Abstract
Aim/hypothesis
To investigate if beta cell neoformation occurs in the transplanted pancreas in patients with type 1 diabetes who received a simultaneous pancreas-kidney (SPK) transplant and later developed recurrence of autoimmunity.
Methods
We examined pancreas transplant biopsies from 9 SPK patients with/without recurrent autoimmunity/recurrent diabetes and 16 non-diabetic organ donors. Tissues were analyzed by immunohistochemistry and immunofluorescence.
Results
Numerous CK-19+ pancreatic ductal cells expressed insulin in 6 SPK recipients with recurrent autoimmunity, of whom 5 developed recurrence of diabetes requiring insulin therapy. These cells expressed the pancreatic-duodenal homeobox-1 transcription factor (PDX-1), implicated in pancreatic development and beta cell differentiation. The number of insulin+ ductal cells varied, being highest in the patient with the most severe beta cell loss and lowest in the normoglycemic patient. We detected insulin+CK-19+PDX-1+ cells expressing Ki-67 in the patient with the most severe beta cell loss, indicating proliferation. We could not detect Ki-67+ beta cells within the islets in any SPK patient. Some insulin+CK-19− ductal cells expressed chromogranin A, suggesting further endocrine differentiation. Insulin+ cells were rarely noted in the pancreas transplant ducts in 3 SPK patients without islet autoimmunity and in 6/16 non-diabetic organ donors; these insulin+ cells never expressed CK-19.
Conclusions/Interpretation
Insulin-expressing pancreatic ductal cells, some apparently proliferating, were found in the transplanted pancreas with recurrent islet autoimmunity/diabetes. Replicating beta cells were not detected within islets. The observed changes may represent attempts at tissue remodelling and beta cell regeneration involving ductal cells in the human transplanted pancreas, possibly stimulated by hyperglycaemia and chronic inflammation.
Keywords: type 1 diabetes, pancreas transplantation, recurrence of autoimmunity, pancreatic ducts, insulin, PDX-1, regeneration
INTRODUCTION
Type 1 diabetes is an autoimmune disease resulting in the destruction of pancreatic beta cells and insulin-dependence. However, residual insulin secretion is often detected at disease onset and marginal amounts of C-peptide are secreted by several patients even many years after diagnosis [1]. Consistently, beta cells are not completely absent in the pancreas of patients with type 1 diabetes [2–4]. A recent meta-analysis suggested that residual beta cell mass at diagnosis is related to age of onset, with younger patients having much more significant destruction than older ones [5]. Beta cells were demonstrated in 88% of autopsy pancreata from 42 patients with disease duration ranging between 4 and 67 years, appearing as single cells or small clusters. While this may simply reflect the survival of a few beta cells, the finding of ongoing beta cell apoptosis and the contemporary presence of non-apoptotic beta cells indirectly suggested beta cell neogenesis [6]. However, replication may be hampered by cytokine-induced damage and apoptosis associated with chronic autoimmunity, to which newly formed beta cells are sensitive [7;8]. In mice, direct beta cell replication appears to be the main mechanism for maintaining beta cell mass [9] in physiologic conditions such as pregnancy [10] and experimentally after pancreatectomy [11] or beta cell depletion induced by transgenically expressed diphtheria toxin [11;12]. Other regenerative mechanisms include regeneration from pancreatic (and perhaps extra-pancreatic) precursor cells and trans-differentiation of other pancreatic (or extra-pancreatic) cell types [13–16]. Trans-differentiation and regeneration were reported in experimental conditions associated with tissue damage or beta cell loss, such as pancreatectomy [17], cellophane wrapping [18], ductal ligation [19;20], streptozotocin treatment [21] and the development of autoimmune diabetes in nonobese diabetic mice [21;22] and diabetes-prone rats [23]. Pancreatic cells with features of ductal and beta cells in pancreatic ducts were originally characterized by electron microscopy [24]. Growing evidence suggests that ductal cells or precursors in the ducts may be involved in beta cell regeneration [17;20;25–30]; for example, human ductal cells transplanted into immunodeficient mice differentiate into new beta cells [30]. Rare insulin+ cells in pancreatic ducts were reported in the pancreas of patients with long standing type 1 diabetes [6], but the phenotype of those cells was not characterized further. Overall, there is growing evidence that pancreatic tissue damage may trigger several regenerative and remodelling mechanisms that may contribute to beta cell neogenesis [9].
Recurrence of autoimmunity and diabetes after pancreas transplantation was originally described in twins and HLA-identical siblings [31]. Other studies contributed evidence that recurrence of autoimmunity can occur regardless of HLA sharing and despite immunosuppression [32]. At our institution we are following approximately 275 patients who received a simultaneous pancreas-kidney (SPK) transplant several years after developing type 1 diabetes. We are monitoring these patients for recurrence of autoimmunity, defined as the reactivation of humoral and/or cellular autoimmune responses, and followed several patients to the full recurrence of type 1 diabetes requiring reinstitution of insulin therapy [33]. Most of these patients had no clinical signs of rejection associated with recurrent diabetes and maintained normal exocrine pancreas and kidney graft function. We could obtain pancreas transplant biopsies from several of these patients, which confirmed the diagnosis of recurrent diabetes revealing variable degrees of insulitis and/or beta cell loss. In this study we investigated if beta cell neoformation occurred in the transplanted pancreas of SPK patients with recurrent autoimmunity and diabetes, by examining the frequency, distribution, localization and phenotype of insulin-expressing cells.
METHODS
Pancreata from transplant biopsies and deceased donors
We obtained open pancreas transplant biopsies from nine patients identified from a cohort of SPK recipients transplanted at the University of Miami. Patients included 2 females and 7 males with a mean age of 44.1 ± SD 4.6 years (Table 1). All transplantation-related research procedures were approved by the University of Miami Institutional Review Board. Informed consent was obtained from all patients (or family members when appropriate) prior to transplantation and again prior to biopsy. We also examined 16 pancreata from non-diabetic, deceased organ donors, identified at the University of Miami and the University of Colorado. These included 7 female and 9 male donors, with a mean age of 40.8 ± SD 14.5 years (data shown in ESM Table 1). Additional features of the non-diabetic organ donors were previously reported [34].
Table 1.
Clinical characteristics of SPK patients.
| Patient | Age (Years) | Sex | Hyperglycaemia | Autoimmunity/Type 1 diabetes Recurrence | Clinical Rejection | GAD AAb | IA-2 AAb | Autoreactive T-Cells | Pancreas Transplant Biopsy | Postprandial C-peptide prior to biopsy (nmol/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulitis | Beta cell loss | Other Features1 | Years after Tx | ||||||||||
| SPK #1 | 38 | M | Yes | Yes/Yes | No | + | + | + | Yes | Yes | mild acute, grade III, minimal chronic rejection | 6, 9 | <0.03 |
| SPK #2 | 39 | M | Yes | Yes/Yes | No | + | + | Not tested | Yes | Yes | minimal acute rejection, grade II | 1.25 | <0.03 |
| SPK #3 | 44 | F | Yes | Yes/Yes | No | + | + | + | Yes | Yes | inflammation of undetermined significance, grade 1 | 10.5 | 0.06 |
| SPK #4 | 41 | M | Yes | Yes/Yes | No | + | + | + | Yes | Yes | no rejection in head and body; mild acute rejection, grade III and evidence of chronic rejection in the tail | 5.5 | 0.06 |
| SPK #5 | 43 | M | Yes | Yes/Yes | No | + | + | + | No | Yes | minimal acute rejection, grade II | 5.5 | 0.075 |
| SPK #6 | 46 | M | No | Yes/No | No | + | + | + | Yes | No | No evidence of rejection | 2 | 0.072 |
| SPK #7 | 44 | F | Yes | No/No | No | − | − | Not tested | No | No | inflammation of undetermined significance, grade 1 | 2 | 0.075 |
| SPK #8 | 50 | M | No | No/No | Yes | − | − | − | No | No | Acute rejection, mild (grade III), early chronic rejection | 9 | 0.057 |
| SPK #9 | 52 | M | Yes | No/No | No | − | − | Not tested | No | No | Unremarkable | 1 | 0.06 |
Clinical features of the SPK patients
As shown in Table 1, patients were assessed for recurrent autoimmunity, recurrent diabetes and rejection. We measured autoantibodies to glutamic acid decarboxylase (GAD) and the tyrosine phosphatase-like protein IA-2 using standardized radioimmunoassays [35]. We tested for the presence of autoreactive CD4 and/or CD8 T cells using MHC class II/I tetramer assays, as described [36;37]. Recurrent type 1 diabetes was diagnosed if the following criteria were met: 1) hyperglycaemia requiring insulin therapy; 2) clinical symptoms of diabetes in the presence of unchanged pancreas transplant exocrine function and kidney transplant function; 3) autoantibodies and/or autoreactive T cells in the circulation; 4) insulitis and/or beta cell loss at biopsy; 5) reduction in C-peptide levels.
Patients #1–6 had ongoing or recent recurrence of islet autoimmunity demonstrated by disease-associated autoantibodies and autoreactive T cells; 5/6 patients had both autoantibodies and autoreactive T cells. For patient #2, autoreactive T cells could not be determined. Patients #1–5 had become diabetic again by the time of biopsy and required insulin therapy. Biopsies showed variable degrees of beta cell loss and/or insulitis. Patient #1 had the most severe beta cell loss and had very little residual insulitis. Patients #2, #3, and #4 had both insulitis and significant beta cell loss. Patient # 5 had no insulitis but several islets showed reduced cellular content and reduced insulin staining. Patient #6 was normoglycemic at the time of his accidental passing, when the pancreas transplant was retrieved with the family's consent, 2 years after transplantation. There was no evidence of beta cell destruction, truly minimal insulitis. He had autoantibodies before and at the time of his passing, when T cells were detected in his peripheral blood, pancreas transplant lymph node and pancreas transplant tissue, reacting against GAD and proinsulin [38]. These data and other features of the biopsies are summarized in Table 1, noting that some inflammatory changes are almost invariably found in pancreas transplant biopsies even in absence of clinical rejection. For comparison, we examined open pancreas transplant biopsies available from three SPK patients (#7–9) without evidence of recurrent autoimmunity. Patients #7 and #8 were normoglycemic and had functioning grafts. Patient #8 could also be tested for GAD autoreactive T cells, which were not detected. This patient had evidence of mild acute and chronic rejection in the pancreas transplant biopsy but largely normal islets and insulin staining 9 years after transplantation. Patient #9 was hyperglycaemic and treated with insulin, although still secreting C-peptide and believed to have developed type 2 diabetes.
Immunohistochemistry and immunofluorescence
Pancreatic tissue from pancreas transplant biopsies and from non-diabetic organ donors was fixed in formalin and paraffin embedded. Five μm-thick sections were cut and mounted on glass slides, which were stained using immunofluorescence and immunohistochemistry standard protocols, as described below.
Primary antibodies
The following primary antibodies were used: mouse anti-insulin (1:100, clone K36AC10, Sigma, St. Louis, MO, USA), guinea pig anti-insulin (pre-diluted, Biogenex, San Ramon, CA, USA), rabbit anti-glucagon (1:50, DAKO, Carpinteria, CA, USA), goat anti-PDX-1 (1:5000, a gift from Dr. C. Wright, including an acid-eluted affinity-purified batch), mouse anti-CK-19 (1:50, Clone RCK108, Biogenex), rabbit anti-CK-19 (1:100, ProteinTech Group, Chicago, IL, USA). We used a rabbit anti-Ki-67 serum (1:50, Zymed Laboratories/Invitrogen, San Francisco, CA, USA) to assess proliferation [39] and both a rabbit serum (1:200, NeoMarkers, Fremont, CA, USA) and mouse monoclonal antibody (1:600, Clone LK2H10, NeoMarkers) to stain for chromogranin A (CgA), a protein found in secretory granules of endocrine cells [40].
Epitope retrieval
For some stains, namely PDX-1, Ki-67 and CgA, epitope retrieval is necessary to successfully stain the tissues. Heat induced epitope retrieval was performed as follows: for PDX-1 staining, slides were boiled in Tris-EDTA (pH 9.0) in a hot plate for 30 minutes (95–100 °C). For Ki-67 staining, tissue sections were digested with trypsin for 10 minutes at 37 °C followed by incubation with citrate buffer (pH 6.0) in a pressure cooker for 5 minutes, 125 °C, using the protocol provided by the manufacturer (Zymed Laboratories/Invitrogen). For CgA staining, tissue sections were incubated with citrate buffer (pH 6.0) in a pressure cooker for 10 minutes, 120 °C. After all the various epitope retrieval treatments, sections were let cool at room temperature for 30 minutes.
Immunohistochemistry
For insulin, glucagon, CK-19 and Ki-67 staining, we used the labelled-streptavidin-biotin (LAB-SA) method and the Histostain Plus kit (Zymed Laboratories/Invitrogen) according to the manufacturer's protocol. Formalin-fixed, paraffin-embedded tissue sections were deparaffinised and dehydrated in a graded series of ethanol washes. Tissue sections were subject to epitope retrieval if required by the specific stain, as described above. After washing in phosphate buffered saline (PBS, pH 7.4), endogenous peroxidase was quenched using a 3% solution of hydrogen peroxidase and methanol. Sections were washed with PBS and incubated with protein block (serum free) reagent (Dako) to block non-specific staining. Slides were incubated with primary antibodies (1–3 hours for insulin, glucagon and CK-19, overnight for Ki-67 at 4 °C). After washing in PBS, sections were incubated with biotinylated secondary antibody, washed again in PBS and incubated with horseradish peroxidase-conjugated streptavidin. The 3-amino, 9 ethyl-carbazole (AEC) substrate revealed specific staining. Slides were counterstained with hematoxylin. For PDX-1 staining, we used the Vectastain ABC Kit (Vector Laboratories, Burlingame, CA, USA), which also uses the peroxidase method but 3, 3'-diaminobenzidine (DAB) as substrate. Slides were incubated with the primary antibody (goat anti-PDX-1) overnight at 4 °C. Negative control reactions included those in which the primary antibody was replaced by PBS or by the appropriate isotype-matched control antibody, those in which the secondary antibody was omitted, and those in which the streptavidin horseradish peroxidase conjugate was omitted to control for residual endogenous peroxidase activity. For Ki-67 staining, tonsil sections were used as positive control.
Immunofluorescence staining
The following double and triple staining combinations were used to assess colocalization: insulin/CK-19, PDX-1/CK-19, insulin/PDX-1, insulin/PDX-1/CK-19, insulin/CK-19/Ki-67 and insulin/CK-19/CgA. After deparaffinization, tissue sections were subject to epitope retrieval if required by the specific stain, as described above. Tissue sections were incubated with protein block (serum free) reagent (Dako) to block non-specific staining. The first primary antibody was applied overnight at 4 °C for Ki-67, PDX-1 and insulin, for 1–2 hours at room temperature for CK-19 and CgA. After washing in PBS, the appropriate secondary antibodies were applied for 1 hour at room temperature (1:400). Double and triple-immunofluorescence stains were carried out sequentially, so the above process was repeated for the second and third primary antibodies. The choice of secondary antibodies was such that the secondary antibodies could only react with their respective primary antibody. For all experiments, control reactions included the omission of the primary antibodies, the replacement of the primary antibody with the appropriate isotype-matched irrelevant antibody, and the omission of the secondary antibody. Secondary antibodies were fluorophore-labelled conjugates: Alexa Fluor 568 goat anti-guinea pig IgG, Alexa-Fluor 488 chicken anti-goat IgG, Alexa-Fluor 647 goat anti-mouse IgG, Alexa-Fluor 488 goat anti-rabbit IgG and Alexa-Fluor 568 goat anti-mouse IgG, all from Invitrogen (Carlsbad, CA, USA).
Microscopy
Slides stained by immunohistochemistry were imaged using a Leica DMLB microscope connected to a Leica DFC-420C digital camera using the IM50 Image Manager software. For immunofluorescence, we used a Zeiss Axiovert 200M inverted microscope. Images were acquired digitally using a Hamamatsu ORCA-ER camera and the Zeiss Axiovision 4.6.3 software. We used the following objectives: Plan-neofluar 10×/0.3 NA, Plan-neofluar 20×/0.5 NA, F-fluar 40×/1.30 oil and Plan-apochromat 63×/1.40 oil. Confocal microscopy was performed with a Zeiss LSM-510 microscope, equipped with the same objectives, to confirm colocalization by Z-stack analysis.
RESULTS
Insulin expression in pancreatic ductal cells
We examined pancreas transplant biopsies from 9 SPK patients, of whom 6 (patients #1–6) had developed recurrent islet autoimmunity. Five of these patients (#1–5) had progressed to full diabetes recurrence with hyperglycaemia requiring reinstitution of insulin therapy by the time of biopsy. Table 1 shows the main clinical characteristics of the SPK patients and their stimulated C-peptide levels at the time of biopsy. In these five patients' biopsies, insulin staining revealed the presence of numerous insulin+ cells in the pancreatic ducts (Fig. 1A, 1B, 1D–M, Table 2). Two antibodies to insulin were used in separate experiments yielding similar results. The biopsy of patient #1, who had completely lost C-peptide secretion, showed the most severe beta cell loss and the most evident insulin expression in ductal cells (Fig. 1A, 1B, 1D–G). Indeed, most of the ducts examined (127/141; 90%) and most ductal cells (1,529/1,608; 95%) stained for insulin (Table 2). Patient #1 had two clinically justified pancreas transplant biopsies, approximately 3 years apart; the first biopsy was obtained one year after the patient had resumed insulin therapy. The presence of insulin+ ductal cells was observed in both biopsy specimens. Insulin expression in ductal cells occurred in a variable number of ducts in patients #2–5 (Fig. 1H–M, and Table 2), ranging from 33% to 85% of the ducts examined. Specifically, 65%, 33%, 82% and 85% of the ducts examined had insulin+ cells and, 35% (400/1,134), 17% (294/1,429), 75% (541/714) and 65% (393/604) of the ductal cells stained for insulin in patients #2–5, respectively. The intensity of the staining varied, but it was usually weaker than in beta cells in nearby islets, even if those beta cells were often in infiltrated (Fig. 1J–L, patient #4) or damaged islets (Fig. 3B, patient #2). There was no apparent predilection for duct type or size. In patient #2, many small ducts or ductal like structures resembling tubular complexes primarily stained for insulin (Fig. 1H). A similar pattern was also observed in patient#1 (Fig. 1G) and in patient #4 (Fig. 1J–L). Larger ducts were stained in other patients, for example patient # 5 (Fig. 1M).
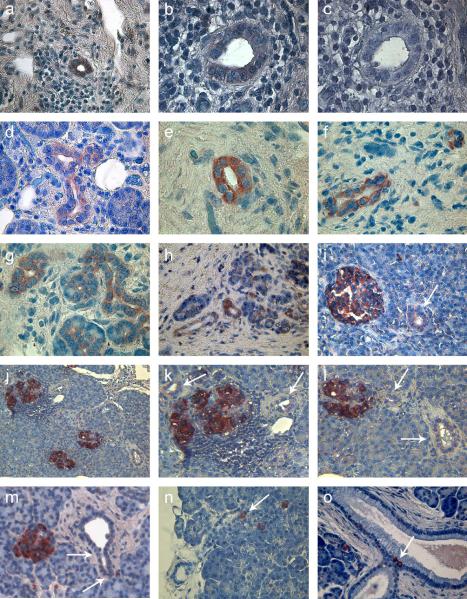
Figure 1. Insulin+ cells in pancreatic ducts.
Insulin expression demonstrated by immunohistochemistry in pancreas transplant biopsies from 6 SPK patients with recurrent autoimmunity (A–N). Patients #1–5 (A–M), who had developed recurrence of diabetes, had the most ductal cells stained for insulin.
Panel A: patient #1, first biopsy; infiltrating lymphocytes are seen surrounding a duct containing insulin+ cells.
Panels B and C: patient#1, second biopsy, serial sections stained for insulin and isotype control, respectively. In B, staining is weak but distinct and clearly above background in C.
Panel D: patient #1, second biopsy, larger duct stained for insulin; staining is present also on the non-luminal side in many cells.
Panel E and F: patient #1, second biopsy, ducts with intense insulin staining.
Panel G: patient #1, a few ducts and some smaller ductal-like structures (lower right corner) are stained for insulin.
Panel H: biopsy of patient #2, showing larger ducts and some smaller ductal-like structures stained for insulin.
Panel I: biopsy of patient #3, insulin staining in a duct (arrow) is weaker than in the nearby islet. Some weaker staining is seen in the acinar tissue, possibly involving smaller ductal-like structures.
Panel J: pancreas transplant biopsy of patient #4, showing three islets, one of which was heavily infiltrated by lymphocytes; Panels K and L are cropped from J to help visualize ducts containing insulin+ cells (arrows). Some weaker and less distinct staining is seen in the acinar tissue, possibly involving smaller ductal-like structures.
Panel M: pancreas transplant biopsy of patient #5; not all ductal cells stained for insulin (arrows); staining is present also on the non-luminal side in many cells.
Panel N: Rare insulin+ cells (arrow) were seen in a minority of ducts in patient #6; he had not yet developed significant insulitis and beta cell destruction and was normoglycemic.
Panel O: Rare insulin+ cells (arrow) were seen in control pancreata. Some single insulin+ cells are also seen in the acinar tissue.
Objective lens: 63× (A, D, F, G), 100× (B, C, E), 40× (H, I, M, N, O), 20× (J; K and L are cropped from J).
Table 2.
Enumeration of insulin-positive ductal cells.
| Subjects | Insulin+ ductal cells/ductal cells examined | Ducts with insulin+ cells/ducts examined |
|---|---|---|
| Donor #1 | 8/173 | 3/7 |
| Donor #2 | 0/148 | 0/6 |
| Donor #3 | 0/160 | 0/6 |
| Donor #4 | 0/443 | 0/16 |
| Donor #5 | 0/98 | 0/5 |
| Donor #6 | 0/114 | 0/5 |
| Donor #7 | 0/392 | 0/14 |
| Donor #8 | 0/208 | 0/8 |
| Donor #9 | 1/162 | 1/6 |
| Donor #10 | 0/139 | 0/6 |
| Donor #11 | 0/152 | 0/6 |
| Donor #12 | 2/231 | 1/8 |
| Donor #13 | 6/188 | 2/7 |
| Donor #14 | 0/77 | 0/4 |
| Donor #15 | 8/1579 | 4/47 |
| Donor #16 | 3/1961 | 3/85 |
| SPK #1 | 1529/1608 | 127/141 |
| SPK #2 | 400/1134 | 37/57 |
| SPK #3 | 294/1429 | 17/52 |
| SPK #4 | 541/714 | 61/74 |
| SPK #5 | 393/604 | 29/34 |
| SPK #6 | 15/2109 | 7/99 |
| SPK #7 | 1/798 | 1/28 |
| SPK #8 | 3/533 | 2/18 |
| SPK #9 | 1/413 | 1/24 |
Figure 3. Co-expression of insulin, CK-19 and PDX-1 in ductal cells of SPK patients with recurrent autoimmunity.
Panel A shows insulin/CK-19 and CK-19/PDX-1 co-expression in the ducts by double immunofluorescence using serial sections from the biopsy of patient #1. Panel B shows expression of PDX-1, insulin and CK-19 in 3 serial sections of the transplanted pancreas of patient #2. A small duct is stained for insulin, CK-19 and PDX-1 in the bottom right corner. A damaged islet with weak insulin staining is also seen. Panel C shows cropped images of the images in B to highlight the staining in the small duct. Panel D shows insulin/CK-19/PDX-1 colocalization by triple immunofluorescence for patient # 6. Objective lens: 40× (A–D).
Patient #6 had evidence of recurrent autoimmunity but had not developed diabetes. The examination of his pancreas transplant biopsy revealed no evidence of beta cell destruction. Only 7% of the ducts examined in his transplant biopsy contained insulin+ cells and these were rare, representing less than 1% of the ductal cells (15/2,109) (Fig. 1N, Table 2). Similarly, insulin+ cells in the ducts were rarely noted in 6/16 control pancreata from non-diabetic organ donors who also tested negative for diabetes-associated autoantibodies (Fig. 1O, Table 2). For additional comparison we examined available pancreas transplant biopsies from 3 SPK patients (#7–9, Table 2) who had no evidence of recurrent autoimmunity. Patients #7 and #8 had functioning grafts and had normal glucose tolerance. Patient #9 had apparently developed type 2 diabetes. Insulin+ cells were rarely seen in the ducts of these patients, mainly consisting of single cells like the ones seen in the non-diabetic organ donors and amounting to less than 0.5% of the ductal cells examined in each patient (range 413–798 cells). The insulin+ cells in the ducts did not co-express glucagon, and we did not identify glucagon+ cells in the ducts of our SPK patients. However, glucagon+ cells were occasionally seen in the ducts of control pancreata (not shown).
Insulin+ cells in the pancreatic ducts of SPK patients with recurrent autoimmunity express CK-19 and PDX-1
In order to better define the phenotype of the insulin+ cells in the ducts and confirm that these were ductal cells, we stained serial sections by immunohistochemistry for insulin and CK-19, a marker of ductal cells, and PDX-1, a transcription factor required for pancreatic development that is expressed in mature beta cells and in ductal cells. We used double or triple immunofluorescence to assess co-expression of insulin with CK-19 and PDX-1. In the SPK patients with recurrent autoimmunity, including patient #6, many but not all of the insulin+ cells in the ducts co-expressed CK-19 (Fig. 2, patient #1; the original image of the corresponding confocal plane from the Z-stack series is provided in ESM Fig. 1). This confirmed that those were ductal cells, in addition to their anatomical localization. The insulin+CK-19+ cells also stained for PDX-1, demonstrating features of both endocrine and ductal cells (Fig. 3). Usually, insulin+CK-19+ ductal cells expressed PDX-1 at higher levels than CK-19+insulin− cells, which could also lack PDX-1 expression (Fig. 3D). As noted, we observed rare insulin+ cells in the ducts in three additional SPK patients (#7–9) without autoimmunity and in 6/16 pancreata from non-diabetic organ donors. In contrast to the SPK patients with recurrent autoimmunity, the insulin+ cells identified in the ducts of the SPK patients without autoimmunity (not shown) and in the control pancreata (Fig. 4A; ESM Fig. 2 shows another image acquired by confocal microscopy) did not express CK-19. Consistent with an endocrine phenotype, those cells expressed PDX-1 (not shown).
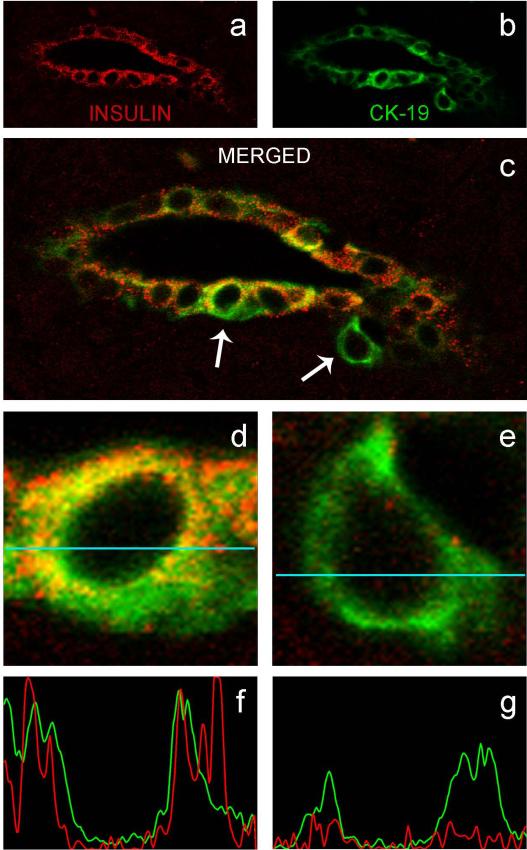
Figure 2. Co-expression of insulin and CK-19 in ductal cells of an SPK patient with recurrent autoimmunity.
Sections are from the biopsy of patient #1. Images were acquired using a confocal microscope with a 40× lens. Panel A, B and C show staining for insulin, CK-19 and merged, respectively. In C, note the cell marked by the right arrow, which stained for CK-19 but did not stain for insulin. The single cells marked by arrows in C are shown enlarged in D and E. Panel D shows an insulin+CK-19+ cell, panel E shows a cell expressing CK-19 only. Colocalization analysis was performed by confocal microscopy and is graphically displayed in F and G, corresponding to the cells shown in D and E, respectively, for the data points collected along the horizontal line (44 μm). Immunofluorescence is not detected when the line goes across the nucleus. Objective lens: 40× (A–C).
Figure 4. Colocalization analysis of insulin, CK-19 and Ki-67.
Panel A demonstrates absence of colocalization of insulin and CK-19 in control pancreata #15. A Ki-67+ cell is shown outside the duct. Panel B shows colocalization of insulin, CK-19 and Ki-67 in the pancreas transplant biopsy of patient #1. There is colocalization of insulin with CK-19 and one cell in the duct also expressed Ki-67. Objective lens: 20× (A) and 40× (B).
Ki-67 expression in insulin+ ductal cells
We investigated if proliferation could be observed in insulin+ cells, in islets or ducts, by staining for the proliferation marker Ki-67. In patient #1, in whom beta cell destruction was the most severe, we observed insulin+CK-19+PDX-1+ cells which, albeit rarely, expressed Ki-67 in the nucleus, indicating proliferation (Fig. 4B; ESM Fig. 3 shows another image acquired by confocal microscopy). Ki-67 expression was not observed in the insulin+ ductal cells of the other 5 SPK patients with recurrent autoimmunity (not shown) and of the non-diabetic pancreas organ donors (Fig. 4A and ESM Fig. 2). We did not observe Ki-67+ beta cells in the islets of any of the pancreas transplant biopsies from the SPK patients with recurrent autoimmunity.
CgA expression in insulin+ ductal cells
While most of the insulin+CK-19+ ductal cells did not express CgA, we observed ductal structures containing insulin+CgA+ cells. Importantly, these cells did not express CK-19. Fig. 5A shows two ducts from the second biopsy of patient #1, one in which cells express insulin together with CK-19 and another in which ductal cells express insulin and CgA in the absence of CK-19. Insulin staining was more intense in the latter duct. Fig. 5B shows coexistence of insulin+CK-19+CgA− cells with insulin+CK-19−CgA+ cells in the same duct (biopsy of patient #5). Fig. 5C shows ductal structures, observed in the biopsy of patient #2, staining for insulin and CgA in the absence of CK-19, which is however expressed in other small ductal structures nearby.
Figure 5. Insulin-positive ductal cells expressing CgA do not express CK-19.
The figure illustrates the exclusive expression of CK-19 and CgA in insulin+ ductal cells (A, patient #1; B, patient # 5; C, patient #2). Some background staining is visible in the nuclei but clear cytoplasmic staining is seen in the cells co-expressing CgA. Weaker cytoplasmic staining but above nuclear background seems also visible in a few other cells that did not stain for CgA. Objective lens: 20× (A, 4× zoom), 40× (B), and 20× (C).
DISCUSSION
Our study of pancreas transplant biopsies provides evidence connecting the ductal and beta cell phenotypes in the human pancreas, further supporting the concept that ductal cells may give origin to new beta cells under certain conditions. We demonstrate the existence of insulin+ ductal cells, confirmed by CK-19 co-expression, in the ducts of the transplanted pancreas, specifically in SPK patients with recurrent autoimmunity and diabetes. These cells expressed PDX-1, a transcription factor required for pancreatic development that is expressed in mature beta cells [41;42] and in ductal cells that do not express insulin [43]. The number of insulin+ ductal cells varied between 33 and 90% of the ductal cells examined. Similarly, 17% to 95% of the ducts had insulin+ cells, indicating that these phenomena were quite extensive. The patient with the most severe beta cell destruction and complete loss of C-peptide secretion at the time of biopsy was the one with the highest number of ducts containing insulin+ cells (patient #1). A consistent feature observed in our SPK patients with recurrent autoimmunity who had also progressed to diabetes by the time of biopsy was that most (although not all, Fig. 2C, E) ductal cells were insulin+ in those ducts that contained insulin+ cells. In contrast, SPK patient #6 who was normoglycemic and without beta cell loss, although with clear evidence of ongoing autoimmunity, had rare cells in the ducts that stained for insulin, CK-19 and PDX-1. Thus, the presence of both hyperglycaemia and autoimmunity may be critical for triggering insulin expression in ductal cells.
By comparison, we rarely observed insulin+ cells in the ducts of non-diabetic organ donors. Importantly, while those cells expressed PDX-1, they did not express CK-19. Similarly, we rarely observed insulin+ cells in the ducts when we examined pancreas transplant biopsies from SPK patients without apparent islet autoimmunity, of whom two were normoglycemic and one had developed type 2 diabetes. Mirroring the observations in non-diabetic pancreas donors, these cells did not stain for CK-19. The findings in these SPK patients without autoimmunity and in the non-diabetic organ donors are consistent with earlier reports of single insulin+ cells in close association with ductal structures in the normal human pancreas [44].
Insulin and PDX-1 expression in CK-19+ ductal cells, with a pattern similar to that of our SPK patients with recurrent autoimmunity and diabetes, was noted in human pancreata from patients with autoimmune chronic pancreatitis with diabetes [45]. This condition is similar to recurrence of autoimmune diabetes in the transplanted pancreas in that both autoimmunity and hyperglycaemia are present. Thus, there are now two studies of human pancreata suggesting that hyperglycaemia and chronic autoimmunity/inflammation may stimulate pancreas remodelling pathways including insulin expression in ductal cells. A similar role for autoimmunity and inflammation in driving beta cell self-replication was suggested by mouse studies [22]. Moreover, autoimmunity and diabetes have been associated with beta cell and islet neoformation in diabetes prone rats [23], with regeneration occurring in tubular complexes which resemble the small ductal structures that stained for insulin in the biopsies of patient #1 (Fig. 1G) and patient # 2 (Fig. 1H).
Another study of patients with pancreatitis of unspecified aetiology reported the presence of cells expressing insulin, or glucagon, or PDX-1 in pancreatic ducts [46]. However, this study did not show co-expression of islet hormones with CK-19 or Ki-67, albeit Ki-67-positive cells were noted in the ducts. Here we find that insulin+CK-19+ cells can express Ki-67, suggesting that these cells are capable of replication. This was noted in the patient with the most severe beta cell destruction, again suggesting a possible link between the replication of insulin+ ductal cells and the severity of beta cell loss/hyperglycaemia. We did not observe Ki-67+ cells among the surviving beta cells in the islets of any of the SPK patients with recurrent autoimmunity. However, we must recognize the limitations associated with the study of biopsy materials, in particular when assessing direct beta cell replication. Moreover, our SPK patients were immunosuppressed with tacrolimus, which inhibits direct beta cell replication in mice [12]. On the other hand, the formation of beta cells from ductal cells may be enhanced by chronic immunosuppression if this antagonizes chronic autoimmunity.
Three of our SPK patients with recurrent autoimmunity (patients #1, 2, and 5) also had ductal cells expressing insulin together with CgA, a protein found in the secretory granules of endocrine cells, including pancreatic beta cells [40]. Importantly, those cells expressing CgA and insulin no longer expressed CK-19, while clearly being present in ductal structures. Various stages of this putative transition were observed in these patients, with some ducts having mixed cell composition (insulin+CgA+CK-19− and insulin+CK-19+CgA−; the latter represented the majority). This observation provides further evidence for the differentiation potential of ductal cells towards an endocrine phenotype, accompanied by an apparent loss of ductal cell features. Among these three patients, patients #1 and #2 had undetectable C-peptide levels, even after stimulation, in the days preceding the biopsy. The absence of C-peptide secretion in these patients seems consistent with the rarity of insulin+ ductal cells expressing CgA, despite a high overall proportion of insulin+ ductal cells. Based on the C-peptide data and on the rarity of this putative transition towards a more mature beta cell phenotype, we speculate that if these cells secrete insulin this may be of negligible clinical significance.
In conclusion, our findings suggest that ductal cells participate in beta cell regenerative processes occurring in the transplanted human pancreas, in the context of hyperglycaemia and recurrent autoimmunity, which may be critical stimuli to trigger pancreas remodelling mechanisms in the adult. Dissecting the mechanisms involved in these remodelling processes may potentially lead to therapeutic exploitation.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the Diabetes Research Institute Foundation and in part by a grant from NIDDK (R01 DK070011-01A1). We acknowledge the support of the University of Miami Analytical Imaging Core, partly funded by the Juvenile Diabetes Research Foundation (Center Grant JDRFI 4-2004-361); George McNamara, PhD, Beata Frydel, PhD and Brigitte Shaw assisted with confocal microscopy. Kevin Johnson assisted with tissue processing and histology. We thank Dr. Christopher Wright and Michael Ray, Vanderbilt University, and Dr. Helena Edlund, University of Umea, for providing PDX-1 anti-sera and for helpful discussion of the results.
ABBREVIATIONS
- AEC
3-amino, 9 ethyl-carbazole
- CgA
chromogranin A
- CK-19
cytokeratin-19
- DAB
3, 3'-diaminobenzidine
- GAD
glutamic acid decarboxylase
- Ki-67
proliferation marker
- LAB-SA
labeled-streptavidin-biotin
- PDX-1
pancreatic duodenal homeobox-1
- SPK
simultaneous pancreas-kidney
Footnotes
Duality of interest The authors declare no duality of interest.
REFERENCES
- 1.Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49:261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 2.Gepts W, De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27:251–261. doi: 10.2337/diab.27.1.s251. [DOI] [PubMed] [Google Scholar]
- 3.Rahier J, Goebbels RM, Henquin JC. Cellular composition of the human diabetic pancreas. Diabetologia. 1983;24:366–371. doi: 10.1007/BF00251826. [DOI] [PubMed] [Google Scholar]
- 4.Lohr M, Kloppel G. Residual insulin positivity and pancreatic atrophy in relation to duration of chronic type 1 (insulin-dependent) diabetes mellitus and microangiopathy. Diabetologia. 1987;30:757–762. doi: 10.1007/BF00275740. [DOI] [PubMed] [Google Scholar]
- 5.Klinke DJ. Extent of Beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS.ONE. 2008;3:e1374. doi: 10.1371/journal.pone.0001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 7.Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50:2323–2331. doi: 10.1007/s00125-007-0794-x. [DOI] [PubMed] [Google Scholar]
- 8.Meier JJ, Ritzel RA, Maedler K, Gurlo T, Butler PC. Increased vulnerability of newly forming beta cells to cytokine-induced cell death. Diabetologia. 2005;49:83–89. doi: 10.1007/s00125-005-0069-3. [DOI] [PubMed] [Google Scholar]
- 9.Bouwens L. Beta cell regeneration. Curr.Diabetes Rev. 2006;2:3–9. doi: 10.2174/157339906775473644. [DOI] [PubMed] [Google Scholar]
- 10.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm.Metab Res. 1997;29:301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 11.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 12.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J.Clin.Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025–1034. doi: 10.1210/en.2004-1576. [DOI] [PubMed] [Google Scholar]
- 14.Lardon J, Bouwens L. Metaplasia in the pancreas. Differentiation. 2005;73:278–286. doi: 10.1111/j.1432-0436.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- 15.Li WC, Yu WY, Quinlan JM, Burke ZD, Tosh D. The molecular basis of transdifferentiation. J.Cell Mol.Med. 2005;9:569–582. doi: 10.1111/j.1582-4934.2005.tb00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner-Weir S, Sharma A. Are there pancreatic progenitor cells from which new islets form after birth? Nat.Clin.Pract.Endocrinol.Metab. 2006;2:240–241. doi: 10.1038/ncpendmet0186. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Zangen DH, Reitz P, et al. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507–513. doi: 10.2337/diabetes.48.3.507. [DOI] [PubMed] [Google Scholar]
- 18.Rafaeloff R, Pittenger GL, Barlow SW, et al. Cloning and sequencing of the pancreatic islet neogenesis associated protein (INGAP) gene and its expression in islet neogenesis in hamsters. J.Clin.Invest. 1997;99:2100–2109. doi: 10.1172/JCI119383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, D'Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes A, King LC, Guz Y, Stein R, Wright CV, Teitelman G. Differentiation of new insulin-producing cells is induced by injury in adult pancreatic islets. Endocrinology. 1997;138:1750–1762. doi: 10.1210/endo.138.4.5049. [DOI] [PubMed] [Google Scholar]
- 22.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–3245. doi: 10.2337/db05-1034. [DOI] [PubMed] [Google Scholar]
- 23.Wang GS, Rosenberg L, Scott FW. Tubular complexes as a source for islet neogenesis in the pancreas of diabetes-prone BB rats. Lab Invest. 2005;85:675–688. doi: 10.1038/labinvest.3700259. [DOI] [PubMed] [Google Scholar]
- 24.Melmed RN, Benitez CJ, Holt SJ. Intermediate cells of the pancreas. I. Ultrastructural characterization. J.Cell Sci. 1972;11:449–475. doi: 10.1242/jcs.11.2.449. [DOI] [PubMed] [Google Scholar]
- 25.Bogdani M, Lefebvre V, Buelens N, et al. Formation of insulin-positive cells in implants of human pancreatic duct cell preparations from young donors. Diabetologia. 2003;46:830–838. doi: 10.1007/s00125-003-1118-4. [DOI] [PubMed] [Google Scholar]
- 26.Bonner-Weir S, Toschi E, Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr.Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 27.Rescan C, Le BS, Lefebvre VH, et al. EGF-induced proliferation of adult human pancreatic duct cells is mediated by the MEK/ERK cascade. Lab Invest. 2005;85:65–74. doi: 10.1038/labinvest.3700204. [DOI] [PubMed] [Google Scholar]
- 28.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54:2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 29.Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet beta-cells from pancreatic duct cells and an increase in functional beta-cell mass. J.Clin.Endocrinol.Metab. 2005;90:3401–3409. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 30.Yatoh S, Dodge R, Akashi T, et al. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland DE, Sibley R, Xu XZ, et al. Twin-to-twin pancreas transplantation: reversal and reenactment of the pathogenesis of type I diabetes. Trans.Assoc.Am.Physicians. 1984;97:80–87. [PubMed] [Google Scholar]
- 32.Tyden G, Reinholt FP, Sundkvist G, Bolinder J. Recurrence of autoimmune diabetes mellitus in recipients of cadaveric pancreatic grafts. N.Engl.J.Med. 1996;335:860–863. doi: 10.1056/NEJM199609193351205. [DOI] [PubMed] [Google Scholar]
- 33.Pugliese A, Allende G, Laughlin E, Dogra R, Ciancio G, Miller J, Nepom GT, Burke GW. Recurrence of autoantibodies and autoreactive T cells in patients with type 1 diabetes following pancreas transplantation. Diabetes. 2004;53:A69. Abstract. [Google Scholar]
- 34.Gianani R, Putnam A, Still T, et al. Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J.Clin.Endocrinol.Metab. 2006;91:1855–1861. doi: 10.1210/jc.2005-1171. [DOI] [PubMed] [Google Scholar]
- 35.Bingley PJ, Bonifacio E, Mueller PW. Diabetes antibody standardization program: first assay proficiency evaluation. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- 36.Reijonen H, Novak EJ, Kochik S, et al. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin E, Burke G, Pugliese A, Falk B, Nepom GT. Recurrence of Autoreactive Antigen-specific CD4+ T cells in Autoimmune Diabetes after Pancreas Transplantation. Clinical Immunology. 2008;128:23–30. doi: 10.1016/j.clim.2008.03.459. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reijonen H, Geubtner K, Allende G, Kwok W, Nepom G, Burke G, Pugliese A. Identification of islet-autoantigen specific CD4+ T-cells in the pancreatic lymph nodes and pancreas of a pancreas-kidney transplant patient with recurrence of autoimmunity. Diabetes. 2006;55:88A. Abstract. [Google Scholar]
- 39.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J.CellPhysiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Lukinius A, Stridsberg M, Wilander E. Cellular expression and specific intragranular localization of chromogranin A, chromogranin B, and synaptophysin during ontogeny of pancreatic islet cells: an ultrastructural study. Pancreas. 2003;27:38–46. doi: 10.1097/00006676-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 42.Gerrish K, Gannon M, Shih D, et al. Pancreatic beta cell-specific transcription of the pdx-1 gene. The role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J.Biol.Chem. 2000;275:3485–3492. doi: 10.1074/jbc.275.5.3485. [DOI] [PubMed] [Google Scholar]
- 43.Heimberg H, Bouwens L, Heremans Y, Van De CM, Lefebvre V, Pipeleers D. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes. 2000;49:571–579. doi: 10.2337/diabetes.49.4.571. [DOI] [PubMed] [Google Scholar]
- 44.Bouwens L, Pipeleers DG. Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia. 1998;41:629–633. doi: 10.1007/s001250050960. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka S, Kobayashi T, Nakanishi K, et al. Evidence of primary beta-cell destruction by T-cells and beta-cell differentiation from pancreatic ductal cells in diabetes associated with active autoimmune chronic pancreatitis. Diabetes Care. 2001;24:1661–1667. doi: 10.2337/diacare.24.9.1661. [DOI] [PubMed] [Google Scholar]
- 46.Phillips JM, O'Reilly L, Bland C, Foulis AK, Cooke A. Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes. 2007;56:634–640. doi: 10.2337/db06-0832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.