Abstract
Flow cytometry and confocal microscopy were used to study the effects of vancomycin, daptomycin, telavancin, and PA1409, a new investigational vancomyquine, on the morphology, membrane potential, and permeability of glycopeptide-susceptible and -resistant Enterococcus faecalis strains. Daptomycin exerted the most pronounced effects on bacterial size and bacterial permeability against susceptible and resistant strains.
Enterococci have emerged as major nosocomial pathogens and now represent the second cause of nosocomial bacteremia (15). Since the first reports in the mid-1980s, infections due to vancomycin-resistant enterococci still represent a therapeutic challenge, because glycopeptide resistance is often associated with multidrug resistance, supporting the need for developing new antibiotics. Recently, several lipopeptides, lipoglycopeptides, and glycopeptide derivatives exhibiting in vitro activity against vancomycin-resistant enterococci have been developed. Their precise mechanisms of action and effects on the bacterial cell wall remain incompletely understood. Daptomycin is a lipopeptide which acts by Ca2+-dependent insertion into the bacterial cytoplasmic membrane, oligomerization with formation of pores, disruption of the membrane functional integrity and membrane depolarization, and triggering of K+ ion leakage, leading to cell death (2). Telavancin, a lipoglycopeptide derivative of vancomycin, binds the d-Ala-d-Ala terminus of N-acetylglucosamine-N-muramylpentapeptide, resulting in inhibition of the transglycosylation and transpeptidation steps of peptidoglycan synthesis; furthermore, it interacts directly with the cell membrane and was shown to provoke rapid concentration-dependent dissipation of cell membrane potential through targeted interaction with the cell wall precursor lipid II in Staphylococcus aureus (7). PA1409 is an investigational vancomyquine obtained by covalent binding between vancomycin and a 4-aminoquinoline. This compound may induce major changes in bacterial membrane protonation (11). In this work, we used flow cytometry and confocal microscopy to analyze the effects of vancomycin, daptomycin, telavancin, and PA1409 on the morphology, membrane potential, and permeability of glycopeptide-susceptible and -resistant Enterococcus faecalis strains.
E. faecalis JH2-2 is susceptible to glycopeptides and β-lactams and is intrinsically resistant to low levels of aminoglycosides (8). E. faecalis BM4316 harbors a 50-kb vanA element that confers VanA-type resistance and was obtained by conjugal transfer from clinical isolate Enterococcus faecium HM1074 to JH2-2 (1). E. faecalis BM4275 harbors a 250-kb chromosomal vanB element conferring VanB-type resistance and was obtained by conjugal transfer of vancomycin resistance from clinical isolate E. faecium BM4120 to JH2-2 (9). All cultures and antibiotic susceptibility testing were performed in brain heart infusion (BHI) broth or agar (Difco Laboratories, Detroit, MI) at 37°C. For all experiments with daptomycin, BHI was supplemented with 50 μg/ml free Ca2+. Vancomycin, telavancin, and the vancomyquine PA1409 were provided by Palumed, Castanet-Tolosan, France. Daptomycin was provided by Novartis, Rueil-Malmaison, France. The MICs of the antibiotics were determined by the macrodilution method according to CLSI recommendations (5). The MIC was defined as the lowest drug concentration at which no growth occurred after 1 day of incubation. Bacterial killing was determined by enumeration on BHI agar before and after exposure to each antibiotic. The generation times of E. faecalis JH2-2, E. faecalis BM4316, and E. faecalis BM4275, determined by optical density growth curves, were 49.5 ± 1.33 (mean ± standard deviation), 49.8 ± 5.54, and 48.5 ± 2.44 min, respectively.
Flow cytometric measurements were performed on a Guava EasyCyte Plus flow cytometer (Millipore, St. Quentin en Yvelines, France) equipped with a 488-nm laser. Optical filters were set up such that red fluorescence was measured above 630 nm and green fluorescence was measured at 520 nm. A suspension of 105 CFU/ml exponentially growing E. faecalis JH2-2, E. faecalis BM4316, or E. faecalis BM4275 was incubated with each antibiotic. Two kinds of experiments were performed at different antibiotic concentrations. For size and membrane potential analyses, bacteria were incubated with each antibiotic at 0.25× and 1× MIC, since for concentrations higher than the MICs, the bactericidal effect by itself may induce nonspecific changes in these parameters; the duration of incubation was 90 min for size analysis and 60 min for membrane potential analysis. For membrane permeability and bacterial killing analyses, bacteria were incubated with each antibiotic at a concentration of 4× MIC for 180 min. Control samples were incubated without antibiotic. For flow cytometric measurements, bacteria were then diluted to a cell density of ca. 104 CFU/ml in phosphate-buffered saline. For size determination, cells were collected on the forward scatter with logarithmic amplifiers for 5,000 events. For membrane potential analysis, cells were stained beforehand (20 min at 37°C in the dark) with a green-fluorescing lipophilic cationic dye, the 3,3′-dihexyloxacarbocyanine iodide DiOC6 (Molecular Probes, Invitrogen, Cergy-Pontoise, France) (12) at a concentration of 0.1 μmol/liter. Since membrane potential varies according to the cell size (13, 14), data were expressed as the ratio of membrane potential according to the size. For permeability determination, cells were stained by using a Live/Dead BacLight kit (Molecular Probes, Invitrogen, Cergy-Pontoise, France) (3). The kit consists of two stains, propidium iodide (PI) and SYTO9, which both stain nucleic acids. The green-fluorescing SYTO9 is able to enter all cells when used alone, whereas the red-fluorescing PI only enters cells with damaged cytoplasmic membranes. The appropriate mixture of the SYTO9 and PI stains enables differentiation between live bacteria with intact cytoplasmic membranes and dead bacteria with permeabilized cytoplasmic membranes. The morphology of the bacteria was observed after staining with the BacLight Live/Dead kit on a Zeiss LSM 510 confocal fluorescence microscope with a 63× objective. The digital images were processed with the LSM Image Browser software (www.zeiss.de/ImageBrowser). Comparisons of bacterial size and membrane potential variations between several antibiotic study groups were performed by using the Kruskal-Wallis test, followed, when significant, by the Mann-Whitney test for comparisons between two groups. The relationship between membrane permeability and decrease in bacterial counts was tested by linear regression analysis. A P value of <0.05 was considered significant.
The MICs of the four antibiotics against vancomycin-susceptible E. faecalis JH2-2, VanA-type E. faecalis BM4316, and VanB-type E. faecalis BM4275 were as follows: vancomycin, 4, 512, and 256 μg/ml, respectively; daptomycin, 2 μg/ml for all three strains; telavancin 1, 4, and 1 μg/ml, respectively; and PA1409, 0.5, 4, and 2 μg/ml, respectively.
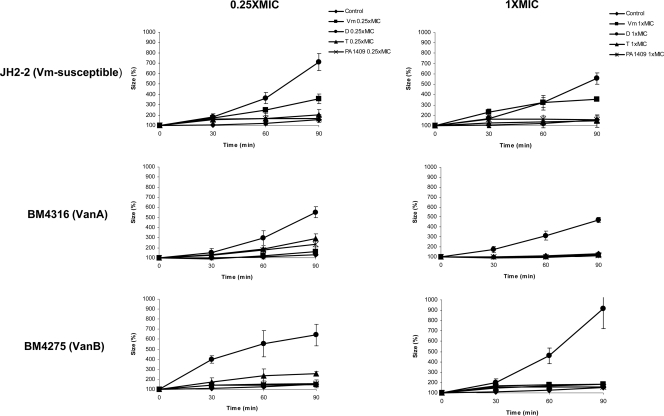
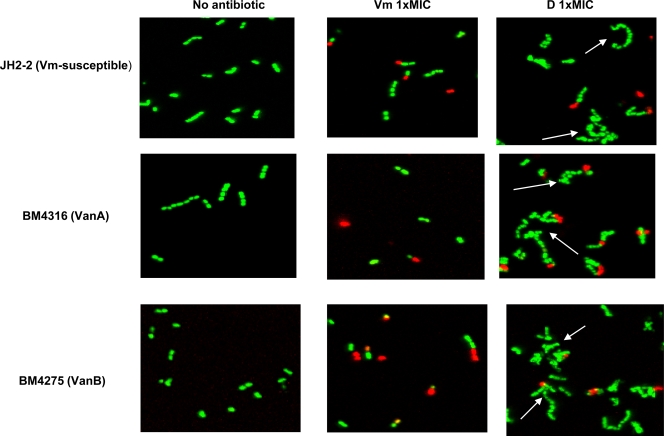
Figure 1 shows the effects on the bacterial size of exposure to 0.25× and 1× MIC of antibiotics. Daptomycin significantly increased the cell size of the three strains at both concentrations after 30, 60, and 90 min of incubation (P = 0.02 versus results for control). Vancomycin at 0.25× and 1× MIC only increased the size of susceptible E. faecalis JH2-2 (P = 0.02 versus results for control), but the increase in size at 0.25× MIC was significantly more pronounced for daptomycin than for vancomycin (P = 0.04). Telavancin and PA1409 had only slight effects on bacterial size, although the size effect of telavancin on VanB-type BM4275 at 0.25× MIC was statistically significant (P = 0.03 versus results for control). Confocal microscopy analysis of bacterial morphology after 60 min of exposure to 1× MIC of daptomycin showed the presence of large elements corresponding to dysmorphic bacterial chains for E. faecalis JH2-2, E. faecalis BM4316, and E. faecalis BM4275 (Fig. 2). The morphological aspect of these elements was different from the bacterial chains that can be observed during bacterial growth, which do not contain dysmorphic elements. Moreover, CFU counts did not increase after 30, 60, and 90 min of exposure to 1× MIC of daptomycin (data not shown), indicating that, as expected, bacterial growth was inhibited at this concentration. Other authors have also observed altered cell morphology after exposure of Gram-positive cocci to daptomycin. McGrath et al. recently analyzed the ultrastructural changes associated with daptomycin resistance in a vancomycin-resistant E. faecalis isolate obtained from a patient treated with daptomycin. This daptomycin-resistant E. faecalis isolate had marked structural changes in the cell envelope, with alterations in cell division and increased cell wall thickness. The cells tended to clump and form aggregates with longer chains, and the percentage of cell divisions was increased (10). Cotroneo et al. observed that after exposure of Staphylococcus aureus ATCC 29213 to 8× MIC of daptomycin for 60 min, >90% of cells displayed altered cell wall morphology consistent with aberrant division septa (6). The morphology of bacteria incubated for 60 min in the presence of 1× MIC of vancomycin (Fig. 2), telavancin, or PA1409 (data not shown) was not modified. There were no statistically significant differences between the antibiotics tested in terms of membrane potential/size ratio of bacteria incubated in the presence of 0.25× and 1× MIC of each antibiotic (data not shown).
FIG. 1.
Flow-cytometric analysis of the bacterial size of vancomycin-susceptible E. faecalis JH2-2, VanA-type E. faecalis BM4316, or VanB-type E. faecalis BM4275 30, 60, and 90 min after exposure to 0.25× or 1× MIC of vancomycin (Vm), daptomycin (D), telavancin (T), or PA1409. Cells were collected on the forward scatter with logarithmic amplifiers for 5,000 events. Each experiment was performed in triplicate, and data are expressed as mean (± standard deviation) rates of increase (%) of bacterial size.
FIG. 2.
Confocal microscopy analysis of the bacterial morphology and membrane permeability of vancomycin-susceptible E. faecalis JH2-2, VanA-type E. faecalis BM4316, or VanB-type E. faecalis BM4275. Cells were incubated for 1 h in the presence of 1× MIC of vancomycin (Vm) or daptomycin (D) or without antibiotic and stained by using a BacLight Live/Dead kit. Green-fluorescent bacteria have an intact membrane; red-fluorescent bacteria have a permeabilized membrane. Dysmorphic bacterial chains (arrows) were only observed after incubation in the presence of daptomycin.
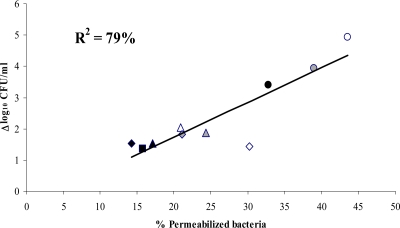
All bacteria grown in the absence of antibiotics fluoresced green after staining with the BacLight Live/Dead kit, indicating that they were alive (Fig. 2). Exposure to each of the four antibiotics increased the percentage of permeabilized bacteria, which fluoresced red (Fig. 2 and data not shown). Figure 3 shows the percentage of permeabilized bacteria and the decrease in bacterial cell counts after 180 min of exposure to 4× MIC of each antibiotic. The higher percentage of permeabilized bacteria was observed after exposure to daptomycin, as was the decrease in bacterial cell counts after the same antibiotic exposure. As shown in Fig. 3, a relationship between the decrease in bacterial counts and the percentage of permeabilized bacteria was evidenced. The proportion of permeabilized bacteria accounted for 79% of the observed variance estimated by the R2 value, suggesting that the bacterial killing provoked by cell wall-active antibiotics may be easily and rapidly estimated by flow cytometry.
FIG. 3.
Relationship between the decrease in bacterial counts (expressed as Δlog10 CFU/ml) and the rates of permeabilization of bacteria after exposure of vancomycin-susceptible E. faecalis JH2-2 (black symbols), VanA-type E. faecalis BM4316 (white symbols), or VanB-type E. faecalis BM4275 (gray symbols) to vancomycin (squares), daptomycin (circles), telavancin (triangles), or PA1409 (diamonds) at 4× MIC for 180 min. Permeabilization was studied by flow cytometry after staining using a BacLight Live/Dead kit. Bacterial counts were determined by enumeration of surviving bacteria on BHI agar. Each experiment was performed 4 times, and data are expressed as the mean (± standard deviation) percentages (%) of permeabilized bacteria and the mean (± standard deviation) difference between log10 CFU/ml counts obtained with and without antibiotic exposure.
In conclusion, daptomycin exerted the most pronounced effect on bacterial size and bacterial permeability and was rapidly bactericidal against vancomycin-susceptible and -resistant E. faecalis. These effects are in accordance with its mechanism of action, which consists of insertion into the cell membrane and formation of pores (2), and with previous data showing a more pronounced and rapid bactericidal effect than that of vancomycin against vancomycin-susceptible and -resistant enterococci (4). The effects of telavancin and PA1409 on the bacterial membrane were comparable to those of vancomycin. Flow cytometry appears to be a useful tool to analyze the physiological and morphological response of E. faecalis strains to cell wall-active antibiotics.
Acknowledgments
We thank Murielle Sanchez and Bernard Meunier, Palumed, Castanet-Tolosan, France, for providing vancomycin, telavancin, and PA1409.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Arthur, M., F. Depardieu, G. Gerbaud, M. Galimand, R. Leclercq, and P. Courvalin. 1997. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J. Bacteriol. 179:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltz, R. H. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13:144-151. [DOI] [PubMed] [Google Scholar]
- 3.Berney, M., F. Hammes, F. Bosshard, H. U. Weilenmann, and T. Egli. 2007. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 73:3283-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brauers, J., M. Kresken, A. Menke, A. Orland, H. Weiher, and I. Morrissey. 2007. Bactericidal activity of daptomycin, vancomycin, teicoplanin and linezolid against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium using human peak free serum drug concentrations. Int. J. Antimicrob. Agents 29:322-325. [DOI] [PubMed] [Google Scholar]
- 5.CLSI. 2009. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Cotroneo, N., R. Harris, N. Perlmutter, T. Beveridge, and J. A. Silverman. 2008. Daptomycin exerts bactericidal activity without lysis of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2223-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefort, A., M. Baptista, B. Fantin, F. Depardieu, M. Arthur, C. Carbon, and P. Courvalin. 1999. Two-step acquisition of resistance to the teicoplanin-gentamicin combination by VanB-type Enterococcus faecalis in vitro and in experimental endocarditis. Antimicrob. Agents Chemother. 43:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath, D., E. M. Barbu, R. Pasqualini, W. Arap, J. P. Quinn, B. E. Murray, and C. Arias. 2010. Resistance to daptomycin in vancomycin-resistant Enterococcus faecalis is associated with alterations of cell membrane potential and surface charge, abstr. C1-079. Abstr. 50th Annu. Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 11.Meunier, B., M. Sanchez, C. Duval, J. Cazelles, R. Leclercq, and C. Rocques. 2009. Vancomyquine PA1409: a new hybrid antibacterial molecule, abstr. F1-2031. 49th Annu. Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 12.Monfort, P., and B. Baleux. 1996. Cell cycle characteristics and changes in membrane potential during growth of Escherichia coli as determined by a cyanine fluorescent dye and flow cytometry. J. Microbiol. Methods 25:79-86. [Google Scholar]
- 13.Novo, D., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55-63. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro, H. M. 2008. Flow cytometry of bacterial membrane potential and permeability. Methods Mol. Med. 142:175-186. [DOI] [PubMed] [Google Scholar]
- 15.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]