Abstract
A library of diarylurea insulin-like growth factor 1 receptor inhibitors was screened for activity against chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of Plasmodium falciparum. The 4-aminoquinaldine-derived diarylureas displayed promising antimalarial potency. Further exploration of the B ring of 4-aminoquinaldinyl ureas allowed identification of several quinaldin-4-yl ureas 4{13, 39} and 4{13, 58} sufficiently potent against both 3D7 and K1 strains to qualify as bone fide leads.
Keywords: Malaria, diarylurea
Malaria affects roughly 250 million people and causes over 800000 deaths annually, with most deaths being attributed to infection by Plasmodium falciparum.1 Drug resistance has been reported against almost all available antimalarial drugs.2−4 Therefore, it remains essential to develop and discover new effective and affordable antimalarial drugs.
Diarylureas derivatives have been reported to have antimalarial activity.5−10 Jiang and co-workers identified a series of diphenylureas in a plasmepsin-directed screening campaign.5 Although the authors proposed the antimalarial activity of diphenylureas as inhibition of plasmepsin, the mechanism of action in P. falciparum remains unclear due to poor correlation between in vitro and in vivo structure−activity relationships (SARs).11−13 Baldwin and co-workers disclosed diarylureas as inhibitors of dihydroorotate dehydrogenase,7 an enzyme in the pyrimidine biosynthetic pathway, although no correlations between enzymatic and cellular SAR were presented.14 Domínguez and co-workers reported that phenylurenyl chalcones had activity against chloroquine-resistant strains of P. falciparum. The phenylurenyl chalcones exerted their antimalarial activity through multiple mechanisms.8 While there is clearly some potential for diarylureas, the mechanism or mechanisms of action remain unclear, and most of these studies revealed either poor correlation between the enzyme target and the cellular potencies or relatively poor potencies or both.
We had previously examined diarylureas as inhibitors of the IGF-1R kinase for the treatment of cancer, an area that has also been explored by industrial groups.15 The work mentioned above led us to screen our existing collection of diarylureas and, with the preliminary data developed from that study, to carry out more extensive structure activity and pharmacophore determination studies to map the elements that are responsible for antimalarial activity. Here, we report the results of these studies against chloroquine-sensitive (3D7) and chloroquine-resistant (K1) P. falciparum strains.
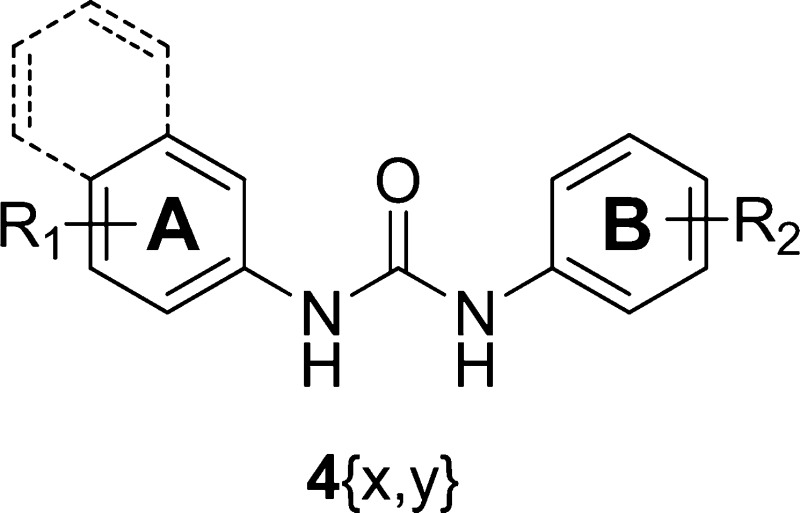
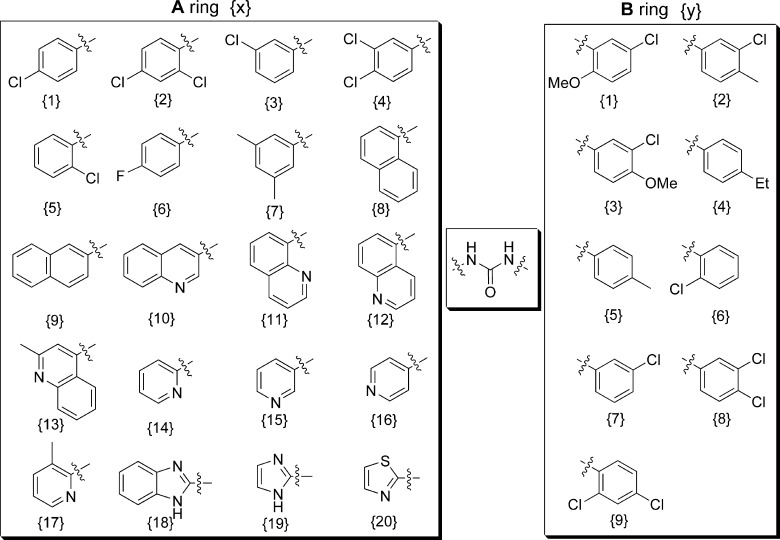
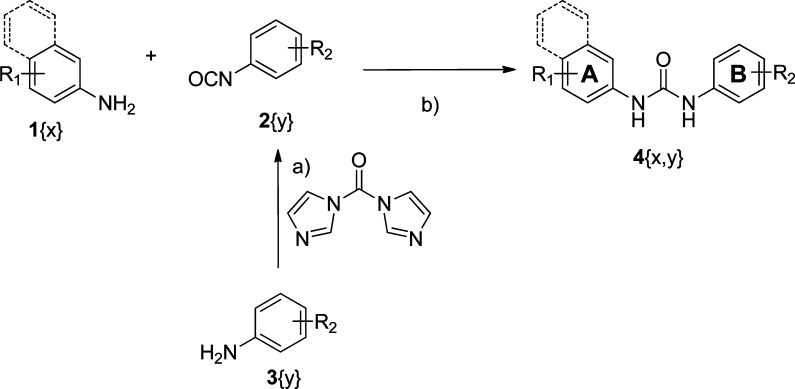
A straightforward synthesis of diarylureas has been previously reported.15 The diarylurea library was generated in parallel by coupling of aryl amines 1{x} (A ring) with aryl isocyanates 2{y} (B ring), as shown in Figure 1. Phenyl isocyanates 2{y} were either commercially available or freshly prepared by treating commercial available aryl amines 3{y} with 1,1′-carbonyldiimidazole (Scheme 1). After all compounds were purified by preparative reverse phase high-pressure liquid chromatography (RP-HPLC), the identification and purity of each compound were estimated by liquid chromatography coupled to mass spectrometry (LC-MS) (Table 1 in the Supporting Information). Novel compounds, not previously reported in the literature, were characterized by both nuclear magnetic resonance spectroscopy (NMR) and LC-MS. This facile synthesis allowed us to produce a library of diarylureas 4{x, y} with over 90% purity.
Figure 1.
Library composition of diarylurea compound chemset 4{x, y}.
Scheme 1. General Synthetic Routes for Diarylureas.
Conditions: (a) DMSO, room temperature, 2 h. (b) DMSO, room temperature, 5−10 min.
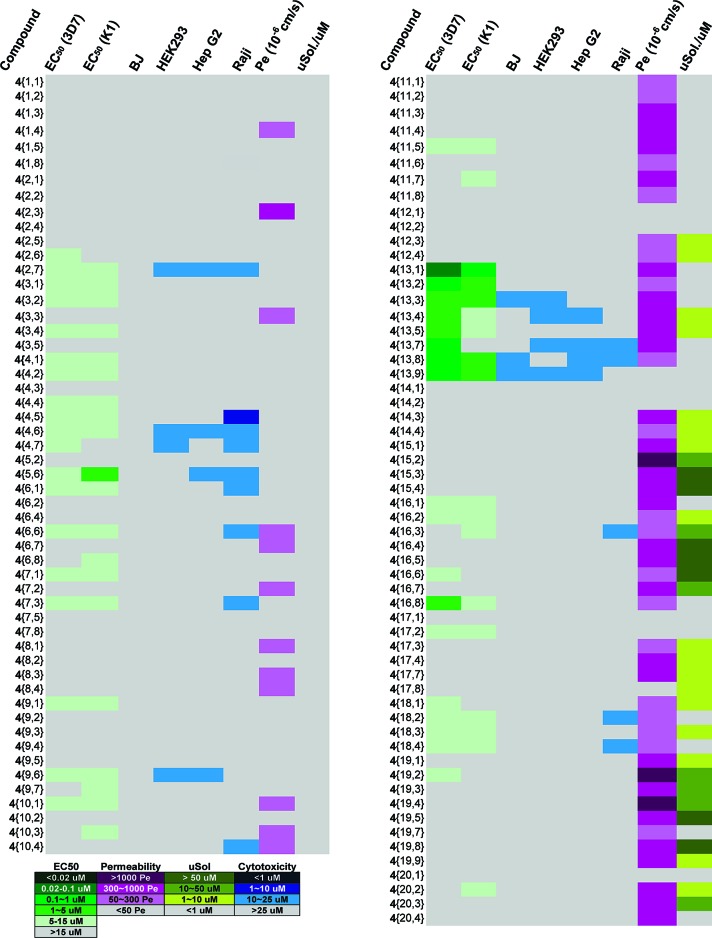
The originally reported IGF-1R targeted library of diarylureas was tested for the antimalarial activity against chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains in replicate dose−response experiments to generate 50% inhibitory concentrations (EC50). Simultaneously, the cytotoxicity of all diarylureas was investigated against four human cell lines [human fibroblast cells (BJ), human embryonic kidney 293 cells (HEK293), human hepatocellular liver carcinoma cells (HEP G2), and human Burkitt's lymphoma cells (Raji)]. In addition, the passive permeability and solubility were examined to provide a survey of structure−property relationships for the series. The results are summarized in a heat map (Figure 2).
Figure 2.
Antimalarial activity, cytotoxicity, permeability, and solubility of diarylurea chemset 4{1−20, 1−9}. (1) Data represent median EC50 values of parasite growth inhibition against 3D7 and K1 strains of P. falciparum, from replicate experiments, shown in a color gradient scale with darker squares indicating a higher antimalarial potency. The positive control for the antimalarial assay was chloroquine (EC50 = 0.047 ± 0.004 μM against 3D7, and EC50 = 1.23 ± 0.07 μM against K1). (2) Cytotoxicity data represent median EC50 values of growth inhibition against BJ, HEK-293, Hep G2, and Raji cell lines, from replicate experiments, and are shown in a color gradient with darker squares indicating a more potent mammalian cytotoxicity. (3) The parallel artificial membrane permeability assay (PAMPA) passive permeability assay results at pH 7.4 are shown in a color gradient with darker purple squares indicating a higher permeability. (4) Aqueous solubility at pH 7.4 in isotonic buffer is shown in olive scale with darker squares indicating a higher solubility.
One class of diarylureas, the 4-aminoquinaldine-derived diarylureas 4{13, 1−9}, showed fairly strong antimalarial potency. All other classes showed weak antimalarial potency at best, although a number of compounds had EC50 values in the range of 5−15 μM—similar to many of the diarylureas reported in other studies.
Diarylureas including the subclasses of diphenyl ureas 4 {1−7, 1−8} or naphthyl phenyl ureas 4 {8−9, 1−7} exhibited weak antimalarial activity. Eleven individual compounds showed modest activities with EC50 values of 5−15 μM against both stains. With the exception of compound 4{4, 5}, these classes were nontoxic against all four mammalian cell lines or weakly toxic with LD50 values between 10 and 25 μM. All compounds in these classes were generally poorly soluble in aqueous buffer at pH 7.4, and some of compounds were reasonably permeable.
The quinoline and the pyridine-containing diarylurea series 4{10−17, 1−9} had more compounds with modest activity and substituents on the B ring influenced the antimalarial activity. For example, compounds 4{13, 1−2} and 4{13, 7−9}, carrying chloro substituents on the B ring, were more potent than 4{13, 4−5}, which lacked chloro groups. The most potent compound 4{13, 1} (EC50 = 0.053 μM vs 3D7) gave an equivalent potency to chloroquine (EC50 = 0.047 ± 0.004 μM vs 3D7) under the same assay conditions in vitro. Although the 4-aminopyridine-derived diarylureas 4{16, 1−8} possessed a pyridine ring structurally equivalent to that in the 4-aminoquinaldine series 4{13, 1−9}, only one compound 4{16, 8} among eight 4-aminopyridine-derived compounds demonstrated an EC50 value lower than 5 μM against 3D7 strain. Thus, the activity requires both the hydrogen bonding and the hydrophobic functions. In general, the 4-aminoquialdine-derived diarylureas showed only weak cytotoxicity. Most compounds 4{10−17, 1−9} had reasonable permeability (>50 × 10−6 cm/s), and the pyridine-derived compounds 4{14−17, 1−8} possessed better aqueous solubility than the quinoline-derived diarylureas 4{10−13, 1−9}.
The set of diarylureas 4{18−20, 1−9} containing two heteroatoms on the A ring was only weakly active. Only the 2-aminobenzimidazole-derived compounds 4{18, 1−4} (EC50 = 5−15 μM) gave measurable potencies. All compounds, except 4{18, 2} and 4{18, 4}, were nontoxic on four mammalian cell lines. Although this set of diarylureas did not show promising antimalarial activity, the presence of benzimidazole, imidazole, and thiazole moieties enhanced both permeability and aqueous solubility of the compounds.
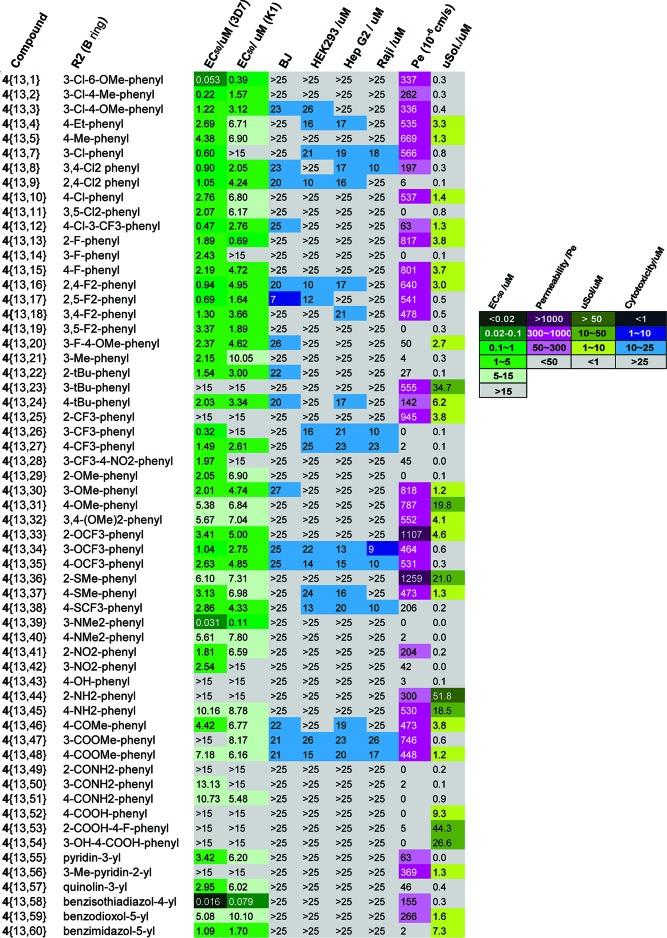
The most promising compounds from the original test contained the 4-aminoquinaldine system. Therefore, this ring was fixed as the A ring and variation of the B ring explored to examine the possibility of improving both the antimalarial potency and the physicochemical properties. All compounds in this series possessed good selectivity against malaria parasites and mammalian cells. The antimalarial potency, cytotoxicity, and physicochemical properties are summarized in Figure 3. Of the substituted phenyl rings surveyed in this series, the chloro or fluoro substitutents (4{13, 1−3} and 4{13, 7−20}) gave the best antimalarial potencies with select compounds, giving EC50 values in the mid or high nanomolar range against the 3D7 strain, equivalent to chloroquine. Some SARs were evident. For example, compound 4{13, 7}, with a 3-Cl substituent on the B ring, gave an EC50 of 0.60 μM, 5-fold more potent than compound 4{13, 10} with a 4-Cl substituent. Compounds 4{13, 8−9} and 4{13, 11}, bearing two chloro substituents on the B ring, displayed weaker antimalarial potency as compared to 4{13, 7}. Introduction of a 2-OMe group to 4{13, 7} afforded 4{13, 1} with an improved EC50 value of 0.053 μM against 3D7. However, moving the methoxy group of 4{13, 1} from the 2- to the 4-position (4{13, 3}) resulted in a 23-fold decrease in potency against 3D7. Compound 4{13, 2} incorporating the 3-Cl and 4-Me groups exhibited an EC50 value of 0.22 μM, which was 3-fold more potent than 4{13, 7}. Notably, compound 4{13, 12} containing the 4-Cl and 3-CF3 substituents displayed an antimalarial potency of 0.47 μM. All compounds 4{13, 13−20}, containing the fluoro substitutents on the B ring, maintained similar levels of potency, in the range of 0.7−3.37 μM. Compounds 4{13, 26} and 4{13, 39}, bearing 3-CF3 and 3-NMe2, respectively, showed EC50 values of 0.32 and 0.031 μM against 3D7, illustrating the preference for electron-donating substitutents. Compounds 4{13, 43−54}, containing hydroxyl, amino, or carbonyl-derived functional groups, showed weak growth inhibitory activities against malaria (5−15 μM) or displayed no activity at the highest assay concentration (15 μM). Next in the study, we explored a series of heteroaromatic rings 4{13, 55−60} as replacements for the phenyl ring. The most promising compound 4{13, 58} carried the benzisothiadiazolyl ring and displayed an EC50 value of 0.016 μM against 3D7, 3-fold more potent than chloroquine. Meanwhile, compound 4{13, 58} was also active against chloroquine-resistant K1 strain (EC50 = 0.079 μM). In general, the entire 4-aminoquinaldine-derived series 4{13, 1−60} showed slightly more potency against chloroquine-sensitive strain 3D7 than chloroquine-resistant strain K1. With respect to structure−property relationships, there was a general correlation between potent antimalarial activity and decreasing aqueous solubility with the compounds with EC50 values below 5 μM showing poor aqueous solubility (<10 μM in 5% DMSO in aqueous buffer). However, the highly active compounds had an aqueous solubility roughly 100−1000-fold higher than their EC50 values, indicating that this is a liability that needs careful management but is not an inherent block to further development. Although we observed a wide range of permeability values, most compounds had reasonable permeability.
Figure 3.
Antimalarial activity, cytotoxicity, permeability, and solubility of the 4-aminoquinaldine-derived diarylurea compounds 4{13, 1−60}. (1) Data represent median EC50 values of parasite growth inhibition against 3D7 and K1 strains of P. falciparum, from replicate experiments, shown in a color gradient scale with darker squares indicating a higher antimalarial potency. The positive control for the antimalarial assay was chloroquine (EC50 = 0.047 ± 0.004 μM against 3D7, and EC50 = 1.23 ± 0.07 μM against K1). (2) Cytotoxicity data represent median EC50 values of growth inhibition against BJ, HEK 293, Hep G2, and Raji cell lines, from replicate experiments, and are shown in a color gradient with darker squares indicating a more potent mammalian cytotoxicity. (3) PAMPA passive permeability assay results at pH 7.4 are shown in a color gradient with darker purple squares indicating a higher permeability. (4) Aqueous solubility at pH 7.4 in isotonic buffer is shown in olive scale with darker squares indicating a higher solubility.
A preliminary analysis of pharmacophores in the series is illustrated with the three most active compounds 4{13, 1}, 4{13, 39}, and 4{13, 58}. The conformers of each compound were generated by systematic conformation search using MOE software with lowest energy conformations being used to generate pharmacophore features (Figure 1 in the Supporting Information). One clear pharmacophore element is the aromatic moiety on the A ring (F2: Aro) and the aromatic nitrogen (F1: Acc) as they relate to the oxygen (F3: Acc), a distance of 4.86 Å from the centroid of the ring. Another important pharmacophore feature of 4{13, 1} and 4{13, 39} appears to be a hydrophobic site (F5: Hyd, in Figure 1 in the Supporting Information), on the B-ring, which overlaid with 3-chloro group and 3-dimethylamino group, respectively. In the case of the fused heteroaromatic ring, it may be the case that a slightly less favorable conformation also fulfills this hydrophobe by rotating the ring inward. These pharmacophore elements can serve as a helpful tool in further designing new diarylureas.
While several antimalarial targets have been proposed for the diarylureas, including plasmepsin II,5 dihydroorate dehydrogenase,7 plasmodial cysteine proteases, and hemoglobin hydrolysis, the mechanism of action of diarylureas in parasites has not been understood clearly.8 To date, we have been unable to confirm any of these targets as the one acted upon by our series of compounds. We have previously investigated the use of diarylurea compounds as inhibitors of the human receptor tyrosine kinase IGF-1R, which is important in all stages of the development of breast and other cancers. The inhibitory potencies of diarylureas against P. falciparum can be compared with potency against the human IGF-1R. Interestingly, while the 4-aminoquinaldine-derived diarylureas were active against IGF-1R, overall, the compounds were significantly less potent (IC50 = 9.7−36.7 μM).15 According to these results, we tentatively suggest that the 4-aminoquinaldine-derived diarylureas may also inhibit kinases present in Plasmodium that contain similar catalytic domains. However, despite attempting to do so, we have been unable to validate any individual malarial kinase as the target of these compounds. Because the 4-animoquinaldine-derived diarylureas likely target kinases of P. falciparum, it is important to know whether those compounds inhibit any kinases of the human host besides IGF-1R. One compound 4{13, 34}, which had some—albeit weak—measurable mammalian cytotoxicity was submitted for comprehensive screening against 402 human kinases by Ambit Biosciences (Table 4 in the Supporting Information). Compound 4{13, 34} bound to only one kinase, DDR1, out of 402 screened human kinases at concentration 10 μM (Figure 2 in the Supporting Information.). Protein kinases of parasite Plasmodium are divergent from their human host.16−18 In the case that the diarylureas target kinases of Plasmodium, it is highly possible that the diarylureas selectively inhibit kinases of Plasmodium over the human host since the most active antimalarial diarylureas essentially failed to bind to mammalian kinases. Further studies will be carried out to validate the targets of diarylureas and understand their action in parasites. Additionally, further structural optimization of diarylureas will be required to overcome concerns for physicochemical properties and cross-resistance.
Herein is reported the evaluation of a library of diarylureas for antimalarial activity against two strains of P. falciparum (3D7 and K1). The most promising subseries of diarylureas contained a 4-aminoquinaldinyl moiety (A ring) as one of the aryl rings of the urea, a novel structural feature in the class. The further investigation on the B ring of this series revealed compounds in this series that were more active against the chloroquine-sensitive strain 3D7 than the chloroqine-resistant strain K1 and with absolute potencies in 3D7 equivalent to chloroquine. The major liability observed in this class of compounds is that they tend to be quite insoluble. To enable clinical development, this liability will need to be addressed in future studies.
Acknowledgments
We are grateful to Taosheng Chen and Jimmy Cui from High Throughput Screening Core, Chemical Biology & Therapeutics Department, St. Jude Children's Research Hospital, for their support in antimalarial and cytotoxicity screening. We are also grateful to High Throughput Synthesis Core and High Throughput Analytical Core, Chemical Biology & Therapeutics Department, St. Jude Children's Research Hospital, for their support in the synthesis and purification of diarylureas.
Supporting Information Available
Synthetic procedures for diarylureas; complete LC-MS characterization data of all diarylureas; complete antimalarial data, cytotoxicity data, permeability, and solubility data for all listed compounds; pharmacophore feature profile; kinase binding profile data; 1H NMR spectra and chromatographic data of novel compounds 4{12, 1−4}, 4{13, 7}, 4{13, 12}, 4{13, 21−24}, 4{13, 27−28}, 4{13, 41−45}, 4{13, 49−50}, 4{13, 60}, 4{19, 1−4}, and 4{19, 8−9}. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the National Institute of Allergy and Infectious Disease at NIH (AI075517), the American Lebanese Syrian Associated Charities (ALSAC), and St. Jude Children's Research Hospital.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- WHO Malarial Report 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Hyde J. E. Drug-resistant malaria. Trends Parasitol. 2005, 2111494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp A. M.; Nosten F.; Yi P.; Das D.; Phyo A. P.; Tarning J.; Lwin K. M.; Ariey F.; Hanpithakpong W.; Lee S. J.; Ringwald P.; Silamut K.; Imwong M.; Chotivanich K.; Lim P.; Herdman T.; An S. S.; Yeung S.; Singhasivanon P.; Day N. P. J.; D.M. N. L.; Socheat D.; White N. J. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessl J. J.; Meshnick S. R.; Trumpower B. L. Modeling the molecular basis of atovaquone resistance in parasites and pathogenic fungi. Trends Parasitol. 2007, 2310494–501. [DOI] [PubMed] [Google Scholar]
- Jiang S.; Prigge S. T.; Wei L.; Gao Y.; Hudson T. H.; Gerena L.; Dame J. B.; Kyle D. E. New Class of Small Nonpeptidyl Compounds Blocks Plasmodium falciparum Development In Vitro by Inhibiting Plasmepsins. Antimicrob. Agents Chemother. 2001, 4592577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leban J.; Pegoraro S.; Dormeyer M.; Lanzer M.; Aschenbrenner A.; Kramer B. Sulfonyl-phenyl-ureido benzamidines: A novel structural class of potent antimalarial agents. Bioorg. Med. Chem. Lett. 2004, 14, 1979–1982. [DOI] [PubMed] [Google Scholar]
- Baldwin J.; Michnoff C. H.; Malmquist N. A.; White J.; Roth M. G.; Rathod P. K.; Phillips M. A. High-throughput Screening for Potent and Selective Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase. J. Biol. Chem. 2005, 280, 21847–21853. [DOI] [PubMed] [Google Scholar]
- Domínguez J. N.; León C.; Rodrigues J.; Domínguez N. G. d.; Gut J.; Rosenthal P. J. Synthesis and Evaluation of New Antimalarial Phenylurenyl Chalcone Derivatives. J. Med. Chem. 2005, 48, 3654–3658. [DOI] [PubMed] [Google Scholar]
- Madapa S.; Tusi Z.; Sridhar D.; Kumar A.; Siddiqi M. I.; Srivastava K.; Rizvi A.; Tripathi R.; Puri S. K.; Keshava G. B. S.; Shukla P. K.; Batra S. Search for new pharmacophores for antimalarial activity. Part I: Synthesis and antimalarial activity of new 2-methyl-6-ureido-4-quinolinamides. Bioorg. Med. Chem. 2009, 17, 203–221. [DOI] [PubMed] [Google Scholar]
- Madapa S.; Tusi Z.; Mishra A.; Srivastava K.; Pandey S. K.; Tripathi R.; Puri S. K.; Batra S. Search for new pharmacophores for antimalarial activity. Part II: Synthesis and antimalarial activity of new 6-ureido-4-anilinoquinazolines. Bioorg. Med. Chem. 2009, 17, 222–234. [DOI] [PubMed] [Google Scholar]
- Francis S. E.; Sullivan D. J. Jr.; Goldberg D. E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997, 51, 97–123. [DOI] [PubMed] [Google Scholar]
- Coombs G. H.; Goldberg D. E.; Klemba M.; Berry C.; Kay J.; Mottram J. C. Aspartic proteases of Plasmodium falciparum and other parasitic protozoa as drug targets. Trends Parasitol. 2001, 17, 532–537. [DOI] [PubMed] [Google Scholar]
- Francis S. E.; Banerjee R.; Goldberg D. E. Biosynthesis and Maturation of the Malaria Aspartic Hemoglobinases Plasmepsins I and II. J. Biol. Chem. 1997, 272, 14961–14968. [DOI] [PubMed] [Google Scholar]
- Seymour K. K.; Lyons S. D.; Phillips L.; Rieckmann K. H.; Christopherson R. I. Cytotoxic Effects of Inhibitors of de Novo Pyrimidine Biosynthesis upon Plasmodium falciparum. Biochemistry 1997, 33, 5268–5274. [DOI] [PubMed] [Google Scholar]
- Anderson M. O.; Yu H.; Penaranda C.; Maddux B. A.; Goldfine I. D.; Youngren J. F.; Guy R. K. Parallel synthesis of diarylureas and their evaluation as inhibitors of insulin-like growth factor receptor. J. Comb. Chem. 2006, 8, 784–790. [DOI] [PubMed] [Google Scholar]
- Ward P.; Equinet L.; Packer J.; Doerig C. Protein kinases of the human malaria paratiste Plasmodium falciparum: The kinome of a divergent eukaryote. BMC Genomics 2004, 5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anamika N. S.; Krupa A. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins 2005, 581180–189. [DOI] [PubMed] [Google Scholar]
- Doerig C.; Meijer L. Antimalarial drug discovery: targeting protein kinases. Expert Opin. Ther. Targets 2007, 113279–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.