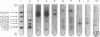Abstract
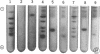
"Ectopic" proteins, not distinguished immunologically from the common alpha subunit of the human glycoprotein hormones, were purified approximately 10,000-fold from a gastric carcinoid tumor (A.L.-alpha) and from tissue culture medium of bronchogenic carcinoma cell lines (ChaGo-alpha). The purified A.L.-alpha was homogeneous by sodium dodecyl sulfate (SDS) gel electrophoresis while the purified ChaGo-alpha showed multiple components, some of which represented aggregated species. In SDS gel electrophoresis, the apparent molecular weights of A.L.-alpha (15,000) and dithioerythritol-reduced ChaGo-alpha (13,000) were significantly lower than those of the alpha subunits of human chorionic gonadotropin (hCG-alpha), luteinizing hormone, follicle-stimulating hormone, or thyroid-stimulating hormone (22,000-23,000). Binding experiments with [35S]-SDS suggested that these apparent differences in molecular weight resulted, at least in part, from diminished binding of the SDS by the normal compared to the ectopic alpha subunits. In gel chromatography, the apparent molecular weights of A.L.-alpha (27,000) and ChaGo-alpha (30,000) were slightly higher than those of normal alpha subunits (23,000-24,000). Both A.L.-alpha and ChaGo-alpha were not distinguished from hCG-alpha in ion-exchange chromatography. The composition of A.L.-alpha was similar to that of hCG-alpha in 13 amino acids but showed decreased phenylalanine and increased valine; glucosamine was identified in both A.L.-alpha and hCG-alpha. Under conditions in which hCG-alpha combined with the hCG beta subunit (hCG-beta) to produce 95% of the expected gonadotropin-binding activity in a rat testis radioreceptor-assay, A.L.-alpha incubation with hCG-beta resulted in only 2% of the expected activity, and ChaGo-alpha incubation with hCG-beta produced no detectable activity. These characteristics of ectopic alpha subunits may reflect abnormalities of neoplastic protein synthesis or carbohydrate attachment which result in polypeptides with chemical and immunologic similarity to normal subunits but with differences in physical and combining properties; alternatively, the ectopic subunits may represent as yet unrecognized alpha precursor forms.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES R. W., GARRISON M. M., HOWARD T. B. Extraction of thyrotrophin from pituitary glands, mouse pituitary tumors, and blood plasma by percolation. Endocrinology. 1959 Jul;65(1):7–17. doi: 10.1210/endo-65-1-7. [DOI] [PubMed] [Google Scholar]
- BOWER B. F., GORDAN G. S. HORMONAL EFFECTS OF NONENDOCRINE TUMORS. Annu Rev Med. 1965;16:83–118. doi: 10.1146/annurev.me.16.020165.000503. [DOI] [PubMed] [Google Scholar]
- Bellisario R., Carlsen R. B., Bahl O. P. Human chorionic gonadotropin. Linear amino acid sequence of the alpha subunit. J Biol Chem. 1973 Oct 10;248(19):6796–6809. [PubMed] [Google Scholar]
- Bishop W. H., Ryan R. J. Human luteinizing hormone and its subunits. Physical and chemical characterization. Biochemistry. 1973 Jul 31;12(16):3076–3084. doi: 10.1021/bi00740a021. [DOI] [PubMed] [Google Scholar]
- Carlsen R. B., Bahl O. P., Swaminathan N. Human chorionic gonadotropin. Linear amino acid sequence of the beta subunit. J Biol Chem. 1973 Oct 10;248(19):6810–6827. [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L., Tsuruhara T. Radioligand-receptor assay of luteinizing hormone and chorionic gonadotropin. J Clin Endocrinol Metab. 1972 Jan;34(1):123–132. doi: 10.1210/jcem-34-1-123. [DOI] [PubMed] [Google Scholar]
- Cheng K. W., Glazer A. N., Pierce J. G. The effects of modification of the COOH-terminal regions of bovine thyrotropin and its subunits. J Biol Chem. 1973 Nov 25;248(22):7930–7937. [PubMed] [Google Scholar]
- Closset J., Hennen G., Lequin R. M. Isolation and properties of human luteinizing hormone subunits. FEBS Lett. 1972 Apr 1;21(3):325–329. doi: 10.1016/0014-5793(72)80194-7. [DOI] [PubMed] [Google Scholar]
- Cornell J. S., Pierce J. G. The subunits of human pituitary thyroid-stimulating hormone. Isolation, properties, and composition. J Biol Chem. 1973 Jun 25;248(12):4327–4333. [PubMed] [Google Scholar]
- Franchimont P., Gaspard U., Reuter A., Heynen G. Polymorphism of protein and polypeptide hormones. Clin Endocrinol (Oxf) 1972 Oct;1(4):315–336. doi: 10.1111/j.1365-2265.1972.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham K. C., Aloj S. M., Edelhoch H. Rates of dissociation and recombination of the subunits of bovine thyrotropin. Arch Biochem Biophys. 1974 Aug;163(2):589–599. doi: 10.1016/0003-9861(74)90518-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J. Rapid and effective removal of sodium dodecyl sulfate from proteins. Biochem Biophys Res Commun. 1971 Nov 5;45(3):662–668. doi: 10.1016/0006-291x(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Liddle G. W., Nicholson W. E., Island D. P., Orth D. N., Abe K., Lowder S. C. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283–314. doi: 10.1016/b978-0-12-571125-8.50009-0. [DOI] [PubMed] [Google Scholar]
- Lipsett M. B. Humoral syndromes associated with cancer. Cancer Res. 1965 Aug;25(7):1068–1073. [PubMed] [Google Scholar]
- Liu W. K., Yang K. P., Nakagawa Y., Ward D. N. The role of the amino group in subunit association and receptor site interaction for ovine luteinizing hormone as studied by acylation. J Biol Chem. 1974 Sep 10;249(17):5544–5550. [PubMed] [Google Scholar]
- Morgan F. J., Canfield R. E. Nature of the subunits of human chorionic gonadotropin. Endocrinology. 1971 Apr;88(4):1045–1053. doi: 10.1210/endo-88-4-1045. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Canfield R. E., Vaitukaitis J. L., Ross G. T. Properties of the subunits of human chorionic gonadotropin. Endocrinology. 1974 Jun;94(6):1601–1606. doi: 10.1210/endo-94-6-1601. [DOI] [PubMed] [Google Scholar]
- NYMAN P., LINDSKOG S. AMINO ACID COMPOSITION OF VARIOUS FORMS OF BOVINE AND HUMAN ERYTHROCYTE CARBONIC ANHYDRASE. Biochim Biophys Acta. 1964 Apr 6;85:141–151. doi: 10.1016/0926-6569(64)90174-9. [DOI] [PubMed] [Google Scholar]
- Pierce J. G. Eli Lilly lecture. The subunits of pituitary thyrotropin--their relationship to other glycoprotein hormones. Endocrinology. 1971 Dec;89(6):1331–1344. doi: 10.1210/endo-89-6-1331. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson A. S., Rosen S. W., Tashijan A. H., Jr, Weintraub B. D. Production of human chorionic gonadotropin in vitro by a cell line derived from a carcinoma of the lung. J Natl Cancer Inst. 1973 Mar;50(3):669–674. doi: 10.1093/jnci/50.3.669. [DOI] [PubMed] [Google Scholar]
- Rathnam P., Saxena B. B. Subunits of luteinizing hormone from human pituitary glands. J Biol Chem. 1971 Dec 10;246(23):7087–7094. [PubMed] [Google Scholar]
- Reichert L. E., Jr, Lawson G. M., Jr, Leidenberger F. L. Influence of alpha- and beta-subunits on the kinetics of formation and activity of native and hybrid molecules of LH and human chorionic gonadotropin. Endocrinology. 1973 Oct;93(4):938–946. doi: 10.1210/endo-93-4-938. [DOI] [PubMed] [Google Scholar]
- Reichert L. E., Jr, Lawson G. M., Jr Molecular weight relationships among the subunits of human glycoprotein hormones. Endocrinology. 1973 Apr;92(4):1034–1042. doi: 10.1210/endo-92-4-1034. [DOI] [PubMed] [Google Scholar]
- Reichert L. E., Jr, Ward D. N. On the isolation and characterization of the alpha and beta subunits of human pituitary follicle-stimulating hormone. Endocrinology. 1974 Mar;94(3):655–664. doi: 10.1210/endo-94-3-655. [DOI] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Rosen S. W., Weintraub B. D. Ectopic production of the isolated alpha subunit of the glycoprotein hormones. A quantitative marker in certain cases of cancer. N Engl J Med. 1974 Jun 27;290(26):1441–1447. doi: 10.1056/NEJM197406272902601. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Li C. H. Human pituitary thyrotropin: isolation and chemical characterization of its subunits. Biochem Biophys Res Commun. 1973 Mar 17;51(2):336–342. doi: 10.1016/0006-291x(73)91262-x. [DOI] [PubMed] [Google Scholar]
- Sairam M. R., Papkoff H., Li C. H. Human pituitary interstitial cell stimulating hormone: primary structure of the subunit. Biochem Biophys Res Commun. 1972 Aug 7;48(3):530–537. doi: 10.1016/0006-291x(72)90380-4. [DOI] [PubMed] [Google Scholar]
- Saxena B. B., Rathnam P. Dissociation phenomenon and subunit nature of follicle-stimulating hormone from human pituitary glands. J Biol Chem. 1971 Jun 10;246(11):3549–3554. [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. 3. Blocks in defective synthesis. J Mol Biol. 1968 Dec;38(3):273–288. doi: 10.1016/0022-2836(68)90386-0. [DOI] [PubMed] [Google Scholar]
- Stockell Hartree A., Thomas M., Braikevitch M., Bell E. T., Christie D. W., Spaull G. V., Taylor R., Pierce J. G. Preparation and properties of subunits of human luteinizing hormone. J Endocrinol. 1971 Sep;51(1):169–180. doi: 10.1677/joe.0.0510169. [DOI] [PubMed] [Google Scholar]
- Swaminathan N., Bahl O. P. Dissociation and recombination of the subunits of human chorionic gonadotropin. Biochem Biophys Res Commun. 1970 Jul 27;40(2):422–427. doi: 10.1016/0006-291x(70)91026-0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Weintraub B. D., Barowsky N. J., Rabson A. S., Rosen S. W. Subunits of human chorionic gonadotropin: unbalanced synthesis and secretion by clonal cell strains derived from a bronchogenic carcinoma. Proc Natl Acad Sci U S A. 1973 May;70(5):1419–1422. doi: 10.1073/pnas.70.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Changing placental concentrations of human chorionic gonadotropin and its subunits during gestation. J Clin Endocrinol Metab. 1974 May;38(5):755–760. doi: 10.1210/jcem-38-5-755. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Rosen S. W. Competitive radioassays and "specific" tumor markers. Metabolism. 1973 Aug;22(8):1119–1127. doi: 10.1016/0026-0495(73)90231-x. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Rosen S. W. Ectopic production of the isolated beta subunit of human chorionic gonadotropin. J Clin Invest. 1973 Dec;52(12):3135–3142. doi: 10.1172/JCI107513. [DOI] [PMC free article] [PubMed] [Google Scholar]