Abstract
The purpose of this study was to investigate the effects of an induced period of post-traumatic epilepsy (PTE) on the histopathological damage caused by traumatic brain injury (TBI). Male Sprague Dawley rats were given a moderate parasagittal fluid-percussion brain injury (1.9–2.1 atm) or sham surgery. At 2 weeks after surgery, seizures were induced by administration of a GABAA receptor antagonist, pentylenetetrazole (PTZ, 30 mg/kg). Seizures were then assessed over a 1-h period using the Racine clinical rating scale. To evaluate whether TBI-induced pathology was exacerbated by the seizures, contusion volume and cortical and hippocampal CA3 neuronal cell loss were measured 3 days after seizures. Nearly all TBI rats showed clinical signs of PTE following the decrease in inhibitory activity. In contrast, clinically evident seizures were not observed in TBI rats given saline or sham-operated rats given PTZ. Contusions in TBI-PTZ-treated rats were significantly increased compared to the TBI-saline-treated group (p < 0.001). In addition, the TBI-PTZ rats showed less NeuN-immunoreactive cells within the ipsilateral parietal cerebral cortex (p < 0.05) and there was a trend for decreased hippocampal CA3 neurons in TBI-PTZ rats compared with TBI-saline or sham-operated rats. These results demonstrate that an induced period of post-traumatic seizures significantly exacerbates the structural damage caused by TBI. These findings emphasize the need to control seizures after TBI to limit even further damage to the injured brain.
Key words: epilepsy, immunohistochemistry, neuronal cell death, traumatic brain injury
Introduction
The importance of brain trauma as a risk factor for the development of epilepsy is well established (Annegers et al., 1998; Lowenstein, 2009; Salazar et al., 1985; Vespa, 2005). Post-traumatic epilepsy (PTE) occurs in up to 30% of severe head injuries (Asikainen et al., 1999). Traumatic brain injury (TBI) patients have a three- to fourfold higher risk of developing epilepsy than the general population (Garga and Lowenstein, 2006). In addition, clinical data indicate that PTE can worsen long-term outcome (Asikainen et al., 1999; Bautista and Glen, 2009; Garga and Lowenstein, 2006; Mazzini et al., 2003). Although efforts have been made to reduce this disabling consequence of TBI, epilepsy after TBI can be pharmacoresistant (Loscher and Schmidt, 2002; Temkin et al., 2001). Thus the development of novel anti-epileptogenic regimens is an active area of investigation (Temkin, 2009).
The pathophysiology of TBI is complex and includes both primary and secondary injury mechanisms. The primary injury to the brain that occurs at the moment of impact initiates secondary, delayed injury cascades that spread via multiple mechanisms, including changes in cerebral blood flow, breakdown of the blood–brain barrier, inflammatory processes, and alterations in oxygen delivery and metabolism (Bramlett and Dietrich, 2004). These secondary injury processes lead to necrotic, apoptotic, and autophagic neuronal cell death and synaptic loss (Bigford et al., 2009; Conti et al., 1998; Gao et al., 2008; Grady et al., 2003; Lenzlinger et al., 2001; Lowenstein et al., 1992; Raghupathi, 2004; Scheff et al., 2005). These patterns of neuronal vulnerability subsequently result in long-term behavioral and cognitive abnormalities, even in the absence of seizure development.
Previous studies have demonstrated patterns of neuronal hyperexcitability and seizure activity after TBI (Atkins, Truettner, et al., 2010; Diaz-Arrastia et al., 2000; Frey, 2003; Golarai et al., 2001; Hunt et al., 2009, 2010; Kharatishvili et al., 2006; Kharatishvili and Pitkanen, 2010; Lowenstein et al., 1992; Santhakumar et al., 2001; Vespa et al., 2010). Within the cerebral cortex and hippocampus, for example, areas of circuit hyperactivity have been identified (Graber and Prince, 2004; Griesemer and Mautes, 2007; Lowenstein et al., 1992). In a model of severe fluid-percussion injury (FPI), seizure activity has been observed using both electrophysiological and behavioral monitoring (D'Ambrosio et al., 2004; D'Ambrosio et al., 2009; Kharatishvili et al., 2006; Lowenstein et al., 1992; Nilsson et al., 1994; Santhakumar et al., 2001).
Consistent with the observations of PTE in models of TBI, decreased seizure threshold has been reported in some models of TBI (Golarai et al., 2001; Statler et al., 2008; Zanier et al., 2003). In one study, a non-convulsant dose of pentylenetetrazole (PTZ), a GABAA receptor antagonist, induced generalized tonic–clonic seizures in animals 15 weeks after the weight-drop contusion injury (Golarai et al., 2001). Zanier and colleagues (2003) reported that the administration of low concentrations of kainic acid 1 h after FPI increased local glucose metabolic rates in the hippocampus and produced a loss of ipsilateral hippocampal CA3, CA4, and hilar neurons. These results suggest that seizure activity after TBI may worsen outcome and exaggerate the primary insult (Asikainen et al., 1999; Mazzini et al., 2003; Skandsen et al., 2008; Vespa, 2005). In patients, the development of PTE is associated with a lower Glasgow Outcome Score, behavioral problems, and hippocampal atrophy (Hitiris et al., 2007; Thapa et al., 2010; Vespa et al., 2010; Wang et al., 2008). Currently however, there is a lack of information on the effects of PTE on quantitative histopathological damage at later time periods after experimental brain trauma. This is particularly important, since the histopathological consequences of TBI evolve over the course of days to months post injury, and thus there may be epochs of neuronal vulnerability in response to the double insult of a post-traumatic seizure (Vespa et al., 2010).
In the present study, rats received a moderate FPI or sham surgery, and 2 weeks later, seizure threshold was assessed by challenging the animals with PTZ. Epileptic seizures were assessed in the three animal groups using a clinical grading scale (Racine, 1972). At 3 days after the PTZ challenge, contusion volume and neuronal cell loss in the cerebral cortex and hippocampus were quantitatively assessed and compared to TBI animals that received vehicle or to sham-operated control animals. We report that post-traumatic seizures significantly worsened structural damage compared to TBI alone.
Methods
Animal groups
All procedures were approved by the University of Miami Animal Care and Use Committee and were carried out according to the NIH Guide for the Care and Use of Laboratory Animals. Animals were maintained at a constant temperature (23–25°C) for at least 7 days before the study on a 12-h light/dark cycle. Rats were allowed free access to water and food, with the exception of 24 h prior to the surgery, where food was withheld overnight.
Surgical preparation and traumatic brain injury model
Twenty-five adult male Sprague Dawley rats (294–384 g) were randomized into three experimental groups: TBI-PTZ group (n = 10), TBI-SAL group (n = 10), and Sham-PTZ group (n = 5). Rats were anesthetized with 5% isoflurane, 70% N2O, and 30% O2, and received a 4.8-mm craniotomy (3.8 mm posterior to the bregma, 2.5 mm lateral to the midline) to affix a plastic hub (3.5 mm inside diameter) over the right parasagittal parietal cortex. Twenty-four hours after the craniotomy, the animals were re-anesthetized with 5% isoflurane, 70% N2O, and 30% O2, and intubated. Pancuronium bromide (0.5 mg/kg, i.v.) was administered to immobilize the rats and assist mechanical ventilation. Arterial blood pressure, blood gases, and blood pH were monitored for 30 min prior to and up to 1 h after TBI to maintain physiological ranges of blood pH between 7.35 and 7.45, pCO2 between 35 and 40 mm Hg, and pO2 between 105 and 140 mm Hg. After stabilization, the animals received a moderate (1.9–2.1 atm) fluid-percussion pulse. The fluid-percussion device was calibrated each time prior to injury to the same reading. The atmospheric pressure was measured at the time of injury for all animals and showed very little variability. Sham-operated controls underwent all surgical steps but did not receive the fluid pulse. Head temperature was monitored with a probe placed in the left temporalis muscle, and core temperature was monitored with a rectally placed thermistor. Self-adjusting feedback warming lamps were used to control head and body temperature at 36.5–37.0°C.
Seizure threshold determination
At 2 weeks after TBI, animals were randomized into TBI-saline or TBI-PTZ treatment groups. Prior to behavioral testing and drug administration, the animals were habituated in a clear plastic cage for 30 min. Animals then received PTZ (30 mg/kg; Sigma-Aldrich, St. Louis, MO) or saline intraperitoneally. This dose of PTZ was based on published data (Golarai et al., 2001) and our own pilot studies showing that this dose is convulsant after TBI but subconvulsant in non-injured animals. After receiving PTZ, each animal was observed for 1 h in a clear plastic cage by an investigator blinded to the treatment groups. During this period of observation, seizure class, duration, onset, and number were measured. Seizure class was scored as follows (Racine, 1972): Class 1, myoclonic (brief shock-like jerks of a muscle or a group of muscles); Class 2, unilateral clonic (rhythmic, rapidly alternating contraction and relaxation of a muscle or muscle group) lasting less than 1 min; Class 3, bilateral clonic lasting less than 1 min; Class 4, bilateral clonic sustained, lasting more than 1 min; Class 5, tonic–clonic (all muscles stiffen with loss of postural control alternating with sustained clonic).
Contusion volume measurements
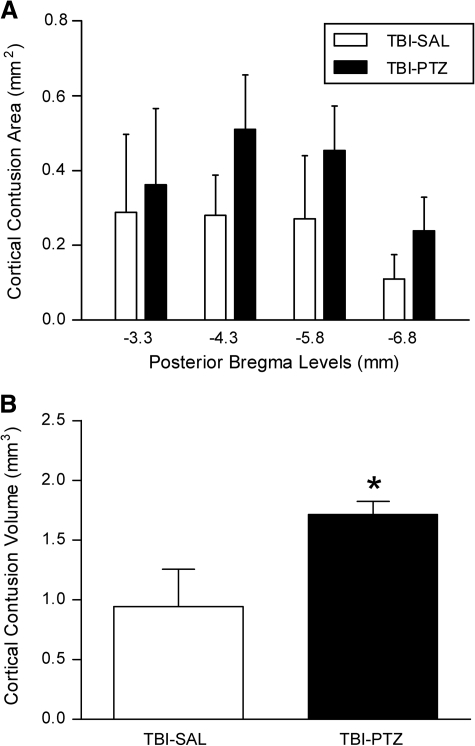
At 3 days after seizure assessment, animals were deeply anesthetized and perfusion-fixed transcardially with cold physiologic saline (75 ml) followed by 4% paraformaldehyde (350 ml) in 0.1M phosphate buffer (pH 7.4) at a pressure of 100–120 mm Hg. Following perfusion, brains were removed and immersed in 4% paraformaldehyde at 4°C for 3 days. Brains were then blocked and embedded in paraffin. Tissue sections 10-μm thick were taken at 150-μm intervals to quantify cortical contusion volume and neuronal survival (Atkins et al., 2007; Dietrich et al., 1994a). The cortical contusion volume was quantified by outlining the entire cortical contusion in sequential hematoxylin and eosin-stained sections (14–20 sections/animal, depending upon the contusion size) using an Olympus BX51TRF microscope (Olympus America, Center Valley, PA) at 20 × magnification and the Neurolucida 7.50.1 software program (MicroBrightField Inc., Williston, VT). The cortical contusion boundaries were well demarcated, and represented an area of shearing damage between the parietal cortex and external capsule and consisted of pyknotic neurons, reactive astrocytes, macrophage infiltration, and disordered white matter tracts. Images representing the cortical contusion at bregma level −5.8 mm are shown and were taken at 20 × magnification by montaging with the Neurolucida 7.50.1 software program.
Neuronal cell counts
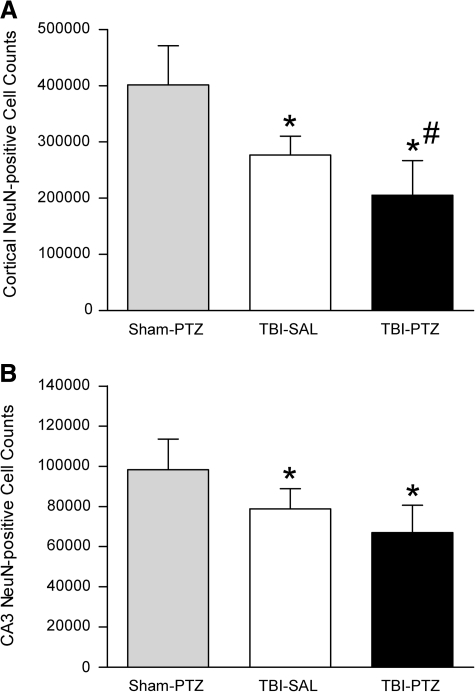
For quantification of neuronal loss, serial sections (10-μm thick, 150-μm intervals) were immunostained for NeuN, a neuronal marker (Atkins et al., 2007). Sections were deparaffinized, rehydrated, and incubated overnight at 4°C with anti-NeuN (1:500; Millipore, Temecula, CA). After washing with 0.1M PBS (pH 7.4) and 0.4% Triton, secondary anti-mouse IgG antibody (1:1000; Vector Laboratories, Burlingame, CA) was applied for 90 min at room temperature. After further washing, ABC Elite (Vector Laboratories) was applied for 90 min, then slides were rinsed with PBS followed by Acetate-Imidasole Buffer (pH 7.2) and reacted with NiDAB (2.5% Nickel Ammonium Sulfate Acetate-Imidasole Buffer, 0.05% DAB, 0.001% H2O2); the reaction was stopped with Acetate-Imidasole Buffer. Slides were then dehydrated, cleared, and cover-slipped for analysis. Images of NeuN-immunostained sections were taken at bregma levels −5.8 mm at 4 × or 60 × and montaged using the Neurolucida 7.50.1 software program.
The parietal cortex overlying the contusion and the hippocampal CA3 region encompassing bregma levels −3.3 to −5.8 mm were identified unequivocally by an investigator blinded to the treatment groups. Between bregma levels −4.6 to −5.8 mm in six serial sections from each animal, the parietal cortex above the cortical contusion was contoured from the external capsule to the pia to encompass all six lamina (grid size 235 × 225 μm) using an Olympus BX51TRF microscope at 4 × . Between bregma levels −4.8 and −5.8 mm in five serial sections from each animal, the hippocampal CA3 pyramidal cell layer was contoured (grid size 170 × 60 μm) using an Olympus BX51TRF microscope at 4 × . Using a 35 × 35 μm counting frame, NeuN-positive cells were counted in 25–40 randomly placed sampling sites within each contoured section (section thickness 8 μm) using StereoInvestigator 7.50.1 software (MicroBrightField, Inc.) with a 60 × , 1.42 NA objective. Q values ranged from 113 to 353 for the parietal cortex and 271 to 496 for the hippocampal CA3 pyramidal cell layer. The CE values for the parietal cortex cell counts ranged from 0.08 to 0.10 (0.09 ± 0.01) for the sham-PTZ group, 0.08 to 0.12 (0.10 ± 0.01) for the TBI-saline group, and 0.10 to 0.15 (0.12 ± 0.02) for the TBI-PTZ group. The CE values for the hippocampal CA3 pyramidal cell layer ranged from 0.09 to 0.13 (0.11 ± 0.02) for the sham-PTZ group, 0.11 to 0.17 (0.14 ± 0.02) for the TBI-saline group, and 0.08 to 0.15 (0.12 ± 0.03) for the TBI-PTZ group.
Statistical analysis
Data are presented as mean ± standard deviation. A Kruskal–Wallis ANOVA on ranks was performed for the seizure behavior data with a post hoc Dunn's t test. A one-way ANOVA was used for the physiological measurements during surgery, contusion volumes, and cortical and hippocampal CA3 neuronal counts. A Student's t test was used for comparison of the atmospheric pressure given to the TBI animal groups. A repeated-measures two-way ANOVA was used for the contusion areas. Tukey HSD tests were used for post hoc analysis of the one-way and two-way ANOVAs. Significance was set at p < 0.05.
Results
For all animal groups, all physiological variables were within normal ranges and no attrition was observed. Body weight, F(2, 22) = 0.472, p = 0.630, and physiological measurements made 15 min after surgery of pCO2, F(2, 22) = 1.087, p = 0.355, pO2, F(2, 22) = 2.573, p = 0.099, mean arterial blood pressure, F(2, 22) = 0.307, p = 0.739, pH, F(2, 22) = 1.162, p = 0.331), and rectal temperature, F(2, 22) = 1.951, p = 0.166, showed no significant differences among groups. There was a slight decrease in head temperature in both TBI-saline treated animals (TBI-SAL) and TBI-PTZ treated animals (TBI-PTZ) as compared to sham-surgery animals 15 min post injury, F(2, 22) = 11.08, p < 0.001; sham 36.98 ± 0.045°C, TBI-SAL 36.86 ± 0.052°C, TBI-PTZ 36.87 ± 0.048°C, but no significant differences between TBI-SAL and TBI-PTZ groups. For the TBI groups, atmospheric pressure levels were not significantly different (TBI-SAL 2.04 ± 0.03 atm, TBI-PTZ 1.97 ± 0.03 atm).
Seizure threshold determination
After injection with PTZ, nearly all TBI rats (90%) exhibited behaviorally evident seizure activity ranging from Class 1 (80%) to Class 2 (10%) and Class 3 (30%) (Racine, 1972). No seizures were observed behaviorally in TBI-saline-treated animals (TBI-SAL) or sham-PTZ-treated animals (Table 1). Thus there was a significant increase in the number of seizures, F(2, 22) = 9.235, p < 0.01, in TBI animals given PTZ compared to TBI animals given saline or sham animals given PTZ. Similarly, the highest seizure class (Class 3) exhibited by the TBI-PTZ animals was significantly higher (p < 0.001) compared to TBI-SAL or sham-PTZ animals.
Table 1.
Characterization of Seizures after Traumatic Brain Injury
| Groups | Number of seizures | Seizure duration (sec) |
|---|---|---|
| Sham-PTZ | 0 ± 0.00 | 0 ± 0.00 |
| TBI-SAL | 0 ± 0.00 | 0 ± 0.00 |
| TBI-PTZ | 2.7 ± 2.41* | 1.9 ± 3.48 |
p < 0.05 sham-PTZ vs. TBI-PTZ; p < 0.01 TBI-SAL vs. TBI-PTZ.
Qualitative histopathology
A well-demarcated cortical contusion near the external capsule in the parietal cortex was identified by hematoxylin and eosin-staining (Fig. 1A,D). This was characterized by extravasation of inflammatory cells, vacuoles, and disordered white matter tracts. A region of selective neuronal dropout in the overlying ipsilateral parietal cortex near the contusion and within the ipsilateral CA3 hippocampal region was also consistently observed using NeuN staining (Fig. 1B,E). Higher magnification of the CA3 hippocampus of TBI animals clearly showed neuronal dropout in this vulnerable brain region (Fig. 1C,F). TBI animals injected with PTZ demonstrated a larger cortical contusion and an increase in neuronal dropout in the parietal cerebral cortex. Higher magnification of the CA3 region of the hippocampus indicated more neuronal loss within this vulnerable area in TBI-PTZ animals compared to TBI-saline animals.
FIG. 1.
Representative images of TBI animals 2 weeks post injury given either saline (A–C) or PTZ (D–F). The contusion in the parietal cortex was well demarcated (dotted lines) when stained with hematoxylin and eosin in both TBI-SAL (A) or TBI-PTZ animals (D). NeuN-immunostaining revealed select neuronal dropout in both the parietal cortex and CA3 region of the hippocampus in both TBI-SAL (B) and TBI-PTZ animals (E). Higher magnification of the CA3 area of hippocampus clearly demonstrated neuronal loss in this vulnerable area in TBI-SAL animals (C) that was exacerbated in TBI-PTZ animals (F). All images were taken at bregma level −5.8 mm. Scale bars (A,D): 500 μm; (B,E): 1 mm; (C,F): 250 μm.
Contusion areas and volumes
There was no pathology evident in sham animals injected with PTZ (not shown). In contrast, all TBI rats had visible, well-demarcated, cortical contusions along the gray/white matter interface. Repeated-measures analysis for cortical contusion areas was significant for group, F(1, 18) = 21.665, p < 0.001, and bregma level, F(3, 18) = 8.831, p < 0.001). Thus TBI animals injected with PTZ had increased contusion areas (Fig. 2A). One-way ANOVA was also significant, F(1, 18) = 27.085, p < 0.001, for overall contusion volume as well. These contusion volumes (TBI-SAL 0.943 ± 0.312 mm3, TBI-PTZ 1.713 ± 0.348 mm3) are smaller than our previous reports of 2–4 mm3 simply because the cortical contusion resolves over time and our previous measurements were made at 3 days post injury (Atkins et al., 2007; Suzuki et al., 2004). Interestingly, TBI-PTZ animals exhibited a greater increase in contusion volume compared to TBI-saline animals (Fig. 2B, p < 0.001). Therefore, seizure activity induced at 2 weeks post TBI appears to exacerbate cortical contusions in TBI animals compared to those TBI rats not exhibiting overt seizures.
FIG. 2.
Quantification of cortical contusion volumes and areas at specific bregma levels at 2 weeks post TBI. (A) Cortical contusion areas were significantly different between group (p < 0.05) and bregma level (p < 0.05). Data indicate that animals administered PTZ 2 weeks after TBI (TBI-PTZ) had a greater amount of damage compared to TBI animals injected with saline (TBI-SAL). (B) Similar results were observed with cortical contusion volumes. Animals that received PTZ after TBI that resulted in seizures produced an increase in cortical contusion volume compared to TBI animals that were only injected with saline and exhibited no seizures (*p < 0.001 TBI-PTZ vs. TBI-SAL). Data represent mean ± standard deviation.
Neuronal cell counts
In NeuN-stained sections of the brain, no apparent patterns of neuronal loss were observed in the sham-operated animals (not shown). The parasagittal FPI model results in significant neuronal death in the ipsilateral parietal cerebral cortex overlying the cortical contusion and in the CA3 region of the hippocampus as compared to sham-operated rats (Atkins et al., 2007; Dietrich et al., 1994; Grady et al., 2003). We chose to focus on bregma levels −4.8 to −5.8 mm of the parietal cortex and CA3 region of the hippocampus for our analysis, since these bregma levels could be easily identified and exhibit the greatest degree of pathology after parasagittal FPI. Analysis using a one-way ANOVA revealed significant differences for both cortical neuronal loss, F(2, 22) = 22.18, p < 0.001 (Fig. 3A), and hippocampal neuronal loss in the CA3 region of the hippocampus from bregma levels −4.8 to −5.8 mm, F(2, 20) =9.821, p < 0.01 (Fig. 3B). Neuronal counts in the parietal cortex and CA3 region of the hippocampus from both TBI animals groups, saline- and PTZ-treated, were significantly decreased compared to sham-PTZ treated animals (parietal cortex p < 0.001, CA3 region of the hippocampus Sham-PTZ vs. TBI-Sal p < 0.05, Sham-PTZ vs. TBI-PTZ p < 0.001). In addition, post hoc analysis indicated that there was greater neuronal cell loss in the parietal cortex of TBI animals administered PTZ compared to TBI animals injected with saline (p < 0.05). Although there was a trend for TBI-PTZ animals to have less NeuN-positive cells in the hippocampal CA3 region compared to TBI-saline animals, this was not significant. These data indicate that PTZ-induced seizures may have contributed to increased neuronal cell loss in the parietal cortex.
FIG. 3.
Quantification of neuronal counts within the parietal cortex and hippocampal CA3 region between bregma levels −3.3 to −5.8 mm at 2 weeks post TBI. (A) NeuN-positive cortical cell counts were significantly decreased (*p < 0.001) in TBI-SAL treated animals and TBI-PTZ treated animals as compared to sham-surgery animals given PTZ (sham-PTZ). Seizure induction resulted in further loss of cortical neurons in TBI-PTZ animals compared to TBI-SAL animals (#p < 0.05). (B) Similar findings were observed in the CA3 hippocampal region. Analysis using a one-way ANOVA of CA3 hippocampal neuronal cell counts was significant for group (p < 0.01). Both TBI groups were significantly different from the sham group (sham-PTZ vs. TBI-Sal *p < 0.05, sham-PTZ vs. TBI-PTZ *p < 0.001). TBI-PTZ animals demonstrated a trend for a decrease in CA3 neuronal counts compared to TBI-saline-administered animals that was not significant. Data represent mean ± standard deviation.
Discussion
Our results demonstrate that the traumatically injured brain is vulnerable to induced periods of PTE as late as 2 weeks following moderate FPI in rats. With the use of the convulsive agent PTZ, we report that an induced period of seizures 2 weeks after TBI significantly aggravated the histopathological consequences of TBI. We report for the first time that overall cortical contusion area and volume were significantly increased in animals that underwent induced seizure activity compared to TBI animals treated with saline and not demonstrating clinically evident seizures. In addition, a significant dropout of NeuN-immunoreactive neurons within the parietal cerebral cortex was also observed. In contrast, we did not observe aggravated loss of hippocampal CA3 neurons after an induced period of seizures 2 weeks post TBI. Taken together, these data demonstrate that there is increased histopathological vulnerability of the post-traumatic brain to periods of seizures induced by PTZ.
The reasons for the reduced threshold of seizure activity after TBI are multifactorial. Following TBI, certain neuronal populations are highly vulnerable that may affect specific neuronal circuits leading to abnormal patterns of neuronal activity (Swartz et al., 2006). These areas include neuronal populations within the dentate gyrus, as well as other hippocampal and cortical regions (Lowenstein et al., 1992; Ross and Soltesz, 2000). In addition, altered blood–brain barrier permeability has also been shown to contribute potentially to PTE in both clinical and experimental studies (D'Ambrosio, Fairbanks, et al., 2004). In the present model, recent studies have shown that the blood–brain barrier is highly permeable to low molecular weight tracers weeks after the primary insult, indicating long-term breakdown of the blood–brain barrier (Lotocki et al., 2009). In addition, post-traumatic inflammatory cascades, including astrocytic activation, are also induced after TBI and may also contribute to the high incidence of PTE in the post-traumatic setting (Somera-Molina et al., 2007). Thus therapeutic interventions that target altered seizure threshold may have to impact multiple pathophysiological events secondary to TBI.
The present study utilized a clinical scale to evaluate seizure activity following the injection of a subthreshold dose of PTZ (Racine, 1972). In contrast to sham-operated animals where little to no evidence of seizure activity was behaviorally observed, animals 2 weeks following moderate FPI commonly showed evidence of seizure activity that could be qualitatively assessed in terms of the animals' behavior. However, since we did not perform electrophysiological measurements, we cannot rule out the possibility that sham-operated animals had subclinical epileptic activity that was not detected behaviorally. Previous studies have correlated these clinical measures of seizure activity to abnormal electrophysiological recordings taken from both cortical and subcortical areas (D'Ambrosio et al., 2004; D'Ambrosio et al., 2009; Hunt et al., 2009, 2010; Kharatishvili et al., 2006; Kharatishvili and Pitkanen, 2010). These studies emphasize that in the clinical situation, periods of PTE could have profound effects on the natural history of the injury process and the long-term consequences of the primary insult (Vespa et al., 2010).
PTE is a relatively common clinical consequence for TBI patients and has been reported to worsen long-term outcome (Frey, 2003; Hitiris et al., 2007; Lowenstein, 2009; Mazzini et al., 2003; Temkin, 2009; Thapa et al., 2010; Vespa et al., 2010; Wang et al., 2008). Clinical studies have demonstrated that spontaneous episodes of PTE occur at variable time periods after TBI and are considered an important second injury mechanism after trauma. In experimental studies, researchers have also reported spontaneous episodes of PTE most commonly after severe TBI in rodent models (Coulter et al., 1996; D'Ambrosio et al., 2004; D'Ambrosio et al., 2009; Hunt et al., 2009, 2010; Kharatishvili et al., 2006; Kharatishvili and Pitkanen, 2010; Lowenstein et al., 1992; Nilsson et al., 1994; Santhakumar et al., 2001). In these studies, PTE is commonly recorded in a subpopulation of rats with injury strategies leading to high mortality. In the present study, we chose to utilize a pharmacological approach by which a sub-threshold dose of an epileptic drug could reproducibly induce clinical features of PTE in rats following more moderate FPI (Coulter et al., 1996; Golarai et al., 2001; Reeves et al., 1997). This study therefore allowed us then to evaluate critically the histopathological consequences of TBI with or without an induced period of PTE.
The exacerbation of histopathological damage following an induced period of PTE is likely due to multiple mechanisms. PTE may have metabolic and hemodynamic consequences that could create further structural damage by aggravating microvascular events leading to increased edema and intracranial pressure (Vespa, McArthur et al., 2003a; Vespa et al., 2003b). Indeed, periods of PTE have been reported to reduce blood flow in local brain regions that are already at risk to ischemia due to the brain trauma (D'Ambrosio et al., 2004). We specifically observed worse pathology with the cortical contusion and neuronal loss in the parietal cortex – areas that are highly associated with breakdown of the blood–brain barrier and inflammation (Cortez et al., 1989; Dietrich 1994; Dietrich et al., 2004; Habgood et al., 2007; Toulmond and Rothwell, 1995). Breakdown of the blood–brain barrier not only occurs during TBI but during seizures as well (Ivens et al., 2007; Oby and Janigro, 2006; Tomkins et al., 2008). In addition, compromise of the blood–brain barrier can itself induce epileptiform discharges, as well as seizures (Seiffert et al., 2004; van Vliet et al., 2007). Thus it is proposed that the present model of induced PTE may be advantageous in clarifying the cellular and molecular mechanisms underlying this increased neuronal vulnerability of the post-traumatic brain to an induced period of abnormal neuronal activation.
In summary, these data emphasize that the post-traumatic brain is extremely sensitive to patterns of abnormal neuronal activation. These studies therefore highlight the need to control or inhibit these events occurring at variable time periods after the primary insult that could have adverse effects on long-term recovery. Treatment strategies that can be initiated in the acute or more chronic phase after TBI need to be investigated to target this potentially devastating second insult.
Acknowledgments
We thank Dr. Beata Frydel for assistance with the histopathological analysis and Jeremy Lytle for editorial assistance. This work was supported by NIH NS030291 and NS042133.
Author Disclosure Statement
No competing financial interests exist.
References
- Annegers J.F. Hauser W.A. Coan S.P. Rocca W.A. A population-based study of seizures after traumatic brain injuries. N. Engl. J. Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Asikainen I. Kaste M. Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: Brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40:584–589. doi: 10.1111/j.1528-1157.1999.tb05560.x. [DOI] [PubMed] [Google Scholar]
- Atkins C.M. Oliva A.A., Jr. Alonso O.F. Pearse D.D. Bramlett H.M. Dietrich W.D. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp. Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C.M. Truettner J.S. Lotocki L. Sanchez-Molano J. Kang Y. Alonso O.F. Sick T.J. Dietrich W.D. Bramlett H.M. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07467.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista R.E. Glen E.T. Seizure severity is associated with quality of life independent of seizure frequency. Epilepsy Behav. 2009;16:325–329. doi: 10.1016/j.yebeh.2009.07.037. [DOI] [PubMed] [Google Scholar]
- Bigford G.E. Alonso O.F. Dietrich W.D. Keane R.W. A novel protein complex in membrane rafts linking the Nr2b glutamate receptor and autophagy is disrupted following traumatic brain injury. J. Neurotrauma. 2009;26:1–18. doi: 10.1089/neu.2008.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett H.M. Dietrich W.D. Pathophysiology of cerebral ischemia and brain trauma: Similarities and differences. J. Cereb. Blood Flow Metab. 2004;24:133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- Conti A.C. Raghupathi R. Trojanowski J.Q. McIntosh T.K. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 1998;18:5663–5672. doi: 10.1523/JNEUROSCI.18-15-05663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez S.C. McIntosh T.K. Noble L.J. Experimental fluid percussion brain injury: Vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271–282. doi: 10.1016/0006-8993(89)91190-6. [DOI] [PubMed] [Google Scholar]
- Coulter D.A. Rafiq A. Shumate M. Gong Q.Z. DeLorenzo R.J. Lyeth B.G. Brain injury-induced enhanced limbic epileptogenesis: Anatomical and physiological parallels to an animal model of temporal lobe epilepsy. Epilepsy Res. 1996;26:81–91. doi: 10.1016/s0920-1211(96)00044-7. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R. Fairbanks J.P. Fender J.S. Born D.E. Doyle D.L. Miller J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R. Hakimian S. Stewart T. Verley D.R. Fender J.S. Eastman C.L. Sheerin A.H. Gupta P. Diaz-Arrastia R. Ojemann J. Miller J.W. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Arrastia R. Agostini M.A. Frol A.B. Mickey B. Fleckenstein J. Bigio E. Van Ness P.C. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch. Neurol. 2000;57:1611–1616. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Busto R. Globus M.Y. Ginsberg M.D. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994a;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Early microvascular and neuronal consequences of traumatic brain injury: A light and electron microscopic study in rats. J. Neurotrauma. 1994b;11:289–301. doi: 10.1089/neu.1994.11.289. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Chatzipanteli K. Vitarbo E. Wada K. Kinoshita K. The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir. 2004;(Suppl. 89):69–74. doi: 10.1007/978-3-7091-0603-7_9. [DOI] [PubMed] [Google Scholar]
- Frey L.C. Epidemiology of posttraumatic epilepsy: A critical review. Epilepsia. 2003;44(Suppl. 10):11–17. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- Gao X. Deng-Bryant Y. Cho W. Carrico K.M. Hall E.D. Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garga N. Lowenstein D.H. Posttraumatic epilepsy: A major problem in desperate need of major advances. Epilepsy Curr. 2006;6:1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golarai G. Greenwood A.C. Feeney D.M. Connor J.A. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J. Neurosci. 2001;21:8523–8537. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber K.D. Prince D.A. A critical period for prevention of posttraumatic neocortical hyperexcitability in rats. Ann. Neurol. 2004;55:860–870. doi: 10.1002/ana.20124. [DOI] [PubMed] [Google Scholar]
- Grady M.S. Charleston J.S. Maris D. Witgen B.M. Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Griesemer D. Mautes A.M. Closed head injury causes hyperexcitability in rat hippocampal CA1 but not in CA3 pyramidal cells. J. Neurotrauma. 2007;24:1823–1832. doi: 10.1089/neu.2006.0237. [DOI] [PubMed] [Google Scholar]
- Habgood M.D. Bye N. Dziegielewska K.M. Ek C.J. Lane M.A. Potter A. Morganti-Kossmann C. Saunders N.R. Changes in blood–brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur. J. Neurosci. 2007;25:231–238. doi: 10.1111/j.1460-9568.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- Hitiris N. Mohanraj R. Norrie J. Sills G.J. Brodie M.J. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Hunt R.F. Scheff S.W. Smith B.N. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp. Neurol. 2009;215:243–252. doi: 10.1016/j.expneurol.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R.F. Scheff S.W. Smith B.N. Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J. Neurophysiol. 2010;103:1490–1500. doi: 10.1152/jn.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivens S. Kaufer D. Flores L.P. Bechmann I. Zumsteg D. Tomkins O. Seiffert E. Heinemann U. Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I. Nissinen J.P. McIntosh T.K. Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140:685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Kharatishvili I. Pitkanen A. Association of the severity of cortical damage with the occurrence of spontaneous seizures and hyperexcitability in an animal model of posttraumatic epilepsy. Epilepsy Res. 2010;90:47–59. doi: 10.1016/j.eplepsyres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Lenzlinger P.M. Hans V.H. Joller-Jemelka H.I. Trentz O. Morganti-Kossmann M.C. Kossmann T. Markers for cell-mediated immune response are elevated in cerebrospinal fluid and serum after severe traumatic brain injury in humans. J. Neurotrauma. 2001;18:479–489. doi: 10.1089/089771501300227288. [DOI] [PubMed] [Google Scholar]
- Loscher W. Schmidt D. New horizons in the development of antiepileptic drugs. Epilepsy Res. 2002;50:3–16. doi: 10.1016/s0920-1211(02)00063-3. [DOI] [PubMed] [Google Scholar]
- Lotocki G. de Rivero Vaccari J.P. Perez E. Sanchez J. Alonso O. Bramlett H.M. Dietrich W.D. Alterations in blood–brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of posttraumatic hypothermia. J. Neurotrauma. 2009;26:1123–1134. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein D.H. Epilepsy after head injury: An overview. Epilepsia. 2009;50(Suppl. 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein D.H. Thomas M.J. Smith D.H. McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzini L. Cossa F.M. Angelino E. Campini R. Pastore I. Monaco F. Posttraumatic epilepsy: Neuroradiologic and neuropsychological assessment of long-term outcome. Epilepsia. 2003;44:569–574. doi: 10.1046/j.1528-1157.2003.34902.x. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Ronne-Engstrom E. Flink R. Ungerstedt U. Carlson H. Hillered L. Epileptic seizure activity in the acute phase following cortical impact trauma in rat. Brain Res. 1994;637:227–232. doi: 10.1016/0006-8993(94)91237-8. [DOI] [PubMed] [Google Scholar]
- Oby E. Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- Racine R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves T.M. Lyeth B.G. Phillips L.L. Hamm R.J. Povlishock J.T. The effects of traumatic brain injury on inhibition in the hippocampus and dentate gyrus. Brain Res. 1997;757:119–132. doi: 10.1016/s0006-8993(97)00170-4. [DOI] [PubMed] [Google Scholar]
- Ross S.T. Soltesz I. Selective depolarization of interneurons in the early posttraumatic dentate gyrus: involvement of the Na(+)/K(+)-ATPase. J. Neurophysiol. 2000;83:2916–2930. doi: 10.1152/jn.2000.83.5.2916. [DOI] [PubMed] [Google Scholar]
- Salazar A.M. Jabbari B. Vance S.C. Grafman J. Amin D. Dillon J.D. Epilepsy after penetrating head injury. I. Clinical correlates: A report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–1414. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Santhakumar V. Ratzliff A.D. Jeng J. Toth Z. Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann. Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Scheff S.W. Price D.A. Hicks R.R. Baldwin S.A. Robinson S. Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J. Neurotrauma. 2005;22:719–732. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- Seiffert E. Dreier J.P. Ivens S. Bechmann I. Tomkins O. Heinemann U. Friedman A. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skandsen T. Ivar Lund T. Fredriksli O. Vik A. Global outcome, productivity and epilepsy 3–8 years after severe head injury. The impact of injury severity. Clin. Rehabil. 2008;22:653–662. doi: 10.1177/0269215508089067. [DOI] [PubMed] [Google Scholar]
- Somera-Molina K.C. Robin B. Somera C.A. Anderson C. Stine C. Koh S. Behanna H.A. Van Eldik L.J. Watterson D.M. Wainwright M.S. Glial activation links early-life seizures and long-term neurologic dysfunction: Evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia. 2007;48:1785–1800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Statler K.D. Swank S. Abildskov T. Bigler E.D. White H.S. Traumatic brain injury during development reduces minimal clonic seizure thresholds at maturity. Epilepsy Res. 2008;80:163–170. doi: 10.1016/j.eplepsyres.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Bramlett H.M. Ruenes G. Dietrich W.D. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Swartz B.E. Houser C.R. Tomiyasu U. Walsh G.O. DeSalles A. Rich J.R. Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Temkin N.R. Preventing and treating posttraumatic seizures: The human experience. Epilepsia. 2009;50(Suppl. 2):10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Temkin N.R. Jarell A.D. Anderson G.D. Antiepileptogenic agents: How close are we? Drugs. 2001;61:1045–1055. doi: 10.2165/00003495-200161080-00002. [DOI] [PubMed] [Google Scholar]
- Thapa A. Chandra S.P. Sinha S. Sreenivas V. Sharma B.S. Tripathi M. Post-traumatic seizures – A prospective study from a tertiary level trauma center in a developing country. Seizure. 2010;19:211–216. doi: 10.1016/j.seizure.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Tomkins O. Shelef I. Kaizerman I. Misk A. Afawi Z. Eliushin A. Gidon M. Cohen A. Zumsteg D. Friedman A. Blood–brain barrier disruption in post-traumatic epilepsy. J. Neurol. Neurosurg. Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- Toulmond S. Rothwell N.J. Interleukin-1 receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- van Vliet E.A. da Costa Araujo S. Redeker S. van Schaik R. Aronica E. Gorter J.A. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Vespa P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “To detect and protect.”. J. Clin. Neurophysiol. 2005;22:99–106. doi: 10.1097/01.wnp.0000154919.54202.e0. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. McArthur D. O'Phelan K. Glenn T. Etchepare M. Kelly D. Bergsneider M. Martin N.A. Hovda D.A. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: A microdialysis study. J. Cereb. Blood Flow Metab. 2003;23:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. McArthur D.L. Xu Y. Eliseo M. Etchepare M. Dinov I. Alger J. Glenn T.P. Hovda D. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P.M. O'Phelan K. Shah M. Mirabelli J. Starkman S. Kidwell C. Saver J. Nuwer M.R. Frazee J.G. McArthur D.A. Martin N.A. Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology. 2003b;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- Wang H.C. Chang W.N. Chang H.W. Ho J.T. Yang T.M. Lin W.C. Chuang Y.C. Lu C.H. Factors predictive of outcome in posttraumatic seizures. J. Trauma. 2008;64:883–888. doi: 10.1097/TA.0b013e31804a7fa4. [DOI] [PubMed] [Google Scholar]
- Zanier E.R. Lee S.M. Vespa P.M. Giza C.C. Hovda D.A. Increased hippocampal CA3 vulnerability to low-level kainic acid following lateral fluid percussion injury. J. Neurotrauma. 2003;20:409–420. doi: 10.1089/089771503765355496. [DOI] [PubMed] [Google Scholar]