Abstract
Traumatic brain injury (TBI) contributes to morbidity in children and boys, and hypotension worsens outcome. Extracellular signal-related kinase (ERK) mitogen-activated protein kinase (MAPK) is upregulated more in males and reduces cerebral blood flow (CBF) after fluid percussion injury (FPI). Increased cerebral perfusion pressure (CPP) via phenylephrine (Phe) sex-dependently improves impairment of the cerebral autoregulation seen after FPI through modulation of ERK MAPK upregulation, which is aggravated in males, but is blocked in females. Activation of ATP- and calcium-sensitive (Katp and Kca) channels produces cerebrovasodilation and contributes to autoregulation, both of which are impaired after FPI. Using piglets equipped with a closed cranial window, we hypothesized that potassium channel functional impairment after FPI is prevented by Phe in a sex-dependent manner through modulation of ERK MAPK upregulation. The Katp and Kca agonists cromakalim and NS 1619 produced vasodilation that was impaired after FPI more in males than in females. Phe prevented reductions in cerebrovasodilation after cromakalim and NS 1619 in females, but reduced dilation after these potassium channel agonists were given to males after FPI. Co-administration of U 0126, an ERK antagonist, and Phe fully restored dilation to cromakalim, calcitonin gene-related peptide (CGRP), and NS 1619, in males after FPI. These data indicate that Phe sex-dependently prevents impairment of Katp and Kca channel-mediated cerebrovasodilation after FPI in females, but aggravates impairment in males, through modulation of ERK MAPK upregulation. Since autoregulation of CBF is dependent on intact functioning of potassium channels, these data suggest a role for sex-dependent mechanisms in the treatment of cerebral autoregulation impairment after pediatric TBI.
Key words: cerebral circulation, newborn, potassium channels, signal transduction
Introduction
Pediatric traumatic brain injury (pTBI) is a global public health concern (Langlois et al., 2005). Boys are disproportionately represented and young children have devastating outcomes after pTBI (Langlois et al., 2005). Systemic hypotension is common after TBI (Coates et al., 2005), resulting in low cerebral perfusion pressure (CPP), and causing cerebral ischemia and poor outcomes, particularly when cerebral autoregulation is impaired (Chaiwat et al., 2009). Since cerebral blood flow (CBF) is dependent on CPP, impaired cerebral autoregulation and CBF may contribute to loss of neuronal cell integrity, and optimal CPP management limits tissue hypoxia due to low CPP. Elevation of mean arterial pressure (MAP) via a commonly used vasopressor such as intravenous phenylephrine decreases brain edema (Ishikawa et al., 2009), increases CPP and CBF, and can improve cerebral autoregulation, thereby improving outcomes. Since ethical constraints preclude mechanistic studies of cerebral autoregulation in children, we used a porcine model of fluid percussion injury (FPI) that mimics many of the pathophysiological features of pTBI to corroborate clinical observations (Armstead and Kurth, 1994). Piglets offer the unique advantage of a gyrencephalic brain containing substantial white matter, which is sensitive to ischemic/TBI damage similarly to the human brain. Our data suggest that the newborn pig is more cerebrohemodynamically sensitive to FPI than the juvenile, and impaired autoregulation post-insult may be caused by an age-dependent decrease in the production of calcitonin gene-related peptide (CGRP; Armstead and Kurth, 1994, Armstead 2000; Armstead and Vavilala, 2007). Adrenomedullin, a member of the CGRP family, prevents impairment of cerebral autoregulation after FPI in piglets (Armstead and Vavilala, 2007).
Relaxation of blood vessels can be mediated by several mechanisms, including those related to cGMP, cAMP, and K+ channels (Faraci and Heistad, 1998). Membrane potential of vascular muscle is a major determinant of vascular tone, and activity of K+ channels is a major regulator of membrane potential (Faraci and Heistad, 1998). Activation or opening of these channels increases K+ efflux, producing hyperpolarization of vascular muscle. Membrane hyperpolarization closes voltage-dependent calcium channels, and causes relaxation of vascular muscle. Direct measurements of membrane potential and K+ channel current in vitro indicate that several types of K+ channels are present in cerebral blood vessels. In addition, a number of pharmacological studies using activators and inhibitors have provided functional evidence that K+ channels, particularly ATP-sensitive (Katp) and calcium-sensitive (Kca) channels, regulate tone of cerebral blood vessels (Faraci and Heistad, 1998). Prototypical Katp agonists are cromakalim and CGRP, while NS 1619 is a Kca agonist. Vasodilation due to these drugs can be used as an index of the intactness of potassium channel function after TBI (Armstead, 1997; Salvucci and Armstead, 2000).
Mitogen-activated protein kinase (MAPK) is a family of at least three kinases (extracellular signal-related kinase [ERK], p38, and c-Jun N-terminal kinase [JNK]; Laher and Zhang, 2001). Our recent studies show that ERK is upregulated more in males and reduces CBF after FPI (Armstead et al., 2009, 2010a). Increased CPP via phenylephrine improves impairment of cerebral autoregulation after FPI through modulation of ERK MAPK upregulation, which is aggravated in males, but blocked in females. In unrelated studies, we observed that pial artery dilation in response to hypotension is dependent on activation of Katp and Kca channels (Armstead, 1999). After FPI, ERK MAPK is upregulated and contributes to reductions in CBF and impaired dilation due to potassium channel agonists such as CGRP (Armstead et al., 2009; Ross and Armstead, 2003). Therefore, we explored the hypothesis that potassium channel functional impairment arising after FPI is prevented by phenylephrine in a sex-dependent manner through modulation of ERK MAPK upregulation.
Methods
Closed cranial window and brain injury procedures
Newborn pigs (1–5 days old, weight 1.0–1.4 kg) of both genders were studied. The brain of a pig this age roughly approximates that of a 6-month- to 1-year-old human child (Dobbing, 1974). All protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The animals were sedated with isoflurane (1–2 MAC). Anesthesia was maintained with α-chloralose (30–50 mg/kg, supplemented with 5 mg/kg/h IV). A catheter was inserted into a femoral artery to monitor blood pressure and to sample blood gas tension and pH. Drugs to maintain anesthesia were administered via a second catheter placed in a femoral vein. The trachea was cannulated, and the animals were ventilated with room air. A heating pad was used to maintain the animals at 37–39°C, and temperature was monitored rectally.
A cranial window was made in the parietal skull of the anesthetized animal. This window consisted of three parts: a stainless steel ring, a circular glass cover-slip, and three ports consisting of 17-gauge hypodermic needles attached to three precut holes in the stainless steel ring. For placement, the dura was cut and retracted over the cut bone edge. The cranial window was placed in the opening and cemented in place with dental acrylic. The volume under the window was filled with a solution similar to CSF, of the following composition: 3.0 mM KCl, 1.5 mM MgCl2, 1.5 mM CaCl2, 132 mM NaCl, 6.6 mM urea, 3.7 mM dextrose, and 24.6 mM NaHCO3. This artificial CSF was warmed to 37°C and had the following chemistry: pH 7.33, Pco2 46 mm Hg, and Po2 43 mm Hg, which was similar to that of endogenous CSF. Pial arterial vessel diameter was measured with a microscope, a camera, a video output screen, and a video microscaler.
The method used to induce brain FPI has been described previously (Wei et al., 1980). A device designed at the Medical College of Virginia was used. A small opening was made in the parietal skull contralateral to the cranial window. A metal shaft was sealed in the opening on top of the intact dura. This shaft was connected to the transducer housing, which in turn was connected to the fluid percussion device. The device itself consisted of an acrylic plastic cylindrical reservoir 60 cm long, 4.5 cm in diameter, and 0.5 cm thick. One end of the device was connected to the transducer housing, whereas the other end had an acrylic plastic piston mounted on O-rings. The exposed end of the piston was covered with a rubber pad. The entire system was filled with 0.9% saline. The percussion device was supported by two brackets mounted on a platform. FPI was induced by striking the piston with a 4.8-kg pendulum. The intensity of the injury (1.9–2.3 atm, with a duration of 19–23 msec) was controlled by varying the height from which the pendulum was allowed to fall. The pressure pulse of the injury was recorded on a storage oscilloscope triggered photoelectrically by the fall of the pendulum. The amplitude of the pressure pulse was used to determine the intensity of the injury.
Protocol
Two types of pial vessels, small arteries (resting diameter 120–160 μm) and arterioles (resting diameter 50–70 μm), were examined to determine whether segmental differences in the effects of FPI could be identified. Typically, 2–3 mL of artificial CSF was flushed through the window over a 30-sec period, and excess CSF was allowed to run off through one of the needle ports. For sample collection, 300 μL of the total cranial window volume of 500 μL was collected by slowly infusing artificial CSF into one side of the window, and allowing the CSF to drip freely into a collection tube on the opposite side. Fourteen experimental groups were studied (all n = 5) (1 and 2) male and female sham controls, (3 and 4) male and female FPI, vehicle pre-treated, (5 and 6) male and female FPI pre-treated with phenylephrine (1 μg/kg/min IV), (7 and 8) male and female FPI pre-treated with phenylephrine and the ERK MAPK antagonist U 0126 (1 mg/kg IV), (9 and 10) male and female FPI vehicle post-treated, (11 and 12) male and female FPI post-treated with phenylephrine, and (13 and 14) male and female FPI post-treated with phenylephrine and U 0126. The dose of phenylephrine was chosen based on that used clinically for treatment of pediatric TBI patients at the University of Washington (unpublished observations). The vehicle for all agents was 0.9% saline, except for the MAPK inhibitor, for which it was dimethyl sulfoxide (100 μL) diluted with 9.9 mL 0.9% saline. In sham control animals, the responses to cromakalim and CGRP, the Katp agonists, and NS 1619, the Kca agonist (10−8 and 10−6 M), were obtained initially, and then again 1 h later in the presence of the agent vehicle. In drug-treated FPI animals, phenylephrine, U 0126, or their combination were administered either 30 min before or 30 min after FPI, and the responses to cromakalim, CGRP, and NS 1619 were obtained at 1 h post-injury.
Enzyme-linked immunosorbent assay (ELISA)
Commercially available ELISA kits were used to quantity CSF adrenomedullin concentrations (an endogenous member of the CGRP family) in sham-control animals 1 h post FPI.
Statistical analysis
Pial artery diameter and CSF adrenomedullin values were analyzed using repeated-measures analysis of variance (ANOVA). If the value was significant, the data were then analyzed by Fisher's protected least significant difference test. An α level of p < 0.05 was considered significant in all statistical tests. Values are represented as mean ± standard error of the mean (SEM) of the absolute value, or as percentage changes from control values.
Results
FPI impairs pial artery dilation in response to Katp and Kca channel agonists more in males compared to females
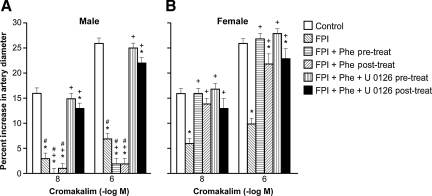
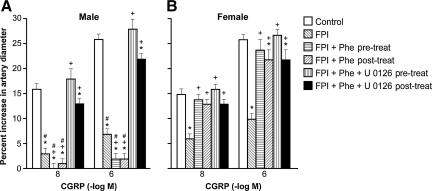
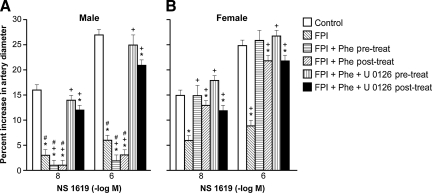
Cromakalim, CGRP, and NS 1619 (10−8 and 10−6) elicited reproducible pial small artery dilation. Vasodilation in response to all three potassium channel agonists was blunted after FPI more in the male than the female (Figs. 1, 2, and 3). Similar observations were made in pial arterioles.
FIG. 1.
Influence of cromakalim (10−8 and 10−6 M) on pial artery diameter in newborn male (A) and female (B) piglets before injury (Control; sham control), 1 h after FPI (FPI), 1 h after FPI treated 30 min prior to injury with phenylephrine (FPI + Phe pre-treat; 1 μg/kg/min IV), 1 h after FPI treated with Phe 30 min after injury (FPI + Phe post-treat), 1 h after FPI treated with Phe + U 0126 (FPI + Phe + U 0126 pre-treat; 1 mg/kg IV) 30 min prior to injury, and 1 h after FPI treated with Phe + U 0126 30 min after injury (FPI + Phe + U 0126 post-treat; all n = 5; *p < 0.05 compared with corresponding control value; +p < 0.05 compared with corresponding FPI untreated value; #p < 0.05 compared with corresponding female value; FPI, fluid percussion injury).
FIG. 2.
Influence of calcitonin gene-related peptide (CGRP) (10−8 and 10− M) on pial artery diameter in newborn male (A) and female (B) piglets before injury (Control; sham control), 1 h after FPI (FPI), 1 h after FPI treated 30 min prior to injury with phenylephrine (FPI + Phe pre-treat; 1 μg/kg/min IV), 1 h after FPI treated with Phe 30 min after injury (FPI + Phe post-treat), 1 h after FPI treated with Phe + U 0126 (FPI + Phe + U 0126 pre-treat; 1 mg/kg IV) 30 min prior to injury, and 1 h after FPI treated with Phe + U 0126 30 min after injury (FPI + Phe + U 0126 post-treat; n = 5; *p < 0.05 compared with corresponding control value; +p < 0.05 compared with corresponding FPI untreated value; # p < 0.05 compared with corresponding female value; FPI, fluid percussion injury).
FIG. 3.
Influence of NS 1619 (10−8 and 10−6 M) on pial artery diameter in newborn male (A) and female (B) piglets before injury (Control; sham control), 1 h after FPI (FPI), 1 h after FPI treated 30 min prior to injury with phenylephrine (FPI + Phe pre-treat; 1 μg/kg/min IV), 1 h after FPI treated with Phe 30 min after injury (FPI + Phe post-treat), 1 h after FPI treated with Phe + U 0126 (FPI + Phe + U 0126 pre-treat; 1 mg/kg IV) 30 min prior to injury, and 1 h after FPI treated with Phe + U 0126 30 min after injury (FPI + Phe + U 0126 post-treat; n = 5; *p < 0.05 compared with corresponding control value; +p < 0.05 compared with corresponding FPI untreated value; #p < 0.05 compared with corresponding female value; FPI, fluid percussion injury).
Phenylephrine prevents loss of pial artery dilation in response to potassium channel agonists after FPI in females, but aggravates this loss in male piglets
Phenylephrine (1 μg/kg/min IV) administered either 30 min prior to or 30 min after FPI, prevented reductions in pial small artery cerebrovasodilation in response to cromakalim, CGRP, and NS 1619 in females, but further reduced (e.g., blocked) dilation with these potassium channel agonists in males after injury (Figs. 1, 2, and 3). Similar observations were made in pial arterioles. Phenylephrine produced comparable increases in MAP in male and female piglets (Armstead et al., 2010b). Phenylephrine increased CPP more in the female than in male piglets, both after FPI and combined hypotension and FPI, a result similar to that of our prior study (Armstead et al., 2010b).
Co-administration of an ERK MAPK antagonist with phenylephrine fully restores potassium channel-mediated cerebrovasodilation in males after FPI
Co-administration of U 0126 (1 mg/kg IV), an ERK antagonist, and phenylephrine, either 30 min before or 30 min after FPI, fully restored pial small artery dilation in response to cromakalim, CGRP, and NS 1619 in males after FPI. No further effect on vascular reactivity to these potassium channel activators after FPI was seen in females given both U 0126 and phenylephrine, compared to the responses noted in animals receiving phenylephrine alone (Figs. 1, 2, and 3). Similar observations were made in pial arterioles.
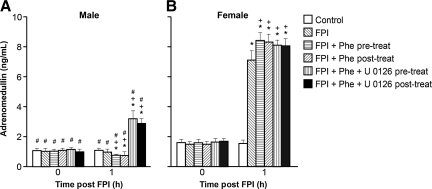
CSF adrenomedullin is upregulated after FPI in the female, but is unchanged in the male
Phenylephrine decreased adrenomedullin concentrations after FPI, contrary to the increases seen in CSF adrenomedullin concentrations after combined phenylephrine and U 0126 in the male, while concentrations were largely unchanged in the female.
Adrenomedullin is an endogenous member of the CGRP family. CSF adrenomedullin concentrations are increased after FPI in the female, but these concentrations are unchanged after FPI in the male (Fig. 4), as reported previously (Armstead et al., 2010b). Phenylephrine decreases CSF adrenomedullin concentrations after FPI in the male, which is contrary to the increases in adrenomedullin seen with combined phenylephrine and U 0126 (Fig. 4). In the female, however, phenylephrine increases CSF adrenomedullin after FPI (Armstead et al., 2010b), and combined phenylephrine and U 0126 have only a minimal additional effect on CSF adrenomedullin concentrations after FPI (Fig. 4).
FIG. 4.
Cerebrospinal fluid (CSF) adrenomedullin as a function of time in newborn male (A) and female (B) piglets before injury (Control; sham control), 1 h after FPI (FPI), 1 h after FPI treated 30 min prior to injury with phenylephrine (FPI + Phe pre-treat; 1 μg/kg/min IV), 1 h after FPI treated with Phe 30 min after injury (FPI + Phe post-treat), 1 h after FPI treated with Phe + U 0126 (FPI + Phe + U 0126 pre-treat; 1 mg/kg IV) 30 min prior to injury, and 1 h after FPI treated with Phe + U 0126 30 min after injury (FPI + Phe + U 0126 post-treat; n = 5; *p < 0.05 compared with corresponding control value; +p < 0.05 compared with corresponding FPI untreated value; #p < 0.05 compared with corresponding female value; FPI, fluid percussion injury).
Blood chemistry
There were no statistically significant differences in blood chemistry between sham control, FPI, and FPI + drug-treated animals before or after in all experiments. The amplitude of the pressure pulse, which is used as an index of injury intensity, was equivalent in male and female FPI-vehicle- and FPI + drug-treated animals (1.9 ± 0.1 atm).
Discussion
Several key findings emerged from this study. First, vasodilation in response to activators of the Katp and Kca channels was observed to be impaired more in the male than in the female after FPI. These data expand prior observations showing a similarly blunted vascular response to potassium channel agonists after FPI in studies that did not consider the role of gender in the effects of FPI on cerebral hemodynamics (Armstead, 1997; Salvucci and Armstead, 2000). Because the intensity level of injury was equivalent between males and females, these data suggest intrinsic gender differences in outcome after pTBI.
Second, the results of this study show that systemic MAP support with phenylephrine robustly protects vasodilation in response to Katp- and Kca-channel agonists in the setting of FPI in the female, but not the male, newborn pig. In fact, the systemic vasopressor phenylephrine worsens the loss of dilator responsiveness to potassium channel agonists after FPI in the male piglet, further indicating sex-dependent differences in outcome when using the same clinically-driven critical pathway for the treatment of pTBI. Recent studies have shown that systemic pressor support with phenylephrine also profoundly protects cerebral autoregulation in the setting of FPI in the female, but not the male, newborn pig (Armstead et al., 2010b). In prior unrelated studies, it was observed that pial artery dilation in response to hypotension is dependent on the activation of Katp and Kca channels (Armstead, 1999). Taken together, the results of the present study provide a mechanistic basis for the recently observed ability of phenylephrine to preserve autoregulation in the female, but not in the male, newborn piglet after FPI.
Our previous recent studies also showed that FPI produces greater increases in intracranial pressure (ICP) and reductions in parietal cortical blood flow and CPP in male compared to female newborn piglets (Armstead et al., 2010a, 2010b). Phenylephrine blunted FPI-associated increases in ICP post-injury in male and female piglets (Armstead et al., 2010b). However, during combined hypotension and FPI, phenylephrine only prevented further increases in ICP in females, but not in males. Phenylephrine increased CPP more in females than males after FPI plus hypotension, despite causing an equivalent increase in MAP in both sexes (Armstead et al., 2010b). The mechanism by which phenylephrine infusion preserves CPP in female piglets is via decreasing ICP, since CPP = MAP – ICP. These data indicate that FPI produces sex-dependent differences in ICP and CPP, while phenylephrine infusion has different efficacy in elevating CPP in male and female piglets.
A third key new finding is that co-administration of an ERK MAPK antagonist with phenylephrine fully restores potassium channel–mediated cerebrovasodilation in males after FPI. Prior studies have shown that FPI-associated upregulation of ERK MAPK contributes to reductions in CBF and impaired cerebral autoregulation (Armstead et al., 2009, 2010a). Phenylephrine blocks ERK MAPK upregulation after FPI in the female, but not in the male, where it actually aggravates such upregulation (Armstead et al., 2010b). The results of this study extend the latter by providing a mechanistic explanation (e.g., impaired potassium channel function) for the cerebral dysregulation seen during hypotension with phenylephrine infusion in the setting of brain injury. These data suggest that therapies for the treatment of brain-injured children should consider the addition of interventions that block ERK MAPK, particularly in boys. Nonetheless, we do acknowledge the caveat that sex-dependent differences in ERK MAPK signaling may not be the only explanation for the observed innate differential sex-based effects of FPI on cerebral hemodynamics.
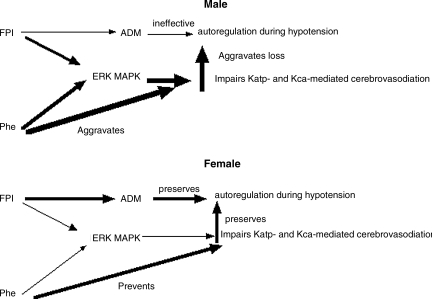
A fourth key finding of the present study relates to adrenomedullin. Our prior studies have indicated a prominent role for impairment of potassium channel function and decreased production of CGRP peptides in the cerebrovascular dysregulation seen during hypotension after FPI (Armstead, 2000; Armstead and Vavilala, 2007). Adrenomedullin is a 52-amino-acid peptide belonging to the CGRP family. In rats, adrenomedullin mRNA expression is upregulated after ischemia and may be cerebroprotective, particularly after stroke (Miyashita et al., 2006; Wang et al., 1995). Adrenomedullin increases CBF and prevents ischemia after middle cerebral artery occlusion (Dogan et al., 1997). Marked increases in CSF adrenomedullin in children occur after severe TBI, and CBF was positively correlated with CSF adrenomedullin levels (Robertson et al., 2001), suggesting that it may be neuroprotective (Baskaya et al., 2005; Juhl et al., 2006). Our recent studies indicate that adrenomedullin prevents sex-dependent impairment of the autoregulation seen during hypotension after FPI, through inhibition of ERK MAPK upregulation (Armstead et al., 2010a). In prior studies, CSF adrenomedullin was found to be upregulated after FPI in the female, but unchanged in the male (Armstead and Vavilala, 2007). In the male, phenylephrine decreases adrenomedullin after FPI, which is contrary to the increase in CSF adrenomedullin seen with combined phenylephrine and U 0126. In the female, phenylephrine increases CSF adrenomedullin levels, while combined U 0126 and phenylephrine had little further effect on adrenomedullin concentration. These data indicate that the combination of U 0126 with phenylephrine contributes to improved cerebral hemodynamic outcomes, both through upregulation of an endogenous dilator system, as well as through inhibition of a signaling system (e.g., ERK MAPK). Figure 5 summarizes these interrelationships.
FIG. 5.
Proposed sex-dependent relationships between exogenous phenylephrine (Phe), endogenous adrenomedullin (ADM), and ERK MAPK, in the setting of fluid percussion brain injury (FPI), in determining the ability of Katp- and Kca-channel agonists to elicit cerebrovasodilation, thereby contributing to autoregulation during hypotension. Arrow thickness reflects the relative degree to which this action occurs (ERK, extracellular signal-related kinase; MAPK, mitogen-activated protein kinase).
In conclusion, phenyleprhine sex-dependently prevents impairment of Katp and Kca channel–mediated cerebrovasodilation after FPI in females, but aggravates this impairment in males, through modulation of ERK MAPK upregulation. Since autoregulation of CBF is dependent on intact functioning of potassium channels, these data suggest a role for sex-dependent mechanisms in the treatment of the impaired cerebral autoregulation seen after pTBI.
Acknowledgments
This research was supported by grant HD57355 from the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Armstead W.M. Age dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7:225–235. [PubMed] [Google Scholar]
- Armstead W.M. Kurth C.D. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J. Neurotrauma. 1994;11:487–497. doi: 10.1089/neu.1994.11.487. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Vavilala M.S. Adrenomedullin reduces gender dependent loss of hypotensive cerebrovasodilation after newborn brain injury through activation of ATP-dependent K channels. J. Cereb. Blood Flow Metab. 2007;27:1702–1709. doi: 10.1038/sj.jcbfm.9600473. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Brain injury impairs ATP-sensitive K+ channel function in piglet cerebral arteries. Stroke. 1997;28:2273–2280. doi: 10.1161/01.str.28.11.2273. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Cines D.B. Bdeir K. Bdeir Y. Stein S.C. Higazi A.A.R. uPA modulates the age dependent effect of brain injury on cerebral hemodynamics through LRP and ERK MAPK. J. Cereb. Blood Flow Metab. 2009;29:524–533. doi: 10.1038/jcbfm.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Hypotension dilates pial arteries by KATP and Kca channel activation. Brain Res. 1999;816:158–164. doi: 10.1016/s0006-8993(98)01146-9. [DOI] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Bdeir K. Kofke W.A. Vavilala M.S. Adrenomedullin prevents sex dependent impairment of autoregulation during hypotension after piglet brain injury through inhibition of ERK MAPK upregulation. J. Neurotrauma. 2010a;27:391–402. doi: 10.1089/neu.2009.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead W.M. Kiessling J.W. Kofke W.A. Vavilala M.S. Impaired cerebral blood flow autoregulation during postraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by ERK MAPK upregulation. Crit. Care Med. 2010b;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaya M.K. Suzuki Y. Anzai M. Seki Y. Saito K. Takayasu M. Shibuya M. Sugita K. Effects of adrenomedullin, calcitonin gene-related peptide, and amylin on cerebral circulation in dogs. J. Cereb. Blood Flow Metab. 1995;15:827–834. doi: 10.1038/jcbfm.1995.103. [DOI] [PubMed] [Google Scholar]
- Chaiwat O. Sharma D. Udomphorn Y. Moore A. Zimmerman J.J. Armstead W.M. Vavilala M.S. Cerebral hemodynamic predictors of poor 6 month Glasgow Outcome Score in severe pediatric traumatic brain injury. J. Neurotrauma. 2009;26:657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates B.M. Vavilala M.S. Mack C.D. Muangman S. Suz P. Sharar S.R. Bulger E. Lam A.M. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit. Care Med. 2005;33:2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- Dogan A. Suzuki Y. Koketsu N. Osuka K. Saito K. Takayasu M. Shibuya M. Yoshida J. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 1997;17:19–25. doi: 10.1097/00004647-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Faraci F.M. Heistad D.D. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol. Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Ito H. Yokoyama K. Mikita K. Phenylephrine ameliorates cerebral cytotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid occlusion with severe hypotension. Anesth. Analg. 2009;108:1631–1637. doi: 10.1213/ane.0b013e31819d94e3. [DOI] [PubMed] [Google Scholar]
- Juhl L. Petersen K.A. Larsen E.H. Olesen J. The in-vivo effect of adrenomedullin on rat dural and pial arteries. Eur. J. Pharmacol. 2006;538:101–107. doi: 10.1016/j.ejphar.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Laher I. Zhang J.H. Protein kinase C and cerebral vasospasm. J. Cereb. Blood Flow Metab. 2001;21:887–906. doi: 10.1097/00004647-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Miyashita K. Itoh H. Arai H. Suganami T. Sawada N. Fukunaga Y. Sone M. Yamahara K. Yurugi-Kobayashi T. Park K. Oyamada N. Sawada N. Taura D. Tsujimoto H. Chao T.H. Tamura N. Mukoyama M. Nakao K. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology. 2006;147:1642–1653. doi: 10.1210/en.2005-1038. [DOI] [PubMed] [Google Scholar]
- Robertson C.L. Minamino N. Ruppel R.A. Kangawa K. Wisniewski S.R. Tsuji T. Janesko K.L. Ohta H. Adelson P.D. Marion D.W. Kochanek P.M. Increased adrenomedullin in cerebrospinal fluid after traumatic brain injury in infants and children. J. Neurotrauma. 2001;18:861–868. doi: 10.1089/089771501750451785. [DOI] [PubMed] [Google Scholar]
- Ross J. Armstead W.M. Differential role of PTK and ERK MAPK in superoxide impairment of KATP and Kca channel cerebrovasodilation. Am. J. Physiol. 2003;285:R149–R154. doi: 10.1152/ajpregu.00003.2003. [DOI] [PubMed] [Google Scholar]
- Salvucci A. Armstead W.M. Vasopressin impairs KATP and Kca channel function after brain injury. Brain Res. 2000;887:406–412. doi: 10.1016/s0006-8993(00)03079-1. [DOI] [PubMed] [Google Scholar]
- Wang X. Yue T.L. Barone F.C. White R.F. Clark R.K. Willette R.N. Sulpizio A.C. Aiyar N.V. Feuerstein G.Z. Ruffolo R.R., Jr. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc. Natl. Acad. Sci. USA. 1995;92:11480–11484. doi: 10.1073/pnas.92.25.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E.P. Dietrich W.D. Povlishock J.T. Navari R.M. Kontos H.A. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ. Res. 1980;46:37–47. doi: 10.1161/01.res.46.1.37. [DOI] [PubMed] [Google Scholar]