Abstract
The purpose of this study was to explore a novel treatment involving removal of free water from ventricular cerebrospinal fluid (CSF) for the reduction of cerebra]l edema. The hypothesis is that removal of free water from the CSF will increase the osmolarity of the CSF, which will favor movement of tissue-bound water into the ventricles, where the water can be removed. Reductive ventricular osmotherapy (RVOT) was tested in a flowing solution of artificial CSF (aCSF) with two end-points: (1) the effect of RVOT on osmolarity of the CSF, and (2) the effect of RVOT on water content of ex vivo cerebral tissue. RVOT catheters are made up of membranes permeable only to water vapor. When a sweep gas is drawn through the catheter, free water in the form of water vapor is removed from the solution. With RVOT treatment, aCSF osmolarity increased from a baseline osmolarity of 318.8 ± 0.8 mOsm/L to 339.0 ± 3.3 mOsm/L (mean ± standard deviation) within 2 h. After 10 h of treatment, aCSF osmolarity approached an asymptote at 344.0 ± 4.2 mOsm/L, which was significantly greater than control aCSF osmolarity (p <<0.001 by t-test, n = 8). Water content at the end of 6 h of circulating aCSF exposure was 6.4 ± 0.9 g H2O (g dry wt)−1 in controls, compared to 6.1 ± 0.7 g H2O (g dry wt)− after 6 h of RVOT treatment of aCSF (p = 0.02, n = 24). The results support the potential of RVOT as a treatment for cerebral edema and intracranial hypertension.
Key words: brain edema, cerebrospinal fluid, hollow fibers, osmotherapy, traumatic brain injury
Introduction
Traumatic brain injury (TBI) continues to be a major cause of death and disability in both civilian and military populations throughout the world. One and one-half million people suffer a TBI each year in the United States alone, and approximately one million of them require an emergency department visit; 500,000 are hospitalized and 50,000 die (Bruns and Hauser, 2003; Maas et al., 2007). This results in direct and indirect costs of at least $56 billion annually in the U.S. alone. Despite the tremendous negative impact of TBI on societies throughout the world, and although numerous multicenter therapeutic trials have been conducted, no specific treatment for TBI is available (Maas et al., 2007; Narayan et al., 2002). The fact that intractable intracranial hypertension in severe TBI is associated with a high mortality rate has been a clinical axiom for decades (Juul et al., 2000; Miller et al., 1977; Rea and Rockswold, 1983).
Cerebral edema, both diffuse and perilesional, contributes substantially to intracranial hypertension. Despite a well-established, step-wise approach to controlling intracranial pressure (ICP), including head-of-bed elevation, CSF drainage, sedation, systemic osmotherapy, the use of paralytic agents, and decompressive craniectomy if necessary, uncontrolled intracranial hypertension remains the number one treatable cause of deterioration and death from severe TBI (Juul et al., 2000; Narayan et al., 2002).
Monitoring of ICP is considered the standard of care for all patients with severe TBI. While placement of an ICP monitor is invasive, the benefits of ICP monitoring are thought to offset this drawback, carrying only a small risk of complications (e.g., infection, hemorrhage, malfunction, obstruction, or malposition), and its use rarely results in increased patient morbidity (Narayan et al., 1981). Ventriculostomy has been considered the standard for ICP monitoring, as it has the advantage of therapeutic drainage of CSF and is relatively inexpensive.
A new approach to the treatment of cerebral edema
We propose exploring a novel method for the reduction of cerebral edema. This treatment involves removal of free water by vaporization of ventricular fluid. The primary hypothesis is that removal of free water from the CSF will increase osmolarity of the CSF, which will favor movement of tissue-bound water to the ventricles, where the water can be removed. The documented increase in tissue osmolarity is a key component of this hypothesis. Post-injury cerebral tissue osmolarity has been measured to be as high as 400 mOsm/L (Hatashita and Hoff, 1986; Hossman et al., 1976; Katayama et al., 1998; Matsuoka and Hossmann, 1982; Tomita et al., 1979). This creates a gradient for movement of fluid into the cells after ischemic injury, leaving large, osmotically-active molecules behind in the extracellular spaces (Odland and Sutton, 1997).
Ventricular osmotherapy to reduce cerebral edema
Intracerebroventricular infusion of therapeutic substances has been studied by several investigators (Doczi et al., 1987; Kawamata et al., 2007; Owen et al., 1989; Pullen, 1985; Rundgren et al., 1990), and there appears to be a rapid and predictable exchange under a variety of experimental conditions. The difficulty with infusion of osmotic therapy is that, in a brain with decreased compliance, even small volumes of infusion can increase pressure. Infusion-withdrawal systems are difficult to balance in low-compliance brains.
There have been two reports of low-volume ventricular osmotherapy specifically aimed at reducing cerebral edema, describing a bolus infusion and continuous infusion of albumin in a rat model. Onal and associates (1997) reported that administration of a single bolus infusion of albumin into the cerebral ventricles at 30 min after a freeze injury resulted in a significant reduction of tissue water content (tissue edema) at 6 h post-injury. This effect was transient, and tissue edema was not reduced in the albumin-treated rats at 24 h post-injury. In an effort to prolong the treatment's effect, Sutton and colleagues (1999) used implanted osmotic minipumps (Alzet; Durect, Cupertino, CA) for continuous intraventricular infusion (1 μL/h) of either artificial CSF (aCSF) or 10% bovine serum albumin (BSA) in a rat model of TBI. At 24 h, water content was determined in the treatment and control groups. The percentage of tissue water content in TBI rats treated with BSA was slightly reduced in all regions assayed, compared to the edema seen in the aCSF-treated rats. An independent t-test comparing the total tissue water content levels in the tissue samples ipsilateral to injury indicated that the BSA-treated rats had significantly (p < 0.03) less tissue water content than the rats treated with aCSF infusion.
These data support our hypothesis that purposefully increasing the osmolarity of CSF after brain injury by even a small amount should increase movement of water into the CSF and reduce cerebral edema. Therapeutic intervention can be described as the rate of delivery of hyperosmolarity per unit of time per gram of brain. The continuous infusion of 10% albumin at 1 μL/h equals 5.6 × 10−8 mmol h−1 g−1, a level that can cause a significant reduction in edema. Application of murine studies to human treatment is difficult, but on the basis of the ratio of the total brain mass, the rate would have to be 8 × 10−5 mmol h−1 to achieve the same effect in humans.
Prevention and treatment of cerebral edema with ventricular water vaporization
The investigators believed that removing water from within the cerebral ventricles would decrease tissue edema. With hollow fiber technology, water can be removed directly from the ventricular CSF and indirectly from the tissue. Vaporization catheters could be placed directly into the cerebral ventricles, similarly to placement of ventriculostomy drainage catheters (Fig. 1). The objective of reductive ventricular osmotherapy (RVOT) is to remove water from the ventricles and subsequently pull fluid from the surrounding edematous brain tissue. If water vaporization rates do not exceed tissue edema reduction rates, the osmolarity and colloid osmotic pressure (COP) of the CSF should remain constant. Likewise, ventricular volume will remain constant.
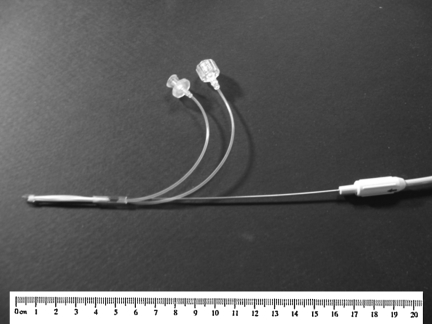
FIG. 1.
Photograph of a prototype reductive ventricular osmotherapy catheter. Airflow inlet and outlet lines are shown. Dimensions as shown: 3 × 3 mm.
Methods
Reductive ventricular osmotherapy ventriculostomy catheter
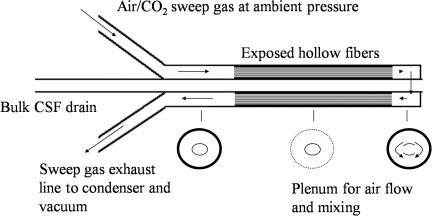
RVOT was tested in a flowing solution of aCSF with two end-points: (1) to measure the effect of free water removal on the osmolarity of CSF, and (2) to measure the effect of free water removal on the water content of cerebral tissue ex vivo. The treatment involves placement of a catheter made up of membranes permeable only to water vapor. When a sweep gas is drawn through the catheter, free water in the form of water vapor is removed from the solution (Fig. 2).
FIG. 2.
Schematic diagram of reductive ventricular osmotherapy catheter. Air flows in one line and through half the hollow fibers, mixes in the plenum at the tip of the catheter, and is drawn back out through the other half by a vacuum (CSF, cerebrospinal fluid).
Reductive ventricular osmotherapy catheter operation
Water removal rate is a function of catheter materials, design, and sweep gas velocity.
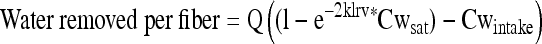 |
where Q is the rate of airflow through the fiber, Cwsat is the equilibrium vapor concentration based on the water vapor pressure of tissue at 37°C, Cwintake is the concentration of water vapor in air entering the system, l is the length of the hollow fiber, r is the radius, and v is the mean velocity of the sweep gas. A free water removal rate of 24 mL/d can be achieved with a 3-mm OD catheter.
The sweep gas selected for the study was a mix of 95% air and 5% carbon dioxide (40 mm Hg partial pressure). Carbon dioxide was added to the sweep gas because the hollow fibers are highly permeable to carbon dioxide. In pilot studies, failure to use a carbon dioxide mix resulted in acidosis. The use of 5% carbon dioxide resulted in a stable pH of 7.4.
A study was performed with RVOT catheters in flowing aCSF in a 10-mL test jig. The jig was constructed with a dual chambered cylinder that would accept an RVOT catheter and ex vivo tissue. After 4 h of RVOT treatment and stabilization of osmolarity, ex vivo cerebral tissue was placed in the test jig and exposed to the flowing aCSF. The effect of RVOT on aCSF osmolarity and ex vivo tissue water uptake were determined.
Reductive ventricular osmotherapy treatment and osmolarity of flowing aCSF in vitro
aCSF was passed through the chambered device at a rate of 10 mL/h. The aCSF was pulsed within the device by means of a reciprocating pump; 30-μL volumes were pulsed 60 times per minute. The effluent exit height was set to 20 mm Hg above the device in order to simulate moderately increased ICP. For controls, a device containing a single 10-mL chamber was constructed; aCSF flow and pulsatile action were the same as that for the treated group.
After introduction of aCSF into the device, treatment of the aCSF was initiated with the RVOT catheter by drawing dry, 5% CO2 + 95% air at 37°C through the catheter by means of a vacuum of 450 mm Hg, which produced airflow at 2.5 L/min. The test jig and RVOT catheter were operated for 4 h prior to placement of the brain tissue. Samples of aCSF were taken every hour for analysis.
Reductive ventricular osmotherapy treatment in ex vivo brain
Ex vivo cerebral tissue from the cortex of a sheep brain was harvested. The sheep were used in an unrelated training exercise under general anesthesia, and had no brain injury. The experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 80-23, revised 1996). The brain samples were harvested immediately after the animal was euthanized. The samples were taken from the middle of a single hemisphere. Consecutive slices were assigned alternately to treatment, control, or sham groups.
After 4 h of pre-equilibrating the aCSF, the brain sections were placed into stainless steel mesh cages and positioned within the test jig. Cross sections of brain (10 × 10 × 2 mm) were cut in a tissue matrix, placed in the cages, and weighed. The sections were alternately designated to treated and control groups. The mesh cages were placed into the test jig, exposing the sections to circulating aCSF. After 6 h of exposure to RVOT or control chambers, the cages were removed and weighed. All studies were performed at 37°C. Dry weights of the tissue were obtained by exposing the tissue to 60°C for 5 days.
In controlled studies of tissue in flowing aCSF, water content of the tissues, tissue surface area, tissue weights (pre- and post-treatment, and dried), and changes in aCSF concentration were measured. Mass transfer coefficients of water in tissue were calculated as well.
Determination of tissue water content with reductive ventricular osmotherapy treatment
Tissue water content is determined by reporting water content per dry tissue weight as standardized for normal water content. True solid component weight at time zero can only be estimated based on water content of normal tissues without aCSF exposure. Therefore, additional sham brain tissue samples were harvested from the same site at the same time as the study samples (alternating control, treatment, and sham). The sham samples were immediately dried without aCSF exposure.
Results
RVOT resulted in an increase in CSF osmolarity when tested in a model of flowing CSF, and further reduced tissue water content in ex vivo cerebral tissue that was exposed to the treated CSF.
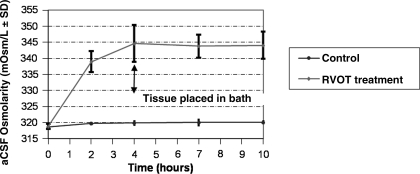
RVOT rapidly increased osmolarity in treated aCSF (Fig. 3) There was a small increase in untreated control aCSF over the 10-h observation period (beginning at 318.6 ± 0.8 SD mOsm/L, to the end-point at 320.0 ± 0.6 mOsm/L). With RVOT treatment, aCSF osmolarity increased from a baseline of 318.8 ± 0.8 mOsm/L, to 339 ± 3.3 mOsm/L (±SD) within 2 h. After 10 h of treatment, aCSF osmolarity approached an asymptote at 344.0 ± 4.2 mOsm/L, which was significantly greater than control aCSF osmolarity (p << 0.001 by t test, n = 8). The best-fit curve for the initial hours of treatment was y = 16.41 * Ln(x) + 318.6 (R2 = 0.99). The rise in osmolarity for the first hours was 20.2 mOsm/L. For a 10-mL volume, this is an increase of 2 × 10−2 mmol h−1, or about 25 times greater than the estimate above.
FIG. 3.
Line chart showing change in osmolarity with reductive ventricular osmotherapy (RVOT) over time. RVOT treatment of flowing cerebrospinal fluid (CSF) significantly increases artificial CSF (aCSF) osmolarity. To simplify modeling, the tissues were not placed in the device until after the 4-h measurement (arrow).
Water content in normal tissue was 3.955 g H2O (g dry wt)−1 (n = 8), which approximates the value of normal brain water content seen in the literature of 4.0 g H2O (g dry wt)−1 in cortical slices without water exposure (Elliot and Pappius, 1956). Therefore, tissue samples were normalized back to 3.955 g H2O (g dry wt)−1, and the relative change in water content was considered for treatment efficacy.
Water content at the end of 6 h of circulating aCSF exposure was 6.4 ± 0.9 g H2O (g dry wt)−1, compared to 6.1 ± 0.7 g H2O (g dry wt)−1 after 6 h of RVOT treatment of aCSF, which is a statistically significant decrease compared to normalized pre-treatment water content (p = 0.02, n = 24).
Increase in mass transfer coefficient
The mass transfer coefficient for water movement in tissues can be calculated based on the following formula:
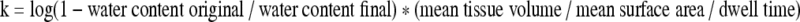 |
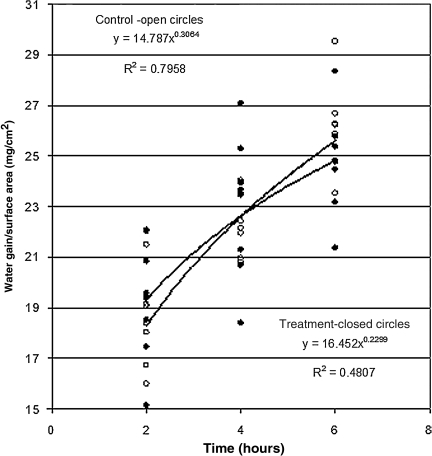
The mass transfer coefficient for treated tissue (2.9 ±0.5 × 10−6 cm2/sec) was greater than that for control tissue (2.5 ± 0.4 ± 10−6 cm2/sec, p = 0.008) based on 47 observations. The values of mass transfer coefficients are consistent with reports in the literature of water diffusion coefficients, and approach the diffusion coefficient of water in dilute non-aqueous liquids of 2.64 ± 10−5 cm2/sec (Cussler, 1997). This finding of increased mass transfer coefficient was confirmed by testing total water gain per surface area at 2, 4, and 6 h (Fig. 4). Though these tissue samples were larger than the first group, and the apparatus was opened every 2 h so that the tissue could be weighed, the mass transfer coefficient calculated for 6-h outcomes for RVOT-treated tissue was greater (2.5 ± 0.4 × 10−6 cm2/sec) than that for control tissue in this series (2.2 ± 0.4 × 10−6 cm2/sec). These values compare favorably with figures found in the literature.
FIG. 4.
Scatterplot showing the rate of water gain per surface area of tissue in control and reductive ventricular osmotherapy (RVOT)-treated artificial cerebrospinal fluid (aCSF). This confirms the findings of an increased mass transfer coefficient in tissue incubated in treated aCSF. The lines represent the best-fit curves.
Discussion
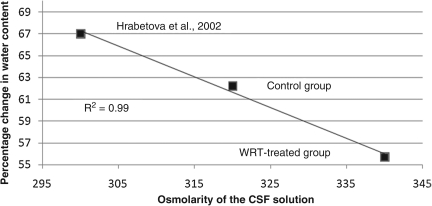
RVOT had a robust effect on aCSF osmolarity in this study. In pilot studies, placement of fresh ex vivo cerebral tissue in saline caused the tissue to gain about 30% in water content. The effect of increased aCSF osmolarity had the intended effect on the increase in water content of the ex vivo tissue exposed to the aCSF. The change in water content was an increase of 62.2 ± 11% in controls, and 55.7 ± 8.3% when normalized to 100% for pre-treatment. Hrabetova and associates (2002) found a 67% increase in water content after 6 h of incubation in 300 mOsm aCSF. These three data points of osmolarity versus water gain represent a linear relationship, with an R2 = 0.99. (Fig. 5).
FIG. 5.
Graph showing water gain of ex vivo cerebral tissue after 6 h of artificial cerebrospinal fluid (aCSF) exposure at different osmolarities. The resultant increase in water gain is inversely proportional to the osmolarity of the aCSF to which the tissue is exposed. The linear trend line is also shown.
The increase in mass transfer coefficient represents a significant finding. Increased extracellular osmolarity results in decreased cellular swelling, as the osmotic gradient would favor movement of water out of the cell. Reduced cell swelling will increase diffusion and convective flow between the cells. Chen and Nicholson (2000) exposed slices of brain to hypersomolar solutions and documented improved diffusion, so one would assume that hyperosmolarity produced by RVOT will increase water transport.
It is important to note that control aCSF was also hyperosmolar, with a beginning level of 320 mOsm/L. A hypertonic solution was chosen as a control because human, post-traumatic CSF is naturally hypertonic, averaging as high as 329 mOsm/L (unpublished data).
Ischemic cerebral tissue has been shown to exhibit hyperosmolarity, with osmolarity as high as 400 mOsm/L being documented (Kawamata et al., 2007). Hyperosmolar tissue may be at least in part responsible for intracranial hypertension, as water is absorbed into cerebral tissue. Systemic osmotherapy is frequently used (Pinto et al., 2006). Systemic levels >320 mOsm/L are to be avoided when using mannitol (Bhardwaj and Ulatowski, 1999), but systemic osmolarity as high as 360 mOsm/L has been tolerated when due to hypertonic saline (Adelson et al., 2003; Peterson et al., 2000; Rockswold et al., 2009).
In children, osmolarity as high as 364.8 mOsm/L was seen after treatment with 3% saline, and in one individual the level reached 431 mOsm/L. These levels were generally well tolerated, although two patients did develop acute renal failure (Khanna et al., 2000). Ventricular osmotherapy may have added value to systemic osmotherapy in the control of ICP.
Therapy aimed at increasing CSF osmolarity by injection or infusion of hyperosmolar solutions to counter this gradient have been successful; however, ventricular osmotherapy is limited by the need for infusion of an osmotic agent in the face of high ICP. RVOT was shown to be efficacious in increasing CSF osmolarity, and was able to do so while simultaneously reducing overall fluid volume.
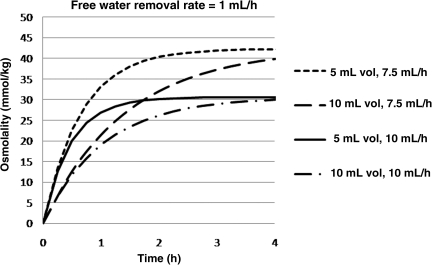
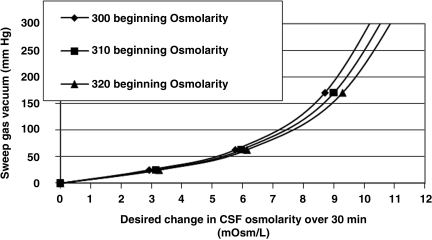
Clinical challenges to ventricular osmotherapy include (1) small ventricular volumes, and (2) potentially high de novo osmolarity. CSF has been shown to be hyperosmolar after TBI (unpublished data). Using computerized modeling, it can be shown that RVOT will actually increase osmolarity at greater rates if therapy is performed in the presence of small ventricular volumes (Fig. 6), and elevated pretreatment CSF osmolarity (Fig. 7).
FIG. 6.
Line graph showing computerized modeling relationship of change in osmolarity as a function of lateral ventricle volume and cerebrospinal fluid (CSF) flow. Small ventricles and low CSF turnover rates require less equilibration time. Clinically, collapsed ventricles will benefit from the greatest increase in osmolarity.
FIG. 7.
Line graph showing computerized modeling to calculate the airflow vacuum level needed to change osmolarity in 30 min based on beginning cerebrospinal fluid (CSF) osmolarity.
Conclusions
RVOT catheters quickly increased osmolarity of aCSF without the need for infusion of osmotic agents. Injured tissue is hyperosmolar, so therapeutic intraventricular or intrathecal osmotic agents must overcome the osmotic gradient to remove free water from tissues. This study also confirmed that an increased CSF osmolarity will reduce tissue water content in ex vivo studies. Problematic conditions of small ventricles or high pre-treatment osmolarity are favorable for RVOT treatment in that osmolarity increases at a faster rate. While in vitro studies must be performed to confirm these findings, the results suggest that RVOT may be a novel and effective method for the reduction of cerebral edema following TBI, thereby improving control of intracranial hypertension.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke, NS43832, and Scott Panter received grant support through this same grant. The authors would like to acknowledge Mary Helen Schmidt of Twin Star Medical for her editorial work and preparation of the manuscript.
Portions of this work were presented in poster form at Neuroscience 2006, Atlanta, Georgia, October 15, 2006.
Author Disclosure Statement
Rick Odland is a principal in Twin Star Medical, Inc., a company that is developing reductive ventricular osmotherapy technology. Twin Star Medical provided hourly support for the writing of this paper. Gaylan Rockswold was a consultant on the grant, and is currently a consultant to Twin Star Medical; he has less than 5% equity in Twin Star Medical.
References
- Adelson P.D. Bratton S.L. Carney N.A. Chesnut R.M. du Coudray H.E. Goldstein B. Kochanek P.M. Miller H.C. Partington M.D. Selden N.R. Warden C.R. Wright D.W. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr. Crit. Care Med. 2003;4:S40–S44. [PubMed] [Google Scholar]
- Bhardwaj A. Ulatowski J.A. Cerebral edema: hypertonic saline solutions. Current treatment options. Neurology. 1999;1:179–187. doi: 10.1007/s11940-999-0002-z. [DOI] [PubMed] [Google Scholar]
- Bruns J. Hauser W.A. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44:S2–S10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- Chen K.C. Nicholson C. Changes in brain cell shape create residual extracellular space volume and explain tortuosity behavior during osmotic challenge. Proc. Natl. Acad. Sci. USA. 2000;97:8306–8311. doi: 10.1073/pnas.150338197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussler E.L. Diffusion: Mass Transfer in Fluid Systems. Cambridge University Press; Cambridge: 1997. p. 113. [Google Scholar]
- Doczi T. Joo F. Szerdahelyi P. Bodosi M. Regulation of brain water and electrolyte contents: the possible involvement of central atrial natriuretic factor. Neurosurgery. 1987;21:454–458. doi: 10.1227/00006123-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Elliot K.A. Pappius H. Water distribution in incubated slices of brain and other tissues. Can. J. Biochem. Physiol. 1956;34:1007–1022. [PubMed] [Google Scholar]
- Hatashita S. Hoff J.T. Role of a hydrostatic pressure gradient in the formation of early ischemic brain edema. J. Cereb. Blood Flow Metab. 1986;6:546–552. doi: 10.1038/jcbfm.1986.100. [DOI] [PubMed] [Google Scholar]
- Hossman K. Pappius H. Feindel W. Dynamics of Brain Edema. Springer-Verlag; Berlin: 1976. pp. 219–227. [Google Scholar]
- Hrabetova S. Chen K.C. Masri D. Nicholson C. Water compartmentalization and spread of ischemic injury in thick-slice ischemia model. J. Cereb. Blood Flow Metab. 2002;22:80–88. doi: 10.1097/00004647-200201000-00010. [DOI] [PubMed] [Google Scholar]
- Juul N. Morris G.F. Marshall S.B. Marshall L.F. Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J. Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- Katayama Y. Mori T. Maeda T. Kawamata T. Pathogenesis of the mass effect of cerebral contusions: rapid increase in osmolality within the contusion necrosis. Acta Neurochir. Suppl. (Wien.) 1998;71:289–292. doi: 10.1007/978-3-7091-6475-4_84. [DOI] [PubMed] [Google Scholar]
- Kawamata T. Mori T. Sato S. Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg. Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- Khanna S. Davis D. Peterson B. Fisher B. Tung H. O'Quigley J. Deutsch R. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit. Care Med. 2000;28:1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- Maas A. Marmarou A. Murray G.D. Teasdale G.M. Steyerberg E.W. Prognosis and clinical trial design in traumatic brain injury: The IMPACT study. J. Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y. Hossmann K.A. Brain tissue osmolality after middle cerebral artery occlusion in cats. Exp. Neurol. 1982;77:599–611. doi: 10.1016/0014-4886(82)90231-x. [DOI] [PubMed] [Google Scholar]
- Miller J.D. Becker D.P. Ward J.D. Sullivan H.G. Adams W.E. Rosner M.J. Significance of intracranial hypertension in severe head injury. J. Neurosurg. 1977;47:503–516. doi: 10.3171/jns.1977.47.4.0503. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Greenberg R.P. Miller J.D. Enas G.G. Choi S.C. Kishore P.R. Selhorst J.B. Lutz H.A., 3rd. Becker D.P. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J. Neurosurg. 1981;54:751–762. doi: 10.3171/jns.1981.54.6.0751. [DOI] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.D. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland R.M. Sutton R.L. Hyperosmosis of cerebral injury. Neurol. Res. 1997;21:500–508. [PubMed] [Google Scholar]
- Onal C. Unal F. Turantan M.I. Uzum G. Hasanoglu A. Kaynar M.Y. The effect of intraventricular albumin in experimental brain oedema. Acta Neurochir. (Wien.) 1997;139:661–668. doi: 10.1007/BF01412002. discussion 668–669. [DOI] [PubMed] [Google Scholar]
- Owen M.D. Matthes R.D. Gisolfi C.V. Effects of CSF ANG II and AVP on sweating in the heat-stressed patas monkey. J. Appl. Physiol. 1989;67:134–140. doi: 10.1152/jappl.1989.67.1.134. [DOI] [PubMed] [Google Scholar]
- Peterson B. Khanna S. Fisher B. Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit. Care Med. 2000;28:1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- Pinto F.C. Capone-Neto A. Prist R.E. Silva M.R. Poli-de-Figueiredo L.F. Volume replacement with lactated Ringer's or 3% hypertonic saline solution during combined experimental hemorrhagic shock and traumatic brain injury. J. Trauma Inj. Infect. Crit. Care. 2006;60:758–764. doi: 10.1097/01.ta.0000214581.89316.73. [DOI] [PubMed] [Google Scholar]
- Pullen R.G. Effect of ventricular tonicity upon cerebrospinal fluid production in rabbits. J. Physiol. 1985;362:273–283. doi: 10.1113/jphysiol.1985.sp015676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea G.L. Rockswold G.L. Barbiturate therapy in uncontrolled intracranial hypertension. Neurosurgery. 1983;12:401. doi: 10.1227/00006123-198304000-00005. [DOI] [PubMed] [Google Scholar]
- Rockswold G.L. Solid C.A. Paredes-Andrade E. Rockswold S.B. Jancik J.T. Quickel R.R. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009 doi: 10.1227/01.NEU.0000359533.16214.04. (in press). [DOI] [PubMed] [Google Scholar]
- Rundgren M. Jonasson H. Hjelmqvist H. Water intake and changes in plasma and CSF composition in response to acute administration of hypertonic NaCl and water deprivation in sheep. Acta Physiol. Scand. 1990;138:85–92. doi: 10.1111/j.1748-1716.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- Sutton R.S. Quist H. Mathews W. Odland R.M. Intraventricular albumin infusion reduces experimental cerebral edema. Abstracts of the 17th Annual National Neurotrauma Society Meeting; Miami, FL. Oct;1999 ; 1999. J. Neurotrauma 16 (abstract). [Google Scholar]
- Tomita M. Gotoh F. Sato T. Yamamoto M. Amano T. Tanahashi N. Tanaka K. Determination of the osmotic potential for swelling of cat brain in vitro. Exp. Neurol. 1979;65:66–70. doi: 10.1016/0014-4886(79)90248-6. [DOI] [PubMed] [Google Scholar]