Abstract
Stromal tumors of the prostate are rare and only a few cases have been described in the literature, including exceptional cases of stromal tumors with unknown malignant potential (STUMP) and a fatal outcome in young patients. Morphologically distinguishing a STUMP from a stromal sarcoma of the prostate (PSS) is still a challenge. We describe the histopathological and immunohistochemical findings in a 34-year-old man with a malignant specialized cell stromal tumor of the prostate that was diagnosed initially as STUMP, and he developed lung metastases within a few months. The patient attended our hospital because of lower urinary tract symptoms, after having excreted tissue through the urethra a few months before. Ultrasonography and urethrocystoscopy examinations showed a mass arising from the verumontanum, and a transurethral resection (TUR) revealed a highgrade spindle cell sarcoma reminiscent of a phyllode tumor of the breast. The tumor cells were immunoreactive for vimentin, progesterone receptor and, focally, CD34. The preliminary histological findings were subsequently confirmed after radical prostatectomy. The patient developed bilateral lung metastases and died 25 months after the initial diagnosis. Although rare in young patients, the challenging differential diagnosis of STUMP and PSS means that a prostate STUMP diagnosis made on the basis of biopsy or TUR specimens also requires urethrocystoscopic monitoring for the early detection of any progression to PSS. Radical prostatectomy should also be carefully considered.
Key words: stromal tumors with unknown malignant potential, stromal tumor, prostatic neoplasm, mesenchymal tumor, stromal sarcoma.
Introduction
Stromal tumors of the prostate are rare and arise from specialized hormone-dependent mesenchymal cells.1 They have a broad spectrum of histological features and, depending on parameters such as cellularity, the number of mitotic figures, necrosis, and the stromal infiltration of periprostatic tissues, are classified as phyllode tumors of the prostate, various subtypes of stromal tumors of uncertain malignant potential (STUMP), or prostatic stromal sarcomas (PSS).2,3 PSS have an immunohistochemical profile that distinguishes them from other prostate sarcomas such as leiomyosarcomas or rhabdomyosarcomas.4 Although some tumors seem to arise centrally near the verumontanum, most develop in the posterior portion of the gland.2,5 The patients may be young and typically present with acute or chronic urin ary obstruction, and possibly hematuria and dysuria. The tumors cause well-known local morbidity, but their management and prognosis are still uncertain.1,2,5
We describe the case of a patient in whom an initial diagnosis of STUMP was made on the basis of tissue excreted through the urethra, but rapid local recurrence and multiple pulmonary metastases suggested a highly malignant PSS. We also review the literature concerning stromal tumors diagnosed in young patients.
Case Report
This 34-year-old man developed progressive urinary obstruction in September 2007. The findings of an ultrasound examination were unremarkable, but intravenous urography and cystography performed elsewhere showed an oval mass within the lumen of the prostatic urethra (Figure 1). The day before he was hospitalized for further tests, the patient observed pieces of tissue during micturition, one of which was available for histopathological examination and led to a diagnosis of prostatic STUMP. The obstructive symptoms resolved following tissue voiding and no treatment was given.
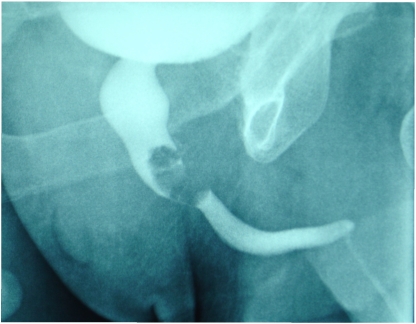
Figure 1.
Intravenous urogram showing the tumor invading the prostatic urethra as a contrast-defective oval mass within the lumen.
The patient first attended our hospital (IRCCS Istituto Clinico Humanitas - Rozzano, Milan, Italy) four months later, when the symptoms recurred. Urethrocystoscopy revealed a multi-lobed mass arising from the verumontanum, and so a transurethral resection (TUR) was performed. Abdominal computed tomog -raphy (CT) and chest radiography did not reveal any distant metastases. An endoscopic evaluation repeated seven months after the initial diagnosis highlighted a local recurrence, which was again treated by means of TUR. Histological investigation confirmed the persistence of the tumor and the patient underwent radical prostatectomy (RP). As the tumor was near the surgical margins in the periprostatic soft tissue, radiotherapy (60 Gy in 30 daily fractions) was delivered to the prostate bed.
Despite this local treatment, 12 months after the first diagnosis and four months after RP, CT revealed multiple metastatic nodules in both lungs, whereas the abdomen and pelvis showed no signs of neoplastic disease. After discussion in the multidisciplinary sarcoma unit and considering the well performance status, the patient underwent lung metastasectomy, which revealed a high-grade polymorphic sarcoma whose features recalled the primary prostatic sarcoma.
In February 2009, the patient experienced lung and pleural progression, and pelvic involvement was diagnosed by means of magnetic resonance imaging. The lung lesions partially responded to palliative chemotherapy with ifosfamide and liposomal doxorubicin, but the pelvis disease progressed symptomatically. Although he was subsequently administered palliative radiotherapy to the left iliac region, the patient died because of disease progression 25 months after the initial diagnosis.
Pathology
Microscopically, the histological specimens taken after both TURs showed a uniform proliferation of spindle and ovoid stromal cells, some of which had atypical nuclei, scattered mitotic figures, and necrotic foci. A malignant stromal tumor of the prostate was diagnosed. After RP, the cut surface of the prostate showed a gray-white polypoid mass protruding into the urethral lumen with numerous hemorrhagic foci (Figure 2), and histological examination revealed a highly malignant mesenchymal neoplasm with multiple areas of necrosis, high mitotic count, and nuclear atypia (Figure 3A). The spindle cell component was intercalated by dilated epithelial structures reminiscent of a malignant phyllode tumor (Figure 3B), and there were tumoral vessels permeating the capsule and pericapsular tissue (Figure 3C). Immunohistochemistry findings showed that the tumoral cells were reactive for vimentin (Figure 3D), progesterone receptor (scattered cells), and CD34. The margins were free, in close proximity to foci of the tumor in periprostatic soft tissue. The final histopathological diagnosis was high-grade prostatic specialized stromal sarcoma.
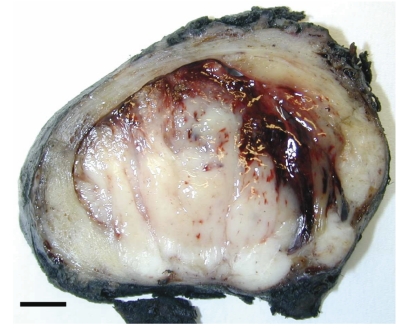
Figure 2.
Whole prostate section. The cut surface shows a gray-white hemorrhagic polypoid mass originating from the peripheral zone of the gland, which completely occluded the urethral space and infiltrated the extra-prostatic tissue.
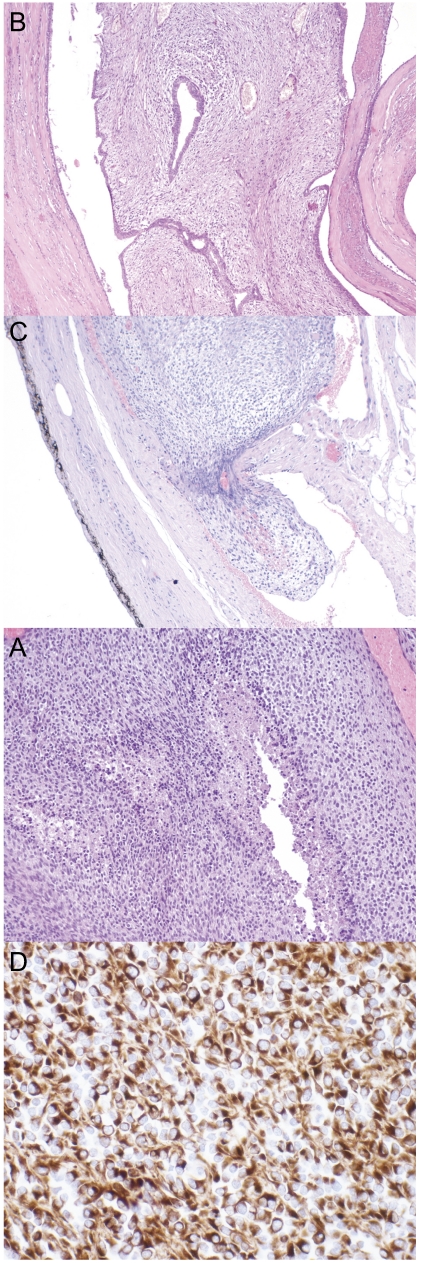
Figure 3.
(A) Malignant stromal cell proliferation with necrosis and areas of high cellularity (magnification: 100×). (B) Some areas of the tumor with cleft-like spaces lined by normal-looking epithelial cells producing a leaf-like (phyllode) pattern (magnification: 100×). (C) Radical prostatectomy revealed the neoplastic invasion of blood vessels in the capsular region of the prostate (magnification: 100×). (D) Neoplastic cells immunoreactive for vimentin demonstrating the mesenchymal origin of the tumor (magnification: 200×).
Discussion
It is not difficult to distinguish a benign stromal lesion from sarcoma of the prostate on the basis of the detection of atypical stromal cells, high cellularity, and invasion but the correct diagnosis of borderline lesions remains a challenge and has led to the introduction of terms such as “unknown malignant potential” and “low malignant potential”. Gaudin et al. identified four distinct STUMP subtypes depending on their histological pattern and the degree of atypia: hypercellular, myxoid, with degenerative atypia, and phyllode-like.5 The histological features of the relatively common phyllode-like subtype have recently been reviewed and may be similar to those of its counterpart in the breast.1 Transformation may occur, but always after several local recurrences.
Our case is notable because of its unusual clinical presentation and the treatment dilemma posed during disease progression. To the best of our knowledge, there are only two reported cases of sarcoma diagnosed on the basis of tumor tissue excreted through the urethra: the first was a pediatric prostatic rhabdomyosarcoma and the second a high-grade stromal sarcoma in a young adult which, as in our case, showed widespread distant metastases a few months after diagnosis.6,7
A review of the literature based on a PubMed search for patients aged less than 40 years with a diagnosis of PSS, STUMP, PSS-PT, or STUMP-PT identified 21 cases described since 1977, about 15–20% of all of the stromal tumors reported worldwide (Table 1).1–6,8–21 This means that STUMP and PSS have been diagnosed in 10 and 11 cases, respectively. Five of the patients with STUMP underwent RP, four enucleation, and one partial prostatectomy (PP), all of whom showed no signs of disease after a median follow-up of 27 months (range: 1–36 months). Nine of the 11 patients with PSS underwent RP, one TUR, and one multi-organ exenteration; five received chemotherapy and one radiotherapy after surgery. Four of these 11 patients (three of whom developed metastases) died as a result of the PSS after a median follow-up of 12 months (range: 3–25 months); one patient was alive with metastatic disease after 26 months; and seven showed no signs of disease a median of 14 months after surgery (range: 1–300 months). Furthermore, the phyllode tumor pattern documented in five patients (four PSS and one STUMP) did not seem to be related to an adverse clinical outcome.
Table 1. Stromal tumors of the prostate in young patients (under 40 years old) reported in the literature from 1977 to 2010.
| N | Ref | Age | Symptoms | Diagnosis | Therapy | F/U (months) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 31 | LUTS | STUMP | Enuc | 30 | NED |
| 2 | 11 | 32 | LUTS | STUMP | PP | 24 | NED |
| 3 | 9 | 23 | Testicular pain | STUMP | Enuc | 9 | NED |
| 4 | 12 | 32 | LUTS | STUMP | Enuc | 36 | NED |
| 5 | 10 | 22 | LUTS | STUMP | RP | 30 | NED |
| 6 | 2 | 27 | Rectal fullness | STUMP | Hormone, RP | 0 | NED after RP |
| 7 | 2 | 39 | LUTS | STUMP | TUR, RP | 18 | NED |
| 8 | 3 | 30 | LUTS, PSA | STUMP | Enuc | 36 | NED |
| 9 | 3 | 27 | LUTS | STUMP | RP | 0 | NED after RP |
| 10 | 17 | 28 | Hemospermia | STUMP-PT | RP | 36 | NED |
| 11 | 20 | 38 | Hematuria | HG PSS-PT | RP, RT | 26 | Lung mts, AWD |
| 12 | 2 | 25 | LUTS | HG PSS | RP, CT | 12 | NED |
| 13 | 19 | 36 | LUTS | PSS-PT | RP, CT | 20 | DOD |
| 14 | 21 | 36 | LUTS | PSS-PT | RP | 14 | NED |
| 15 | 1 | 36 | Hematuria, LUTS | LG PSS-PT | RP | 101 | NED |
| 16 | 1 | 25 | LUTS | LG PSS-PT | RP | 300 | NED |
| 17 | 6 | 31 | TEU | HG PSS | Extenteration | 4 | Multiorgan mts, DOD |
| 18 | 13 | 20 | LUTS | HG PSS | RP | 12 | NED |
| 19 | 14 | 38 | LUTS | HG PSS | RP | 0 | NED after RP |
| 20 | 18 | 19 | LUTS | LG PSS-PT | RP, CT | 48 | NED |
| 21 | 15 | 33 | Perineal pain | HG PSS | TUR, CT | NA | Lung mts, DOD after CT |
| 22 | Current case | 34 | TEU | HG PSS | RP, RT, CT | 25 | Lung mts, DOD |
LUTS, lower urinary tract symptoms; PSA, prostate specific antigen; TEU, tissue excreted through urethra; STUMP, stromal tumor of uncertain malignant potential; PT, phyllodes tumor; HG, high-grade malignancy; PSS, prostatic stromal sarcoma; LG, low-grade malignancy; Enuc, enucleation; PP, partial prostatectomy; RP radical prostatectomy; TUR, transurethral resection; RT, radiation therapy; CT chemotherapy; NA not available; NED, not evidence of disease; Mts, distant metastasis; AWD, alive with disease; DOD, dead of disease.
Although not exhaustive because of the rarity of the neoplasm, these data suggest that a clear sarcomatous transformation leads to an increased incidence of metastases and death; however, as 10 patients with STUMP were alive and without disease during follow-up, it can be hypothesized that such tumors are curable surgically in the majority of young patients and have a benign/very low grade malignant course. As the name indicates, the malignant potential of STUMPs is unclear: some grow slowly (if at all) over a period of several years whereas others infiltrate the prostate extensively, invade adjacent rectal tissue, and often develop recurrences after surgery.1,2,5,22 Some STUMPs may dedifferentiate and progress to PSS,2,5,15,19,23 generally over years; in other cases STUMP and PSS may be present in the same tissue specimens as separate foci or merging into each other.
It may not be possible to determine whether a neoplastic lesion is malignant or not on the basis of biopsy or TUR samples because of sampling problems. In the case of biopsy mater ial, some authors have recommended the use of the term “uncertain malignant potential” in cases that do not satisfy the traditional criteria of malignancy: for instance, marked cellularity, a high degree of mitotic activity, nuclear atypia, necrosis, or extracapsular invasion;5 however, others claim that classifying a tumor as stromal proliferation with unknown malignant potential might only confuse urologists and therefore not be very helpful. Herawi and Epstein found that seven (14%) of the 50 stromal tumors of the prostate they analyzed were STUMPs associated with sarcoma: three of these patients developed metastases during follow-up while there was no evidence of disease in the remaining four patients, thus demonstrating that only the histological subtypes of STUMP harboring foci of sarcoma do not correlate with clinical behavior.2
It is possible that this was true in our case. After the first recurrence in which a small focus of clear high-grade sarcoma was detected, the patient underwent RP and the specimen showed locally advanced disease – although rapidly implemented, this aggressive treatment failed to control the disease, and multiple bilateral lung metastases developed within a few months. We conclude that all cases of STUMP should be closely monitored clinically by means of both imaging and urethroscopy in order to be able to identify any progression to PSS early, and that radical prostatectomy should be carefully considered as well in young patients even if only a focal area of high-grade stromal sarcoma is found in TUR material.
References
- 1.Bostwick DG, Hossain D, Qian J, et al. Phyllodes tumor of the prostate: long-term follow up study of 23 cases. J Urol. 2004;172:894–9. doi: 10.1097/01.ju.0000134580.71261.57. [DOI] [PubMed] [Google Scholar]
- 2.Herawi M, Epstein JI. Specialized stromal tumors of the prostate: a clinicopathologic study of 50 cases. Am J Surg Pathol. 2006;30:694–704. doi: 10.1097/00000478-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; Lyon, France: 2004. pp. 218–49. (World Health Organization Classification of Tumours.) [Google Scholar]
- 4.Sexton WJ, Lance RE, Reyes AO, et al. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166:521–5. doi: 10.1016/s0022-5347(05)65974-5. [DOI] [PubMed] [Google Scholar]
- 5.Gaudin PB, Rosai J, Epstein JI. Sarcomas and related proliferative lesions of specialized prostatic stroma: a clinicopathologic study of 22 cases. Am J Surg Pathol. 1998;22:148–62. doi: 10.1097/00000478-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Froehner M, Bartholdt E, Meye A, et al. Adult prostate sarcoma diagnosed from tissue spontaneously excreted through the urethra. Urol Oncol. 2004;22:119–20. doi: 10.1016/S1078-1439(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 7.Perry MJ, Cahill DJ, Denham PL, Naerger HGA. Embryonal rhabdomyosarcoma passed per urethra. J Urol. 2002;167:2167–2167. [PubMed] [Google Scholar]
- 8.Attah EB, Powell ME. Atypical stromal hyperplasia of the prostate gland. Am J Clin Pathol. 1977;67:324–7. doi: 10.1093/ajcp/67.4.324. [DOI] [PubMed] [Google Scholar]
- 9.Kendall AR, Stein BS, Shea FJ, et al. Cystic pelvic mass. J Urol. 1986;135:550–3. doi: 10.1016/s0022-5347(17)45733-8. [DOI] [PubMed] [Google Scholar]
- 10.Kevwitch MK, Walloch JL, Waters WB, Flanigan RC. Prostatic cystic epithelialstromal tumors: a report of 2 new cases. J Urol. 1993;149:860–4. doi: 10.1016/s0022-5347(17)36234-1. [DOI] [PubMed] [Google Scholar]
- 11.Manivel C, Shenoy BV, Wick MR, Dehner LP. Cystosarcoma phyllodes of the prostate. A pathologic and immunohistochemical study. Arch Pathol Lab Med. 1986;110:534–68. [PubMed] [Google Scholar]
- 12.Reese JH, Lombard CM, Krone K, Stamey TA. Phyllodes type of atypical prostatic hyperplasia: a report of 3 new cases. J Urol. 1987;138:623–6. doi: 10.1016/s0022-5347(17)43280-0. [DOI] [PubMed] [Google Scholar]
- 13.Lara C, Borrero JJ, Porras V, Giraldez J. Prostatic stromal sarcoma in a 20-year-old patient. Arch Esp Urol. 2005;58:947–9. doi: 10.4321/s0004-06142005000900012. [DOI] [PubMed] [Google Scholar]
- 14.Chang YS, Chuang CK, Ng KF, Liao SK. Prostatic stromal sarcoma in a young adult: a case report. Arch Androl. 2005;51:419–24. doi: 10.1080/014850190947822. [DOI] [PubMed] [Google Scholar]
- 15.Segawa N, Hamada S, Takahara K, et al. Prostatic stromal sarcoma: a case report. Hinyokika Kiyo. 2008;54:29–34. [PubMed] [Google Scholar]
- 16.Young JF, Jensen PE, Wiley CA. Malignant phyllodes tumor of the prostate. A case report with immunohistochemical and ultrastructural studies. Arch Pathol Lab Med. 1992;116:296–9. [PubMed] [Google Scholar]
- 17.Mawlawi H, Ould Heamane DS, Ferlicot S, et al. Phyllodes tumour of the prostate in a 28-year-old man. Prog Urol. 2007;17:1379–81. doi: 10.1016/s1166-7087(07)78582-2. [DOI] [PubMed] [Google Scholar]
- 18.Sakura M, Tsukamoto T, Yonese J, et al. Successful therapy of a malignant phyllodes tumor of the prostate after postoperative local failure. Urology. 2006;67:845–845. doi: 10.1016/j.urology.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 19.De Raeve H, Jeuris W, Wyndaele JJ, Van Marck E. Cystosarcoma phyllodes of the prostate with rhabdomyoblastic differentiation. Pathol Res Pract. 2001;197:657–62. doi: 10.1078/0344-0338-00142. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Beltran A, Gaeta JF, Huben R, Crohan GA. Malignant phyllodes tumor of the prostate. Urology. 1990;35:164–7. doi: 10.1016/0090-4295(90)80068-x. [DOI] [PubMed] [Google Scholar]
- 21.Kameoka H, Kumakawa K, Uchida H, et al. Phyllodes tumor of the prostate: a case report. Nippon Hinyokika Gakkai Zasshi. 2002;93:52–7. doi: 10.5980/jpnjurol1989.93.52. [DOI] [PubMed] [Google Scholar]
- 22.Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20:148–58. doi: 10.1038/modpathol.3800676. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe M, Yamada Y, Kato H, et al. Malignant phyllodes tumor of the prostate: retrospective review of specimens obtained by sequential transurethral resection. Pathol Int. 2002;52:777–83. doi: 10.1046/j.1440-1827.2002.01417.x. [DOI] [PubMed] [Google Scholar]