Abstract
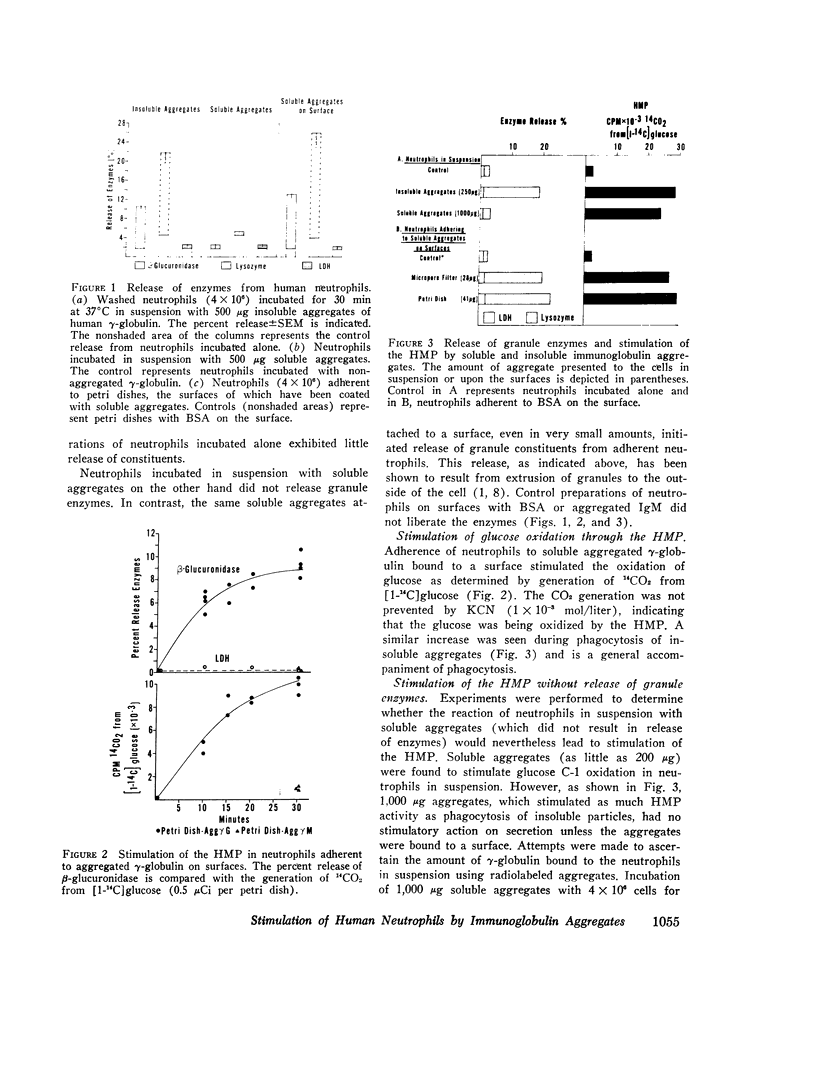
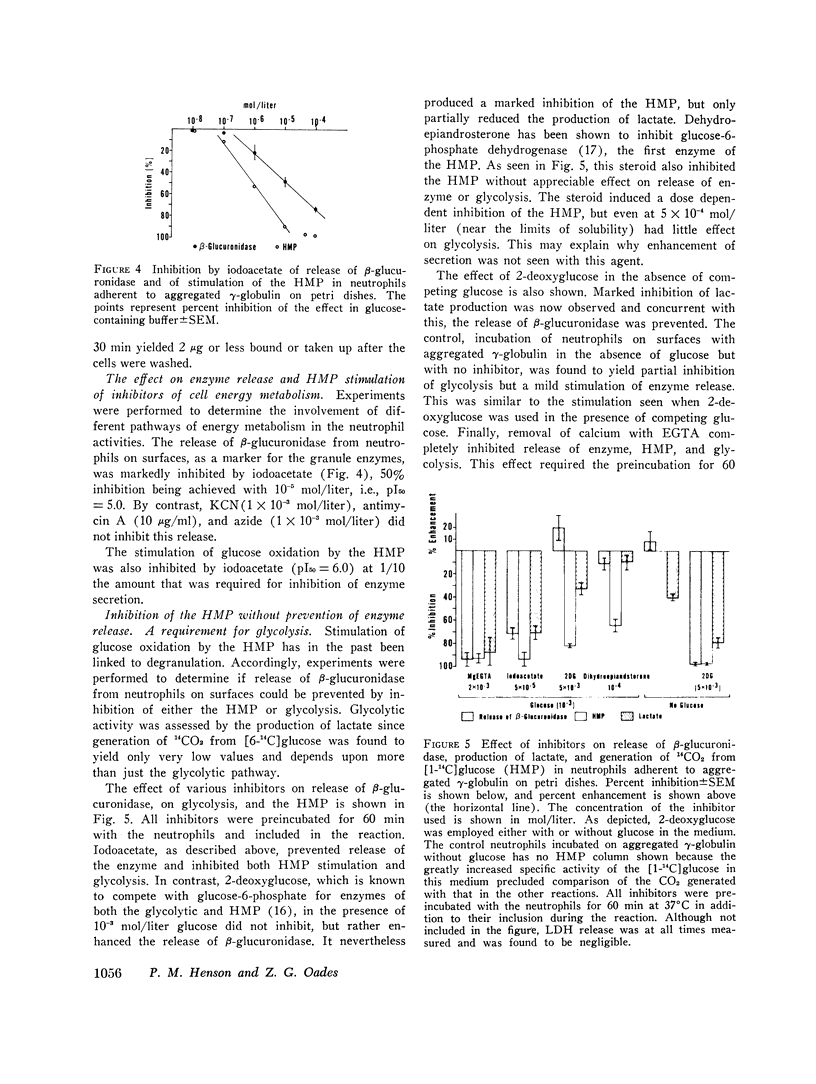
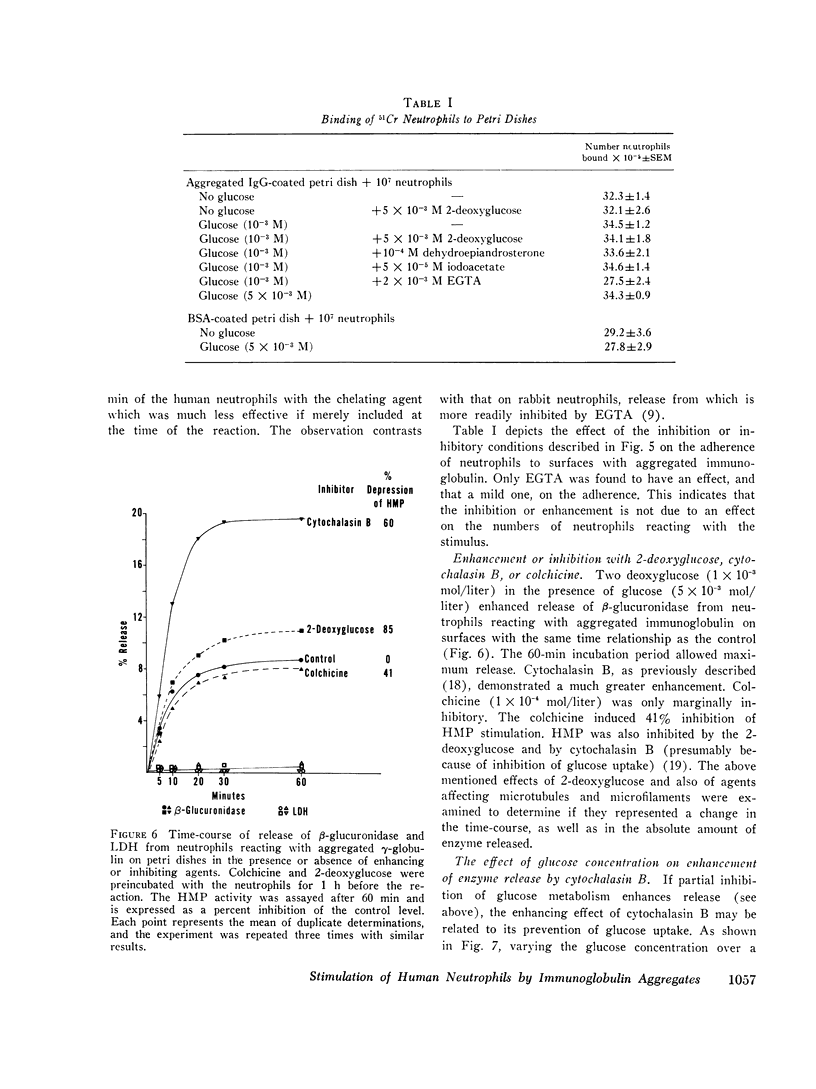
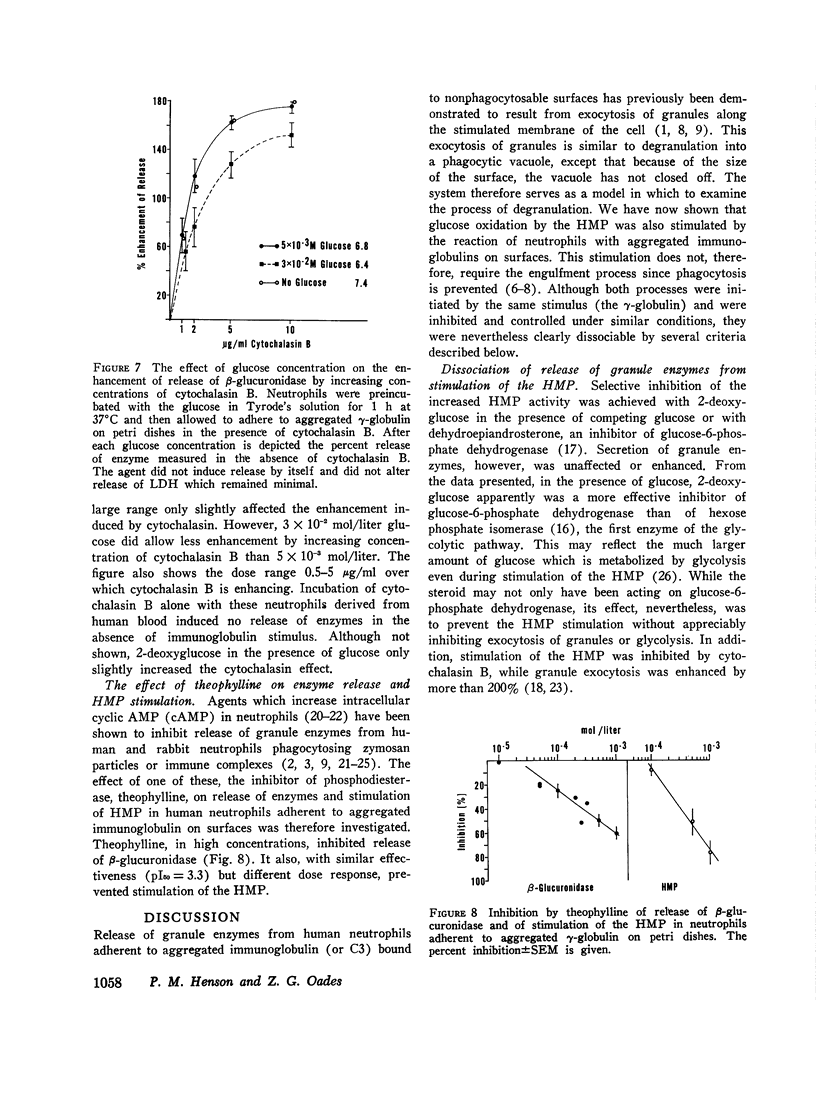
Reaction of human neutrophils with aggregated immunoglobulin on nonphagocytosable surfaces results in secretion of granule enzymes (exocytosis of granules) and stimulation of glucose oxidation by the nexose monophosphate pathway (HMP). The role of HMP stimulation in the enzyme secretion and some requirements for the two neutrophil activities have been examined. It was found that (a) HMP stimulation could be selectively inhibited under conditions where release of granule enzymes remained unchanged or was enhanced, for example, by reduced glucose concentration or by 2-deoxyglucose. (b) Removal of Ca++ and addition of agents which increased the intracellular levels of cyclic AMP (cAMP), however, prevented both activities, while colchicine had greater inhibitory activity on HMP stimulation than upon secretion. (c) Neutrophils incubated in suspension with particulate aggregated gamma-globulin phagocytosed the particles and exhibited a stimulated HMP and released granule enzymes. In contrast, incubation in suspension with soluble aggregated gamma-globulin resulted in the stimulated HMP only. Granule evzymes were not liberated. 300-fold less soluble aggregates bound to a surface, however readily induced exocytosis of granules from adherent neutrophils. This demonstrates the importance of surface effects in the induction of secretion from neutrophils. Aggregated immunoglobulin reacting with neutrophil Fc receptors thus induces both degranulation (exocytosis) and increased HMP activity. The pathways leading to these events are separable although apparently sharing some common steps, including the initiating events.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBAN S., SCHULZE H. O. The effects of 2-deoxyglucose on the growth and metabolism of cultured human cells. J Biol Chem. 1961 Jul;236:1887–1890. [PubMed] [Google Scholar]
- BECK W. S. Occurrence and control of the phosphogluconate oxidation pathway in normal and leukemic leukocytes. J Biol Chem. 1958 May;232(1):271–283. [PubMed] [Google Scholar]
- Becker E. L., Henson P. M. In vitro studies of immunologically induced secretion of mediators from cells and related phenomena. Adv Immunol. 1973;17:93–193. doi: 10.1016/s0065-2776(08)60732-4. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Bodel P., Malawista S. E. Phagocytosis by human blood leucocytes during suppression of glycolysis. Exp Cell Res. 1969 Jul;56(1):15–23. doi: 10.1016/0014-4827(69)90386-3. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Lichtenstein L. M., Weissmann G., Zurier R. Effects of cholera enterotoxin on adenosine 3',5'-monophosphate and neutrophil function. Comparison with other compounds which stimulate leukocyte adenyl cyclase. J Clin Invest. 1973 Mar;52(3):698–708. doi: 10.1172/JCI107231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan T. D. Mechanisms of phagocytosis in human polymorphonuclear leucocytes. Immunology. 1966 Feb;10(2):137–148. [PMC free article] [PubMed] [Google Scholar]
- Cooper M. R., DeChatelet L. R., McCall C. E., LaVia M. F., Spurr C. L., Baehner R. L. Complete deficiency of leukocyte glucose-6-phosphate dehydrogenase with defective bactericidal activity. J Clin Invest. 1972 Apr;51(4):769–778. doi: 10.1172/JCI106871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. H., Malawista S. E. The nitroblue tetrazolium reaction in human granulocytes adherent to a surface. Yale J Biol Med. 1972 Apr;45(2):119–132. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Stimulation of human neutrophil leukocyte aerobic glucose metabolism by purified chemotactic factors. J Clin Invest. 1974 Feb;53(2):591–599. doi: 10.1172/JCI107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins D. Biopolymer membrane: a model system for the study of the neutrophilic leukocyte response to immune complexes. J Immunol. 1971 Aug;107(2):344–352. [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions in vitro. 3. Pharmacologic modulation of lysosomal constituent release. Clin Immunol Immunopathol. 1974 Jan;2(2):141–152. doi: 10.1016/0090-1229(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions in vitro: effect of cytochalasin B. J Immunol. 1973 Jan;110(1):294–296. [PubMed] [Google Scholar]
- Henson P. M. Interaction of cells with immune complexes: adherence, release of constituents, and tissue injury. J Exp Med. 1971 Sep 1;134(3 Pt 2):114s–135s. [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. Mechanisms of release of granule enzymes from human neutrophils phagocytosing aggregated immunoglobulin. An electron microscopic study. Arthritis Rheum. 1973 Mar-Apr;16(2):208–216. doi: 10.1002/art.1780160211. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Enhancement of immunologically induced granule exocytosis from neutrophils by cytochalasin B. J Immunol. 1973 Jan;110(1):290–293. [PubMed] [Google Scholar]
- Henson P. M. Pathologic mechanisms in neutrophil-mediated injury. Am J Pathol. 1972 Sep;68(3):593–612. [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Ignarro L. J. Neutral protease release from human leukocytes regulated by neurohormones and cyclic nucleotides. Nat New Biol. 1973 Oct 3;245(144):151–154. doi: 10.1038/newbio245151a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- LEVY H. R. The interaction of mammary glucose 6-phosphate dehydrogenase with pyridine nucleotides and 3beta-hydroxyandrost-5-en-17-one. J Biol Chem. 1963 Feb;238:775–784. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Pancake S. J., Noseworthy J., Karnovsky M. L. Measurement of rates of phagocytosis: the use of cellular monolayers. J Cell Biol. 1969 Jan;40(1):216–224. doi: 10.1083/jcb.40.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Zatti M., Patriarca P., Cramer R. Effect of specific antibodies on the metabolism of guinea pig polymorphonuclear leukocytes. J Reticuloendothel Soc. 1971 Jan;9(1):67–85. [PubMed] [Google Scholar]
- Scott R. E. Effects of prostaglandins, epinephrine and NaF on human leukocyte, platelet and liver adenyl cyclase. Blood. 1970 Apr;35(4):514–516. [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Dukor P., Zurier R. B. Effect of cyclic AMP on release of lysosomal enzymes from phagocytes. Nat New Biol. 1971 Jun 2;231(22):131–135. doi: 10.1038/newbio231131a0. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]





