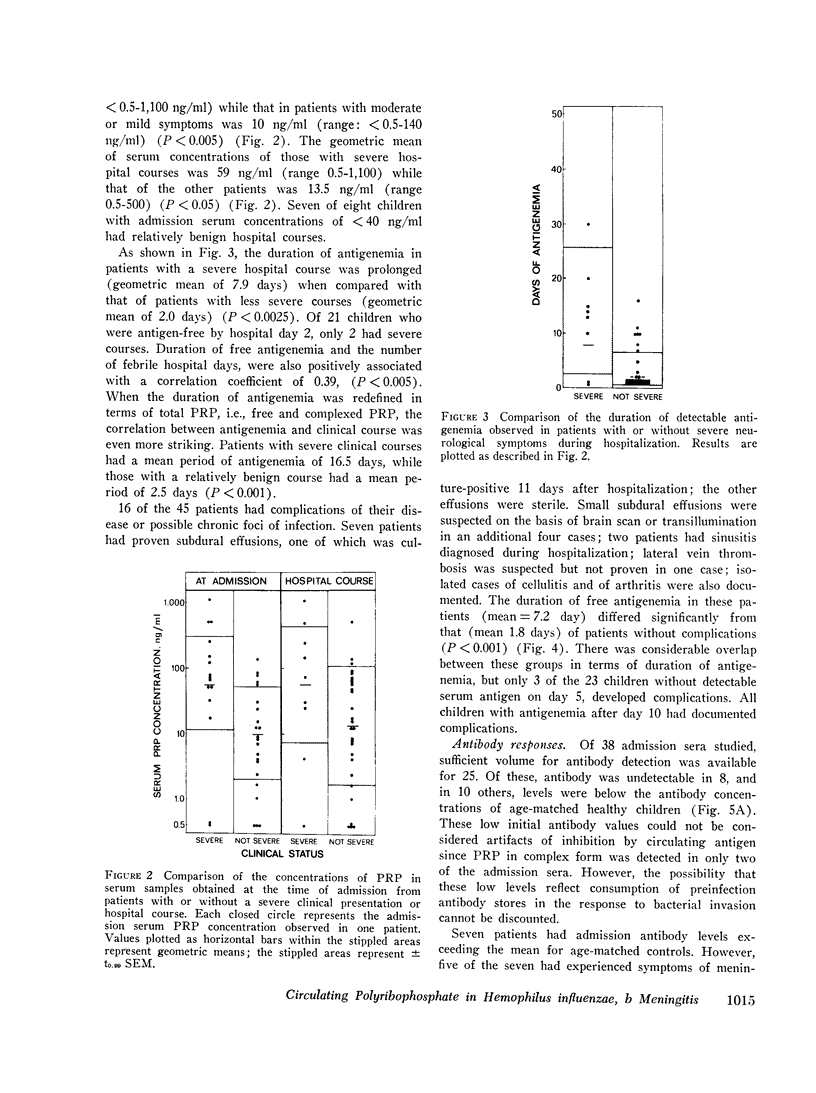
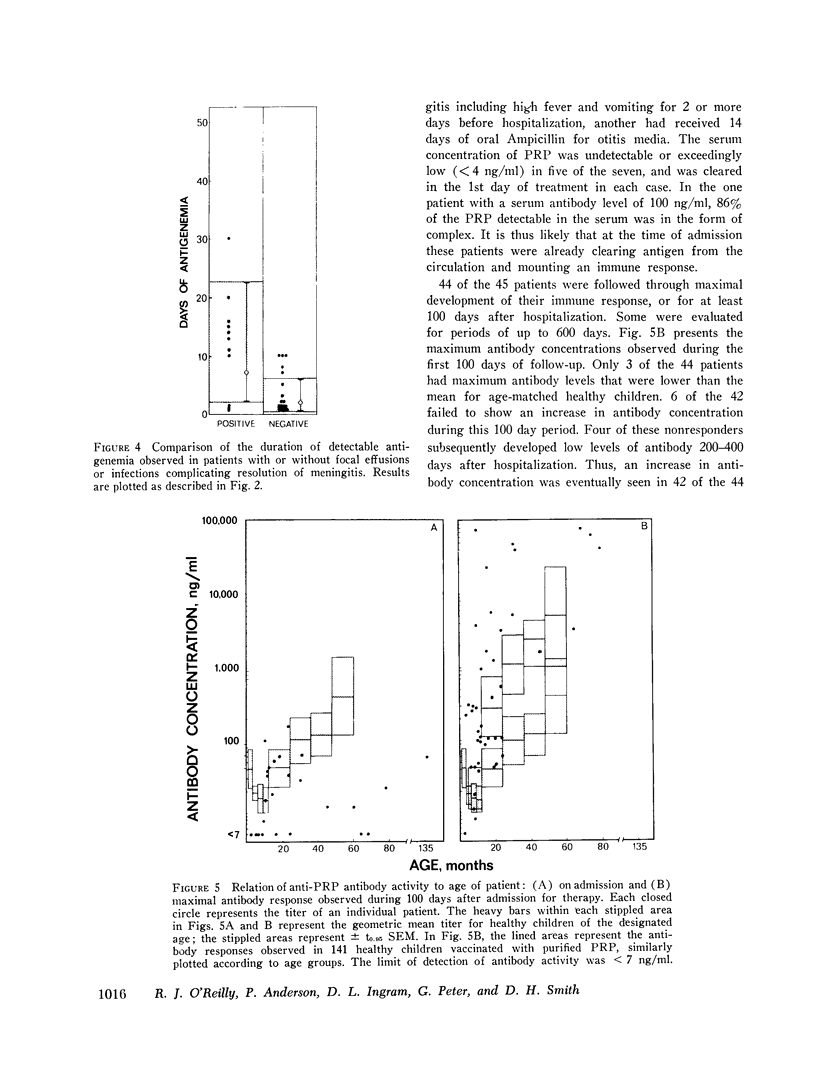
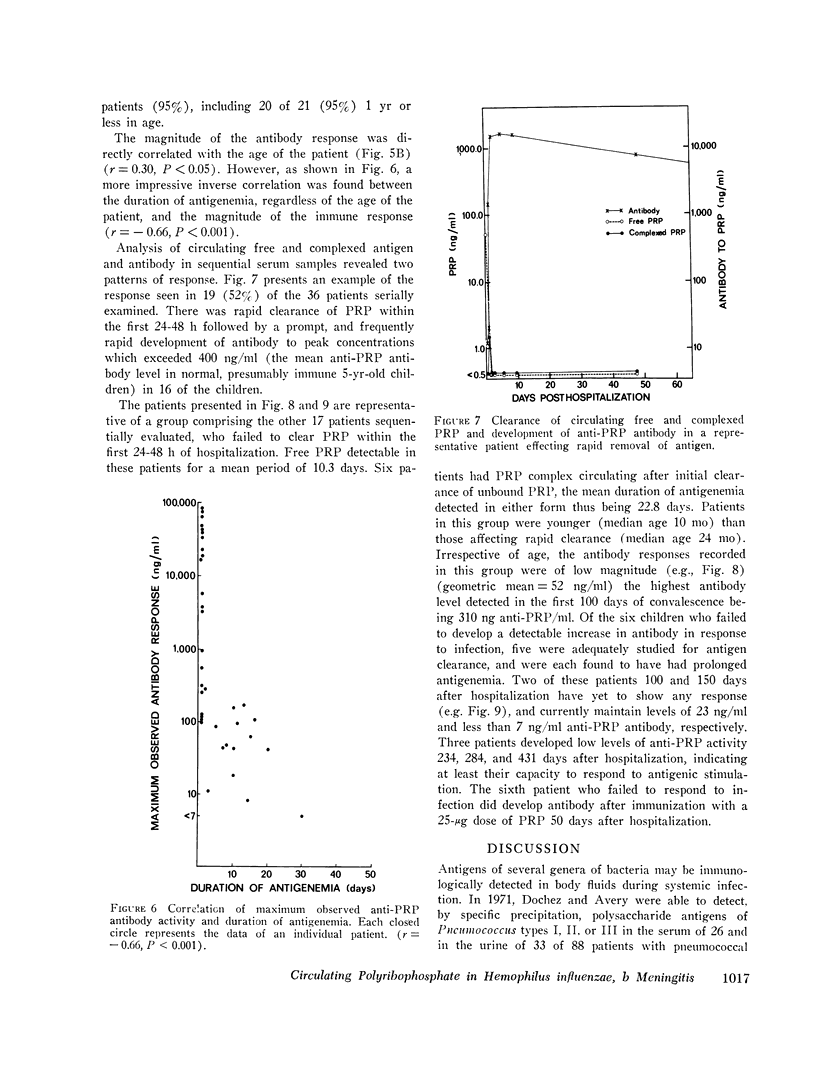
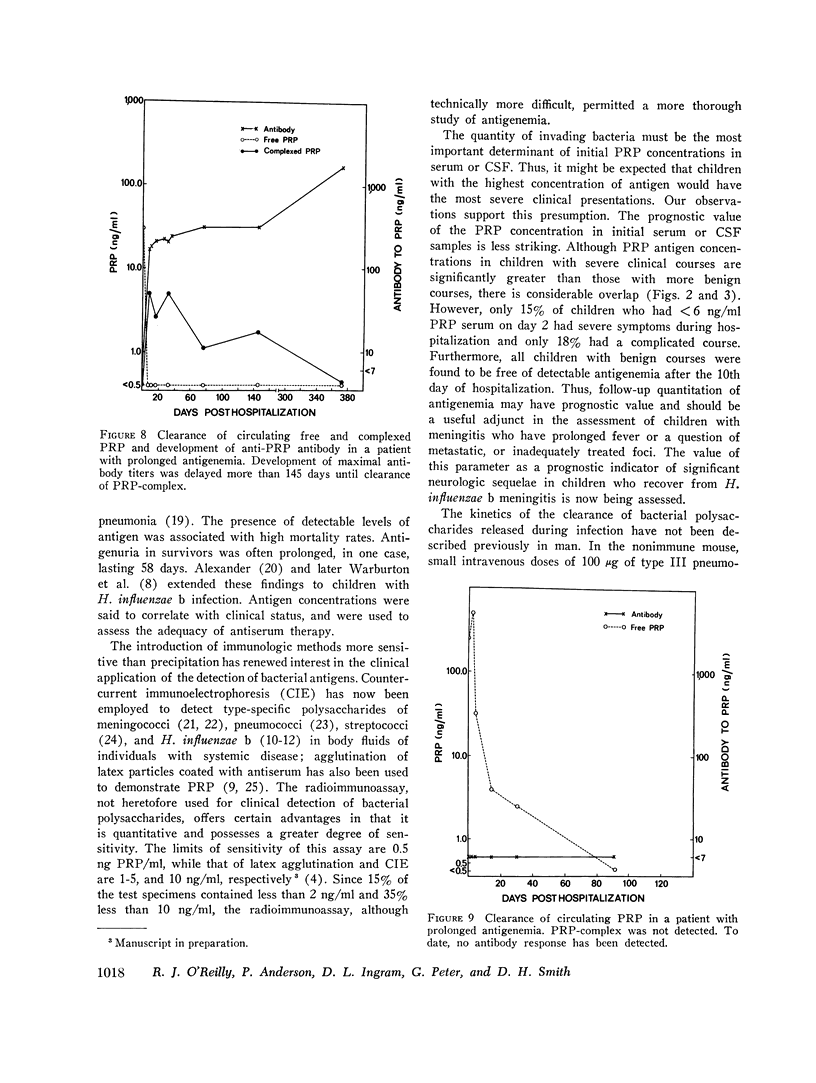
In systemic infections caused by Hemophilus influenzae, type b, the capsular polysaccharide, polyribophosphate, is released into the circulation. Polyribophosphate was quantitated in serial serum and cerebrospinal fluid samples from 45 children with H. influenzae, type b meningitis by means of a radiolabeled antigen-binding inhibition assay. Polyribophosphate was regularly found in acute serum and cerebrospinal fluid samples and could be detected in unbound form for periods of 1-30 days after initiation of effective therapy. Complexes of polyribophosphate dissociable with acid and pepsin were detected in serum samples from 17 patients, in one case for a period of 145 days after hospitalization. Polyribophosphate levels and patterns of clearance were studied in relation to hospital course and antibody response. Patients with prolonged antigenemia had protracted fevers and severe neurological symptoms during hospitalization, frequently with focal complications.Antipolyribophosphate antibody responses were detected during the first 100 days of convalescence by radioimmunoassay in 79% of the patients studied, including 60% of the children 1 yr or less in age. The intensity of antibody response although clearly related to the age of the patient, was more reliably predicted by the efficiency of antigen clearance. Antibody responses were uniformly of low magnitude in patients with prolonged antigenemia, irrespective of age. Paients who failed to develop antibody to polyribophosphate after meningitis also exhibited impaired antigen clearance. These studies suggest that mechanisms necessary for clearance of polyribophosphate may influence the development and intensity of the humoral immune response and raise the possibility of developmental deficiencies in the clearance system in infants and children.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Peter G., Johnston R. B., Jr, Wetterlow L. H., Smith D. H. Immunization of humans with polyribophosphate, the capsular antigen of Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):39–44. doi: 10.1172/JCI106794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byfield P. Increased plaque-forming cell response to pneumococcal polysaccharide type 3-coated sheep erythrocytes after priming with erythrocytes but not with polysaccharide. Cell Immunol. 1972 Apr;3(4):616–622. doi: 10.1016/0008-8749(72)90123-2. [DOI] [PubMed] [Google Scholar]
- Coonrod J. D. Physical and immunologic properties of pneumococcal capsular polysaccharide produced during human infection. J Immunol. 1974 Jun;112(6):2193–2201. [PubMed] [Google Scholar]
- Coonrod J. D., Rytel M. W. Detection of type-specific pneumococcal antigens by counterimmunoelectrophoresis. I. Methodology and immunologic properties of pneumococcal antigens. J Lab Clin Med. 1973 May;81(5):770–777. [PubMed] [Google Scholar]
- Coonrod J. D., Rytel M. W. Determination of aetiology of bacterial meningitis by counter-immunoelectrophoresis. Lancet. 1972 May 27;1(7761):1154–1157. doi: 10.1016/s0140-6736(72)91376-1. [DOI] [PubMed] [Google Scholar]
- Dajani A. S. Rapid identification of beta hemolytic streptococci by counterimmunoelectrophoresis. J Immunol. 1973 Jun;110(6):1702–1705. [PubMed] [Google Scholar]
- Dorff G. J., Coonrod J. D., Rytel M. W. Detection by immunoelectrophoresis of antigen in sera of patients with pneumococcal bacteraemia. Lancet. 1971 Mar 20;1(7699):578–579. doi: 10.1016/s0140-6736(71)91169-x. [DOI] [PubMed] [Google Scholar]
- Edwards E. A. Immunologic investigations of meningococcal disease. I. Group-specific Neisseria meningitidis antigens present in the serum of patients with fulminant meningococcemia. J Immunol. 1971 Feb;106(2):314–317. [PubMed] [Google Scholar]
- FARR R. S. A quantitative immunochemical measure of the primary interaction between I BSA and antibody. J Infect Dis. 1958 Nov-Dec;103(3):239–262. doi: 10.1093/infdis/103.3.239. [DOI] [PubMed] [Google Scholar]
- FELTON L. D., KAUFFMANN G., PRESCOTT B., OTTINGER B. Studies on the mechanism of the immunological paralysis induced in mice by pneumococcal polysaccharides. J Immunol. 1955 Jan;74(1):17–26. [PubMed] [Google Scholar]
- FELTON L. D., PRESCOTT B., KAUFFMANN G., OTTINGER B. Pneumococcal antigenic polysaccharide substances from animal tissues. J Immunol. 1955 Mar;74(3):205–213. [PubMed] [Google Scholar]
- Gotschlich E. C. A simplification of the radioactive antigen-binding test by a double label technique. J Immunol. 1971 Sep;107(3):910–911. [PubMed] [Google Scholar]
- Gotschlich E. C., Goldschneider I., Artenstein M. S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969 Jun 1;129(6):1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green I., Paul W. E., Benacerraf B. The behavior of hapten-poly-L-lysine conjugates as complete antigens in genetic responder and as haptens in nonresponder guinea pigs. J Exp Med. 1966 May 1;123(5):859–879. doi: 10.1084/jem.123.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S., Peter G., Howie V. M., Ploussard J. H., Smith D. H. Acquisition of type-specific antibodies to Hemophilus influenzae type b. J Pediatr. 1972 Feb;80(2):204–208. doi: 10.1016/s0022-3476(72)80579-1. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M., Whittle H. C., Dominic-Rajkovic O. Counter-current immunoelectrophoresis in the diagnosis of meningococcal infections. Lancet. 1971 Sep 4;2(7723):519–521. doi: 10.1016/s0140-6736(71)90439-9. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. IMMUNOLOGICAL UNRESPONSIVENESS TO PROTEIN ANTIGENS IN RABBITS. I. THE DURATION OF UNRESPONSIVENESS FOLLOWING A SINGLE INJECTION AT BIRTH. Immunology. 1964 Jul;7:449–461. [PMC free article] [PubMed] [Google Scholar]
- HUMPHREY J. H., TURK J. L. Immunological unresponsiveness in guinea pigs. I. Immunological unresponsiveness to heterologous serum proteins. Immunology. 1961 Oct;4:301–309. [PMC free article] [PubMed] [Google Scholar]
- Halliday W. J. Immunological paralysis of mice with pneumococcal polysaccharide antigens. Bacteriol Rev. 1971 Sep;35(3):267–289. doi: 10.1128/br.35.3.267-289.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Jacob M. J., Elson J. Studies on immunological paralysis. 3. Recirculation and antibody-neutralizing activity of 14C-labelled type 3 pneumococcal polysaccharide in paralysed mice. Clin Exp Immunol. 1970 Oct;7(4):583–596. [PMC free article] [PubMed] [Google Scholar]
- Ingram D. L., Anderson P., Smith D. H. Countercurrent immunoelectrophoresis in the diagnosis of systemic diseases caused by Hemophilus infleunzae type b. J Pediatr. 1972 Dec;81(6):1156–1159. doi: 10.1016/s0022-3476(72)80252-x. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Humphrey J. H. Synthetic antigens composed exclusively of L- or D- amino acids. II. Effect of optical configuration on the metabolism and fate of synthetic polypeptide antigens in mice. Immunology. 1968 Feb;14(2):225–234. [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Kind P. D. Enhancing effect of bacterial endotoxins on bone marrow cells in the immune response to SRBC. J Immunol. 1972 May;108(5):1453–1455. [PubMed] [Google Scholar]
- KAPLAN M. E., COONS A. H., DEANE H. W. Localization of antigen in tissue cells; cellular distribution of pneumococcal polysaccharides types II and III in the mouse. J Exp Med. 1950 Jan 1;91(1):15-30, 4 pl. doi: 10.1084/jem.91.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R., Smith A. L., Averill D. R., Smith D. H. Haemophilus influenzae meningitis in infant rats after intranasal inoculation. J Infect Dis. 1974 Feb;129(2):154–162. doi: 10.1093/infdis/129.2.154. [DOI] [PubMed] [Google Scholar]
- Mpairwe Y. Immunity to Haemophilus influenzae type B: the nature of the bactericidal antibody in human blood. J Med Microbiol. 1971 Feb;4(1):43–49. doi: 10.1099/00222615-4-1-43. [DOI] [PubMed] [Google Scholar]
- Newman R. B., Stevens R. W., Gaafar H. A. Latex agglutination test for the diagnosis of Haemophilus influenzae meningitis. J Lab Clin Med. 1970 Jul;76(1):107–113. [PubMed] [Google Scholar]
- Norden C. W., Melish M., Overall J. C., Jr, Baum J. Immunologic responses to Hemophilus influenzae meningitis. J Pediatr. 1972 Feb;80(2):209–214. doi: 10.1016/s0022-3476(72)80580-8. [DOI] [PubMed] [Google Scholar]
- SELL S. H. Some observations on acute bronchiolitis in infants. Am J Dis Child. 1960 Jul;100:7–15. doi: 10.1001/archpedi.1960.04020040009005. [DOI] [PubMed] [Google Scholar]
- SMITH R. T., BRIDGES R. A. Immunological unresponsiveness in rabbits produced by neonatal injection of defined antigens. J Exp Med. 1958 Aug 1;108(2):227–250. doi: 10.1084/jem.108.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARK O. K. Studies on pneumococcal polysaccharide. II. Mechanism involved in production of immunological paralysis by type I pneumococcal polysaccharide. J Immunol. 1955 Feb;74(2):130–133. [PubMed] [Google Scholar]
- Schmidtke J. R., Dixon F. J. Immune response to a hapten coupled to a nonimmunogenic carrier. Influence of lipopolysaccharide. J Exp Med. 1972 Aug 1;136(2):392–397. doi: 10.1084/jem.136.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975 May 22;292(21):1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Rodrigues L. P., Parke J. C., Jr, Robbins J. B. Immunity to disease caused by Hemophilus influenzae type b. II. Specificity and some biologic characteristics of "natural," infection-acquired, and immunization-induced antibodies to the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971 Oct;107(4):1081–1089. [PubMed] [Google Scholar]
- Shackelford P. G., Campbell J., Feigin R. D. Countercurrent immunoelectrophoresis in the evaluation of childhood infections. J Pediatr. 1974 Oct;85(4):478–481. doi: 10.1016/s0022-3476(74)80448-8. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- TURK D. C., GREEN C. A. MEASUREMENT OF ANTIBODIES REACTING WITH CAPSULAR ANTIGENS OF HAEMOPHILUS INFLUENZAE. J Clin Pathol. 1964 May;17:294–296. doi: 10.1136/jcp.17.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG V. M., GILLEM H. C., AKEROYD J. H. Sensitization of infant red cells by bacterial polysaccharides of Escherichia coli during enteritis. J Pediatr. 1962 Feb;60:172–176. doi: 10.1016/s0022-3476(62)80032-8. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., LEIDY G., FITZGERALD P. L., ALEXANDER H. E., CHARGAFF E. Polyribophosphate, the type-specific substance of Hemophilus influenzae, type b. J Biol Chem. 1953 Aug;203(2):695–704. [PubMed] [Google Scholar]