Abstract
The human T-cell lymphotropic virus type 1 (HTLV-1), endemic in defined geographical areas around the world, is recognized as the etiologic agent of adult T-cell leukemia/lymphoma (ATL), or HTLV-1. ATL is a rare adult onset T-cell malignancy that is characterized by the presence of ATL flower cells with T-cell markers, HTLV-1 antibodies in the serum, and monoclonal integration of HTLV-1 provirus in affected cells. Ocular manifestations associated with HTLV-1 virus infection have been reported and include HTLV-1 uveitis and keratoconjunctivitis sicca, but reports of ocular involvement in ATL are exceedingly rare. This article describes the ocular manifestations and pathology of ATL. We also report for the first time a case of a 34-year-old male with systemic ATL and prominent atypical lymphoid cell infiltration in the choroid. To our knowledge, this is the first report defining prominent choroidal involvement as a distinct ocular manifestation of ATL. ATL may masquerade as a variety of other conditions, and molecular techniques involving microdissection and PCR have proven to be critical diagnostic tools. International collaboration will be needed to better understand the presentation and diagnosis of this rare malignancy.
Key words: adult T-cell leukemia/lymphoma, human T-lymphotrophic virus type 1, pathology, eye.
Introduction
The human T-cell lymphotropic virus type 1 (HTLV-1) was first isolated in 1980 from two T-cell lymphoblastoid cell lines and the blood of a patient with cutaneous T-cell lymphoma.1 This was the first retrovirus to be causally linked to human disease, and it is now recognized as the etiologic agent of HTLV-1 associated myelopathy/tropical spastic paraparesis, HTLV-1 associated uveitis, various additional autoimmune and inflammatory diseases, and HTLV-1 associated adult T-cell leukemia/lymphoma (ATL).2 HTLV-1 infection is endemic in southwestern Japan, the Caribbean, and Central Africa. HTLV-1 carriers are also prevalent in South America, Papua New Guinea, and the Solomon Islands. It has been estimated that there are 10–20 million HTLV-1 infected individuals worldwide.3
HTLV-1 infection occurs primarily through the transmission of infected cells with integrated HTLV-1 provirus via breast feeding, contaminated blood, and sexual contact. In the host, the virus initially spreads from cell to cell through viral synapses and later proliferates by clonal expansion of the infected T-cells.4 The HTLV-1 genome includes gag, pol, and env, three genes that are common to all retroviruses, and a unique pX region that contains 4 open reading frames (ORFs). Several regulatory genes are encoded in the pX region, among which tax, rex, and HTLV-1 basic leucine zipper factor (HBZ) have been shown to play critical roles in HTLV-1 mediated leukemogenesis. ATL has an extended latency period between infection and the onset of ATL symptoms, and less than 5% of HTLV-1 infected individuals develop ATL.5,6 This suggests that multiple complex molecular events are required in ATL pathogenesis.7
Case Report
Choroidal manifestations of ATL are extremely rare. Cases involving choroidal thickening and the infiltration of a few scattered atypical lymphoid cells have been reported.8,9 Recently, a 34-year-old Jamaican male with a history of systemic ATL was seen at the National Cancer Institute at the National Institutes of Health (NIH). A previous computed tomography scan had shown large masses in the abdomen, chest, and neck, and echocardiography revealed a 20×20 mm mass in the interventricular septum. The patient was initially treated with CHOP chemotherapy [cyclophosphamide, hydroxydaunorubicin (doxorubicin), oncovin (vincristine), and prednisone/prednisolone], followed by CMED (cyclophosphamide, methotrexate, etoposide and dexamethasone), with minimal response. He developed fever, chills, nausea, and vomiting, which progressed to respiratory failure over 10 days, before being admitted to the NIH for decitabine and vorinostat treatment. He was intubated and placed on levophed for hypotension. Blood cultures revealed Enterococcus faecalis and Candida tropicalis sepsis. Despite aggressive antimicrobial therapy, the patient expired, and an autopsy was performed in accordance with established protocols.
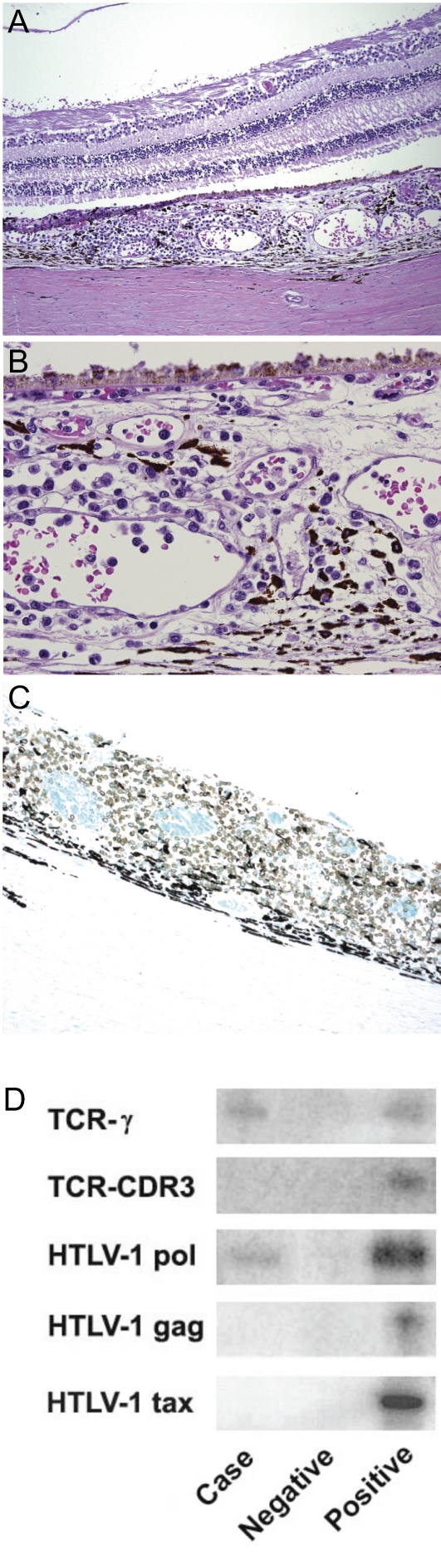
The autopsy revealed large ATL masses in the neck, mediastinum, mesentery, retroperitoneum, and myocardium. Autopsied eye examination disclosed aggregates of moderate small to medium sized atypical lymphocytes in the posterior choroid of both eyes (Figure 1A and B), more prominently temporally. Occasional small choroidal vessels were packed with atypical CD3+ (Figure 1C), CD25+, and CD20− lymphoid cells.
Figure 1.
Atypical lymphocytes in the posterior choroid of both eyes. (A) Atypical lymphoid infiltrate in the choroid. The retina and subretinal space are not involved. (B) High power shows that the atypical lymphocytes vary in size. They are larger than mature lymphocytes. The clumps of these cells in the vessels indicate hematologic spread of the tumor. (C) These tumor cells are CD3 positive (shown by the dark color in this stain). (D) The microdissected atypical lymphoid cells in the choroid are positive for T-cell receptor (TCR)- γ gene rearrangement and the HTLV-1 pol gene. TCR-CDR3 gene rearrangement and the HTLV-1 genes gag and tax were not detected.
Atypical lymphoid cells were microdissected from the choroid. PCR showed T-cell receptor (TCR)-γ gene rearrangement and the presence of the HTLV-1 gene pol in the microdissected lymphoma cells (Figure 1D). Interestingly, the HTLV-1 gene tax was undetectable. The results confirmed monoclonal expansion and the presence of HTLV-1 provirus. For the first time, we report prominent atypical lymphoid cell infiltration in the choroid with unremarkable conjunctiva, cornea and retina in an ATL patient. Substantial choroidal involvement can be regarded as a new ocular manifestation of ATL.
Adult T-cell leukemia/lymphoma
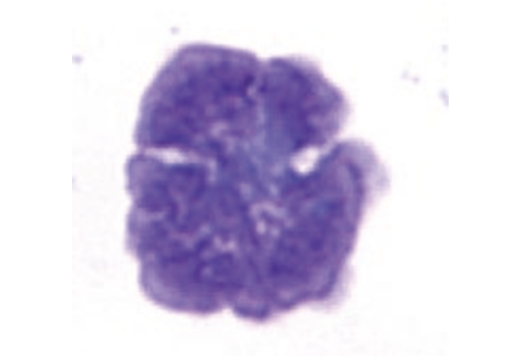
ATL is an adult onset T-cell malignancy that is diagnostically defined by morphologically atypical lymphoid cells with basophilic cytoplasm, multilobed nuclei, and T-cell markers; HTLV-1 antibodies in the serum; and molecular demonstration of monoclonal integration of HTLV-1 provirus in affected cells.10 The incidence of ATL is estimated to be 61/100,000 HTLV-1 carriers, and the crude lifetime risk for developing ATL is 7.29% for males and 3.78% for females.11 ATL is classified into 4 subtypes: acute, lymphoma, chronic, and smoldering.12 In all but the lymphoma subtype, ATL flower cells (Figure 2) are present in the peripheral blood. The canonical subtype is the acute, accounting for 55–75% of all ATL cases.2 Lymphadenopathy, hepatosplenomegaly, skin lesions, osteolytic bone lesions, and hypercalcemia are common.13 Systemic lymphadenopathy without peripheral blood involvement defines the lymphoma subtype. Patients with chronic ATL have lymphocytosis, ≥5% atypical T-lymphocytes, skin lesions, and no hypercalcemia or organomegaly. Smoldering ATL is defined by the presence of atypical T-lymphocytes without lymphocytosis or organ involvement, though patients may present with skin or pulmonary lesions.
Figure 2.
ATL flower cell in the peripheral blood. Characteristic ATL cells have been described as flower cells, with many nuclear convolutions and lobules. They are medium to large lymphoid cells with irregular nuclei and basophilic cytoplasm.
The acute and lymphoma subtypes are resistant to chemotherapy and are considered to be aggressive forms of ATL with poor prognosis. The chronic and smoldering subtypes are considered to be indolent, though they may progress to an aggressive form within several years.14 The median survival times for acute, lymphoma, chronic, and smoldering ATL are estimated to be 4–6 months, 9–10 months, 17–24 months, and ≥34 months, respectively.15 ATL primarily affects the skin, lungs, lymph nodes, liver, spleen, central nervous system, bones, and gastrointestinal tract.12 Affected patients are also immune compromised and susceptible to opportunistic infections. Although the involvement of other organs is rare, several cases of ocular involvement have been reported. The ocular manifestations and pathology of ATL are discussed below.
Ocular manifestations and pathology
The first case of orbital involvement was reported by Lauer et al in 1988.16 A 41-year-old Jamaican male developed proptosis and a large orbital tumor 3 years after being diagnosed with systemic ATL. Histological analysis showed large lymphoid cell infiltrates and necrosis. The ATL cells had large nuclei with prominent nucleoli, scanty cytoplasm, many polysomes, and a few swollen mitochondria. Bilateral orbital tumor, causing exophthalmos and eyelid edema, has also been reported as the first presenting manifestation of ATL in a 64-year-old Japanese male who did not have any other systemic symptoms.17 Biopsy revealed atypical lymphoid cells in the orbital adipose tissues that were positive for the T-cell markers CD3 and CD5. Nodular, mass, and erythematous eyelid lesions and eyelid lymphoma have also been reported.8,18–21
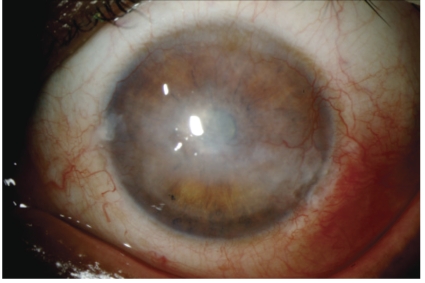
Corneal manifestations of ATL include peripheral ulcerative keratitis, immunoprotein keratopathy (hypergammaglobulinemia), central stromal opacities or deposits, and corneal haze, thinning, scarring, edema, and neovascularization (Figure 3).8,18 Bilateral conjunctival masses have also been reported in a 29-year-old Jamaican male.19 From a conjunctival biopsy, infiltration of CD3 and CD4 positive atypical lymphoid cells with scanty cytoplasm and multilobed nuclei was observed in the substantia propria. Yellowish conjunctival nodular lesions have manifested with aqueous tear-deficient keratoconjunctivitis sicca and conjunctival injection.18 Yellow-white lesions have also been observed along the limbal conjunctiva and into the cornea, and lesions near the corneal limbus have shown calcification.21 Biopsy of the conjunctival lesion revealed leukemic T-cell infiltration in the subepithelial layer.
Figure 3.
Corneal manifestations of ATL. Image shows keratitis with central stromal opacities and neovascularization.
Recurrent episcleritis and ocular hyperemia are signs that have been reported to precede the manifestation of systemic ATL.22 Lens manifestations include nuclear sclerosis18 and diffuse opacities.21 Several groups have reported vitreous opacities due to ATL cell infiltration.9,20,23–25 ATL patients also present with anterior uveitis. Iritis, cyclitis, aqueous cells and flare, keratic precipitates, and synechiae are common manifestations.20,23,24 In a 33-year-old Japanese female with a 10 year history of systemic ATL, anterior uveitis was reported to be the first ocular manifestation.8 Aqueous cells, fibrin membrane, hyphema, hemorrhages, anterior chamber opacity, and mass lesions on the iris were observed. Optic nerve manifestations of ATL include optic disc edema and optic nerve thickening, and direct infiltration of ATL cells in the optic nerve has been reported as well.9,24,26
Kohno et al. reported a case of retinal involvement in a 38-year-old Japanese male with ATL.9 In addition to cells in the anterior chamber and vitreous opacities, the retina was edematous, detached, atrophic, and necrotic with total disorganization of the retinal structure. Multiple white lesions could be seen in the peripheral retina, and the retinal vessels showed white sheathing indicative of vasculitis. Microscopic findings revealed atypical lymphoid infiltration around the retinal veins, near breaks in the inner limiting membrane, in the subretinal space, and between the retinal pigment epithelium (RPE) and Bruch's membrane. The malignant cells were positive for T-cell markers and had irregular pleomorphic nuclei, peripheral chromatin condensation, and prominent lysosomes. A case of retinal infiltration with atrophic lesions in the retinal vessels and the choriocapillaris has demonstrated that ATL cells can enter the retina via the choroidal circulation.23 Retinal sheathing, venous dilation and vasculitis, edema, folds, lesions, and exudates with cotton wool spots have also been seen.24,25,27 Additionally, the retina may be affected by opportunistic infections such as cytomegalovirus retinitis. Such a case has been reported, and histological analysis showed cytomegalic cell infiltration and widespread retinal necrosis and disorganization.20
A case of retinal and subretinal infiltration without vitreous involvement has also been reported in a 38-year-old male of Caribbean origin.28 Funduscopic examination revealed pale subretinal lesions and cotton-wool spots. Histological analysis of the chorioretinal biopsy specimen showed RPE atrophy and areas of RPE proliferation. Pleomorphic lymphocytes with scanty cytoplasm, hyperchromatic and convoluted nuclei, prominent nucleoli, little endoplasmic reticulum, and many lysosomes were observed in the subretinal space and the outer retina. ATL cell infiltration in all retinal layers, particularly in the neuroretina, has also been demonstrated in a 51-year old male with acute ATL.24 Rarely, ATL may manifest as a necrotizing granulomatous retinal vasculitis. This was reported in a 40-year-old Jamaican female who was referred to the National Eye Institute at the NIH.29 Fundus examination showed vitreous cells with haze, posterior vitreous detachment, infiltrates near the retinal vessels, vascular sheathing, deep retinal lesions, and macular edema. Retinal biopsy revealed necrotizing granulomatous retinal vasculitis and a few areas of retinal neovascularization. Molecular analysis revealed TCRγ gene rearrangement and the presence of the HTLV-1 gene pol in the infiltrating cells, allowing for a definitive diagnosis of ATL.
Conclusions
In addition to the current case, microdissection and PCR techniques have been successfully used to diagnose ATL involving ocular manifestations in previously reported cases.19,29 Analysis for multiple integrations of HTLV-1 proviral DNA using Southern blot may also be useful, as it has been shown that multiple integrations are correlated with more aggressive clinical manifestations and the involvement of nontraditional tissues, such as the retina and the uvea.24 Ocular manifestations of ATL are uncommon (Table 1) and have also been broadly regarded as markers of poor prognosis.19 Elevated levels of of soluble IL-2 receptor alpha (sIL-2α ) may suggest direct ocular infiltration of ATL cells, as T leukemic cells constitutively express and secrete soluble forms of this protein into the vitreous. Consequently, vitreous levels of sIL-2Rα may be an indicator of ocular infiltration and prognosis as well.25 Opportunistic infectious ocular lesions, such as cytomegalovirus retinitis, are also associated with poor prognosis. One study observed that 4 out of the 5 patients who developed such lesions died within several months.20 Early detection using both clinical and molecular markers to diagnose ATL and identify signs of poor prognosis may permit earlier interventions. Because ATL is so rare, international collaboration will be necessary to elucidate and characterize the various factors that influence ocular manifestations of this devastating tumor.
Table 1. Ocular manifestations of HTLV-1 infection.
| ATL | Non-ATL | |
|---|---|---|
| Orbit | Proptosis, exophthalmos, tumor, ATL cell infiltration, necrosis16,17 | |
| Eyelid | Nodular mass, erythematous lymphomatous lesions8,18–21 | |
| Cornea | Peripheral ulcerative keratitis, immunoprotein keratopathy (hypergammaglobulinemia), central stromal opacities/deposits, haze, thinning, scarring, edema, neovascularization18 | |
| Conjunctiva | Conjunctival mass, nodular lesion, aqueous tear-deficient keratoconjunctivitis sicca, conjunctival injection, calcified lesion18,19,21 | |
| Sclera | Recurrent episcleritis, ocular hyperemia22 | |
| Lens | Nuclear sclerosis, diffuse opacities18,21 | |
| Vitreous | Opacity due to ATL cell infiltration9,20,23–25 | HTLV-1-associated uveitis (HAU): Vitritis, snowball opacities HTLV-I-associated myelopathy/Tropical spastic paraparesis (HAM/TSP): vitreous opacity20,30 |
| Uvea | Anterior uveitis, iritis, cyclitis, aqueous cells, aqueous flare, keratic precipitates, synechiae, fibrin membrane, hyphemia, hemorrhages, mass lesions on iris, ATL cell infiltration of choroid8,20,23,24 | HAU: Iritis, cyclitis, aqueous cells, aqueous flare, posterior synechiae, keratic precipitates HAM/TSP: anterior uveitis20,30 |
| Optic nerve | Optic disc edema, optic nerve thickening, ATL cell infiltration9,25,26 | Cotton wool spots near optic disc20 |
| Retina | Edema, detachment, atrophy, necrosis, lesions, vascular sheathing, vasculitis, ATL cell infiltration, CMV retinitis, cotton-wool spots9,20,23–25,27,29 | HAU: cotton-wool spots, exudates, hemorrhages vasculitis HAM/TSP: cotton-wool spots20,30 |
| Subretina/RPE | Lesions, cotton-wool spots, RPE atrophy, RPE proliferation28 | |
| Systemic | Lymphadenopathy, hepatosplenomegaly, lesions (skin, lungs, bone), hypercalcemia, ATL cells in peripheral blood, opportunistic infections12–15 | HAM/TSP: upper motor neuron myelopathy, bladder dysfunction20 |
References
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuh M, Beilke M. The human T-cell leukemia virus type 1 (HTLV-1): new insights into the clinical aspects and molecular pathogenesis of adult T-cell leukemia/lymphoma (ATLL) and tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM) Microsc Res Tech. 2005;68:176–96. doi: 10.1002/jemt.20231. [DOI] [PubMed] [Google Scholar]
- 3.de Thé G, Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9:381–6. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- 4.Verdonck K, Gonzalez E, Van Dooren S. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–81. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves DU, Proietti FA, Ribas JG, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;;23:577–89. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–92. [PubMed] [Google Scholar]
- 7.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–85. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 8.Mori A, Deguchi HE, Mishima K, et al. A case of uveal, palpebral, and orbital invasions in adult T-Cell leukemia. Jpn J Ophthalmol. 2003;47:599–602. doi: 10.1016/j.jjo.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Kohno T, Uchida H, Inomata H, et al. Ocular manifestations of adult T-cell leukemia/lymphoma. A clinicopathologic study. Ophthalmology. 1993;100:1794–9. doi: 10.1016/s0161-6420(13)31398-0. [DOI] [PubMed] [Google Scholar]
- 10.Takatsuki K. Discovery of adult T-cell leukemia. Retrovirology. 2005;2:16–16. doi: 10.1186/1742-4690-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga Y, Iwanaga M, Soda M, et al. Trends in HTLV-1 prevalence and incidence of adult T-cell leukemia/lymphoma in Nagasaki, Japan. J Med Virol. 2010;82:668–74. doi: 10.1002/jmv.21738. [DOI] [PubMed] [Google Scholar]
- 12.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87) Br J Haematol. 1991;79:428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 13.Jaffe ES, Blattner WA, Blayney DW, et al. The pathologic spectrum of adult T-cell leukemia/lymphoma in the United States. Human T-cell leukemia/lymphoma virus-associated lymphoid malignancies. Am J Surg Pathol. 1984;8:263–75. doi: 10.1097/00000478-198404000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Yasunaga J, Matsuoka M. Human T-cell leukemia virus type I induces adult T-cell leukemia: from clinical aspects to molecular mechanisms. Cancer Control. 2007;14:133–40. doi: 10.1177/107327480701400206. [DOI] [PubMed] [Google Scholar]
- 15.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453–9. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer SA, Fischer J, Jones J, et al. Orbital T-cell lymphoma in human T-cell leukemia virus-I infection. Ophthalmology. 1988;95:110–5. doi: 10.1016/s0161-6420(88)33231-8. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa T, Ogata N, Takahashi K, et al. Bilateral orbital tumor as initial presenting sign in human T-cell leukemia virus-1 associated adult T-cell leukemia/lymphoma. Am J Ophthalmol. 2005;140:327–9. doi: 10.1016/j.ajo.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 18.Buggage RR, Levy-Clarke GA, Smith JA. New corneal findings in human T-cell lymphotrophic virus type 1 infection. Am J Ophthalmol. 2001;131:309–13. doi: 10.1016/s0002-9394(00)00881-3. [DOI] [PubMed] [Google Scholar]
- 19.Buggage RR, Smith JA, Shen D, Chan CC. Conjunctival T-cell lymphoma caused by human T-cell lymphotrophic virus infection. Am J Ophthalmol. 2001;131:381–3. doi: 10.1016/s0002-9394(00)00865-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohba N, Matsumoto M, Sameshima M, et al. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol. 1989;33:1–12. [PubMed] [Google Scholar]
- 21.Takahashi K, Sakum T, Onoe S, et al. Adult T-cell leukemia with leukemic cell infiltration in the conjunctiva. A case report. Doc Ophthalmol. 1993;83:255–60. doi: 10.1007/BF01204326. [DOI] [PubMed] [Google Scholar]
- 22.Goto K, Sugita M, Okada K, et al. Recurrent episcleritis associated with adult T cell leukaemia. Br J Ophthalmol. 1993;77:743–4. doi: 10.1136/bjo.77.11.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata A, Miyazaki T, Tanihara H. Intraocular infiltration of adult T-cell leukemia. Am J Ophthalmol. 2002;134:616–8. doi: 10.1016/s0002-9394(02)01651-3. [DOI] [PubMed] [Google Scholar]
- 24.Shibata K, Shimamoto Y, Nishimura T, et al. Ocular manifestations in adult T-cell leukemia/lymphoma. Ann Hematol. 1997;74:163–8. doi: 10.1007/s002770050276. [DOI] [PubMed] [Google Scholar]
- 25.Sugita S, Takase H, Yoshida T, et al. Intraocular soluble IL-2 receptor alpha in a patient with adult T cell leukaemia with intraocular invasion. Br J Ophthalmol. 2006;90:1204–6. doi: 10.1136/bjo.2006.092809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto N, Kiyosawa M, Kawasaki T, et al. Successfully treated optic nerve infiltration with adult T-cell lymphoma. J Neuroophthalmol. 1994;14:81–3. [PubMed] [Google Scholar]
- 27.Merle H, Donnio A, Gonin C, et al. Retinal vasculitis caused by adult T-cell leukemia/lymphoma. Jpn J Ophthalmol. 2005;49:41–5. doi: 10.1007/s10384-004-0144-1. [DOI] [PubMed] [Google Scholar]
- 28.Kumar SR, Gill PS, Wagner DG, et al. Human T-cell lymphotropic virus type I-associated retinal lymphoma. A clinicopathologic report. Arch Ophthalmol. 1994;112:954–9. doi: 10.1001/archopht.1994.01090190102028. [DOI] [PubMed] [Google Scholar]
- 29.Levy-Clarke GA, Buggage RR, Shen D, et al. Human T-cell lymphotropic virus type-1 associated t-cell leukemia/lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. 2002;109:1717–22. doi: 10.1016/s0161-6420(02)01132-6. [DOI] [PubMed] [Google Scholar]
- 30.Mochizuki M, Watanabe T, Yamaguchi K, et al. Uveitis associated with human T-cell lymphotropic virus type I. Am J Ophthalmol. 1992;114:123–9. doi: 10.1016/s0002-9394(14)73974-1. [DOI] [PubMed] [Google Scholar]