Abstract
The aim of this study was to evaluate the plasma and intracellular pharmacokinetics of raltegravir in HIV-infected patients receiving once-daily raltegravir. Five HIV-infected patients on stable therapy with lopinavir-ritonavir monotherapy whose HIV-1 RNA load was <50 copies/ml were included in this open-label, pilot study. Raltegravir was added to the antiretroviral regimen at a dose of 800 mg once daily from days 0 to 10. On day 10, a full pharmacokinetic profile was obtained for each participant. Raltegravir concentrations in plasma and peripheral blood mononuclear cells (PBMCs) were determined by high-performance liquid chromatography with a fluorescence detector and by liquid chromatography-tandem mass spectrometry (LC-MS/MS), respectively. The values of the raltegravir pharmacokinetic parameters in plasma and PBMCs were calculated by noncompartmental analysis. Raltegravir was well tolerated, and all participants completed the study. No differences in the times to the maximum concentration of raltegravir in plasma or the raltegravir half-lives were observed between plasma and PBMCs. The geometric mean raltegravir maximum concentration, the concentration at the end of the dosing interval, and the area under the concentration-time curve during the dose interval in plasma versus PBMCs were 2,640 ng/ml (range, 887 to 10,605 ng/ml) versus 199 ng/ml (range, 82 to 857 ng/ml) (geometric mean ratio [GMR], 13.30; 95% confidence interval [CI], 3.11 to 56.89; P = 0.003); 89 ng/ml (range, 51 to 200 ng/ml) versus 7 ng/ml (range, 2 to 15 ng/ml) (GMR, 13.21; 95% CI, 3.94 to 44.26; P = 0.001); and 12,200 ng·h/ml (range, 5,152 to 30,130 ng·h/ml) versus 909 ng·h/ml (range, 499 to 2,189 ng·h/ml) (GMR, 13.43; 95% CI, 5.13 to 35.16; P < 0.001), respectively. Raltegravir does not accumulate in PBMCs, with intracellular concentrations being about 1/10 of the concentrations in plasma. Despite once-daily dosing, mean raltegravir concentrations at the end of the dosing interval in plasma and PBMCs exceeded the reported protein-binding-adjusted 95% inhibitory concentration (IC95) and IC50 for wild-type viral strains, respectively.
The armamentarium for the treatment of HIV infection has recently increased by the addition of new antiretroviral drugs, with some of them belonging to new families targeting new steps in the virus life cycle.
Raltegravir is the first integrase approved for the treatment of HIV-infected patients (2, 6, 7, 16). It has shown extremely high antiretroviral potency and efficacy in both extensively pretreated and antiretroviral-naïve HIV-infected patients (5, 9-12, 19), with rates of adverse events being close to those observed with placebo (5, 9, 11). Consequently, just a few months after its approval, raltegravir is currently considered among the preferred options for the initial treatment for HIV-infected patients (16).
Antiretroviral therapy with raltegravir in antiretroviral-naïve HIV-infected patients is followed by a steep decline in the HIV-1 RNA level in plasma, with the time to achieve undetectable levels of viral load being shorter than the times for other antiretrovirals (9, 12, 14). However, no clear relationship between the magnitude of the decrease in viral load and raltegravir concentrations in plasma was identified in dose-finding studies in which HIV-infected patients were administered raltegravir at dosages of 100, 200, 400, or 600 mg twice daily, despite raltegravir exposure increasing with dose up to 400 mg twice daily (10, 12). Although 400 mg twice daily was selected for further drug development and was approved by the regulatory authorities, the lack of a pharmacokinetic/pharmacodynamic (PK/PD) relationship and the efficacy data from phase II and III trials have raised interest in once- instead of twice-daily dosing of raltegravir.
Different hypotheses to explain the lack of a PK/PD relationship for raltegravir have been proposed, including speculation that the response might be better linked to intracellular rather than plasma raltegravir concentrations. In addition, raltegravir accumulation within peripheral blood mononuclear cells (PBMCs) might contribute to the persistence of its antiviral effect (postantibiotic effect), despite the drug being removed from the extracellular medium, as observed in some in vitro experiments (12a). Nevertheless, there is little information about raltegravir intracellular pharmacokinetics, especially when it is dosed once instead of twice daily.
Our objective was, therefore, to evaluate the plasma and intracellular pharmacokinetics of raltegravir in HIV-infected patients receiving raltegravir at 800 mg once daily.
MATERIALS AND METHODS
Study design.
This was an open-label, pilot study with five HIV-infected patients attending the HIV clinic at the Hospital Universitari Germans Trias i Pujol in Badalona, Spain, whose HIV-1 RNA load was <50 copies/ml and who were receiving antiretroviral monotherapy with lopinavir-ritonavir at 400/100 mg twice-daily for at least 4 weeks prior to their inclusion.
After enrollment, raltegravir was added to the antiretroviral regimen at a dosage of 800 mg once daily from days 0 to 10. Raltegravir was administered in the morning, without regard to food intake, except on day 10, when it was administered in the fasted state.
Demographic and clinical variables and the drugs concomitantly used were recorded. Safety was evaluated by clinical evaluation and laboratory assessment (blood counts and chemistry) on days 0 and 10. In addition, serial blood samples were collected at 0, 1, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, and 24 h postdosing on day 10.
The trial was performed according to the stipulations of the Declaration of Helsinki, the protocol was approved by the Ethics Committee of the Hospital Universitari Germans Trias i Pujol, and patients gave informed consent before enrollment (Clinicaltrials.gov NCT00995241).
Sample treatment.
Blood samples for the measurement of raltegravir concentrations were collected and placed into potassium- and EDTA-containing 10-ml tubes, and the tubes were processed within 1 h after collection and kept at 4°C. Samples were first centrifuged at 390 × g for 10 min. The supernatant was then transferred to 10-ml tubes and centrifuged at 1,090 × g for 10 min, and the isolated plasma was stored at −80°C until analysis. The remaining cellular part was diluted with 0.9% NaCl at 4°C up to a total volume of 10 ml. Subsequently, it was transferred to a 50-ml conical tube containing 5 ml of Ficoll gradient (Lymphoprep) at 4°C, and it was centrifuged at 720 × g for 30 min. Thereafter, the PBMC layer was washed twice using 0.9% NaCl solution at 4°C, and the final pellet was stored at −80°C for further analysis.
Raltegravir determinations.
Raltegravir concentrations in plasma were determined by high-performance liquid chromatography (HPLC) with a fluorescence detector (Multifluorescence detector 2475; Waters), according to the method described by Poirier et al. (17). Briefly, chromatographic separation was performed on a Sunfire C18 column (particle size, 5 μm; 4.6 by 150 mm; Waters). The mobile phase was phosphate buffer-acetonitrile (25 mM, pH 3). The fluorescence detector was set at 299 and 396 nm for excitation and emission wavelengths, respectively. The drug was extracted from plasma by liquid-liquid extraction with tert-butyl methyl ether. The method was linear over the range from 10 to 5,000 ng/ml. The intra- and interday coefficients of variation were less than 10%.
Raltegravir concentrations in PBMCs were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS), substantially by the method described by Else et al. (3). The assay was validated according to FDA guidelines, with specific attention given to matrix effects. Calibration standards and quality controls were prepared in blank human PBMCs. Calibration curves were prepared over the range from 0.4 to 150 ng/ml of lysate. The intra- and interday coefficients of variation were less than 10%. The number of PBMCs in each clinical sample was determined using a DNA detection procedure which was substantially based on the method of Benech et al. (1).
Pharmacokinetic analysis.
The values of the pharmacokinetic parameters for raltegravir in plasma and PBMCs were calculated for each individual using a noncompartmental approach by means of WinNonlin software (version 2.0; Pharsight, Mountain View, CA). The area under the concentration-time curve during the dose interval (AUCτ) was calculated by means of the linear trapezoidal rule. Concentrations immediately before dosing (C0s), maximum concentrations (Cmaxs), times to Cmaxs (Tmaxs), and the concentrations at the end of the dosing interval (Cτ) were obtained by inspection of the concentration data. The terminal-phase elimination rate constant (λz) was obtained by linear regression analysis of the terminal log-linear portion of the concentration-time curve. The intracellular distribution ratio was calculated as the ratio between the raltegravir concentrations in PBMCs and plasma.
Statistical analysis.
Statistical analysis was carried out using SPSS (version 15.0) statistical software (Chicago, IL). Raltegravir pharmacokinetic parameters were described using the geometric mean (range). The correlation between raltegravir concentrations in plasma and PBMCs was evaluated by means of the Pearson's correlation coefficient. Raltegravir C0s and Cτs were compared using the Wilcoxon nonparametric test. Comparisons between raltegravir pharmacokinetic parameters in plasma and PBMCs were performed using the geometric mean ratio (GMR) and the 95% confidence interval (CI).
RESULTS
Five Caucasian male patients were included in the study. Raltegravir was well tolerated, and all participants completed the study. The median age was 40 years (range, 24 to 47 years), and the median body mass index was 24.4 kg/m2 (range, 18.2 to 25.5 kg/m2). According to the inclusion criteria, all patients were on antiretroviral treatment with lopinavir-ritonavir monotherapy and had an HIV-1 RNA load of <50 copies/ml. The median CD4+ T cell count was 711 cells/mm3 (range, 444 to 862 cells/mm3). A median of 10.6 million PBMCs (range, 0.9 to 16.5 million) was isolated from blood samples at each time point.
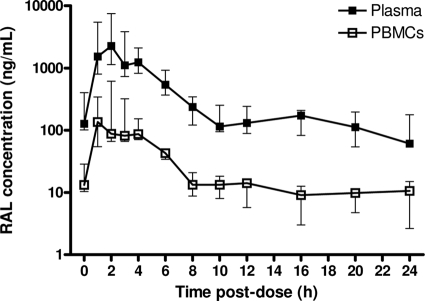
Figure 1 shows the raltegravir concentrations in plasma and PBMCs over the dosing interval. There was large interpatient variability in raltegravir concentrations in both plasma and PBMCs, particularly during the absorption phase. Raltegravir Cmaxs were observed both in plasma and in PBMCs 2 h after drug intake, with a secondary increase occurring 4 h after intake in two patients. Peak concentrations were followed by a biphasic decline over the dosing interval. There were no statistically significant differences between the raltegravir concentrations immediately before and 24 h after dosing in either plasma or PBMCs (for C0 versus Cτ, P = 0.080 and P = 0.225, respectively). Overall, raltegravir concentrations in plasma and PBMCs were well correlated (r = 0.977, P < 0.001).
FIG. 1.
Raltegravir time-concentration curve in plasma and PBMCs. Data are represented as medians and interquartile ranges. RAL, raltegravir.
For the pharmacokinetic parameters for raltegravir, the geometric mean AUCτs were 12,200 ng·h/ml (range, 5,152 to 30,130 ng·h/ml) in plasma and 909 ng·h/ml (range, 499 to 2,189 ng·h/ml) in PBMCs (GMR, 13.43; 95% CI, 5.13 to 35.16; P < 0.001). The maximum concentrations were 2,640 ng/ml (range, 887 to 10,605 ng/ml) in plasma and 199 ng/ml (range, 82 to 857 ng/ml) in PBMCs (GMR, 13.30; 95% CI, 3.11 to 56.89; P = 0.003). Cτs were 89 ng/ml (range, 51 to 200 ng/ml) in plasma and 7 ng/ml (range, 2 to 15 ng/ml) in PBMCs (GMR, 13.21; 95% CI, 3.94 to 44.26; P = 0.001). There were no differences in raltegravir terminal-phase half-lives between plasma and PBMCs (16.0 and 11.2 h, respectively; P = 0.164). The median raltegravir intracellular distribution ratios were 0.07 (range, 0.05 to 0.10) for AUCτ, 0.08 (range, 0.06 to 0.09) for Cmax, and 0.18 (range, 0.01 to 0.25) for Cτ.
DISCUSSION
Results from this study show that raltegravir does not accumulate within PBMCs. Intracellular and plasma concentrations of raltegravir were well correlated, with the concentrations in PBMCs being less than 1/10 of the concentrations in plasma.
The plasma raltegravir pharmacokinetic profile was consistent with that described by others (8, 10). Raltegravir was rapidly absorbed, and drug concentrations after Cmax decreased in a biphasic manner, with an initial rapid decay occurring during the first 8 h after dosing and a slower decline occurring thereafter. Of note, despite once- instead of twice-daily dosing, the mean raltegravir Cτs in plasma and PBMCs exceeded the reported protein-binding-adjusted 95% inhibitory concentration (IC95) and IC50 for wild-type viral strains, respectively (20). The findings support the possibility of once- instead of twice-daily dosing for raltegravir for HIV-infected patients with no integrase inhibitor resistance-associated mutations, and a randomized clinical trial evaluating the efficacy of this dosing regimen in antiretroviral-naïve HIV-infected patients is currently ongoing.
Cell-associated drug concentrations are critically dependent on different factors, including the total volume of whole blood collected, the method used for PBMC isolation (Ficoll density gradient versus cell preparation tubes) (15, 18), and the skill level of the laboratory personnel in harvesting cells from the Ficoll gradient, among others. As a result, different data may appear until there is greater standardization in sample treatment. Recently, ter Heine et al. (21) reported that raltegravir could not be detected in cells, which is clearly different from our findings. However, the intracellular distribution ratio of raltegravir in our study is in agreement with that observed by Fayet-Mello et al. (4), who also reported raltegravir concentrations in PBMCs about 1/10 of the concentrations in plasma. Moreover, this ratio is also in agreement with the free fraction of raltegravir in plasma previously described (2). Although raltegravir has been shown to be a substrate for influx transporters organic iron transporter 1 (OAT1) and peptide transporter 1 (PEPT1) (13), these proteins are not expressed in PBMCs, and therefore, our results suggest that raltegravir enters PBMCs mainly through simple diffusion. The physicochemical characteristics of raltegravir (slight lipophilicity and low molecular weight) could also support passive diffusion from serum to PBMCs. In the same way, despite raltegravir being a substrate for P-glycoprotein (P-gp) in in vitro cell models (13), the lack of intracellular accumulation of raltegravir in the present study, in which P-gp activity was inhibited by administration of lopinavir-ritonavir, suggests that raltegravir is a minor substrate for this efflux transporter, which has a limited impact on the intracellular pharmacology of raltegravir in vivo.
One conclusion that can be drawn from the low intracellular accumulation of raltegravir is that its antiretroviral efficacy seems to be more related to its high intrinsic antiretroviral potency rather that to its distribution in PBMCs. Once it is bound to the preintegration complex, raltegravir blocks the integration step and viral replication in a functionally irreversible manner (12a). This may result in maximal inhibition of viral replication even at low raltegravir concentrations. Consequently, the absence of a PK/PD relationship for raltegravir could simply be explained by the fact that raltegravir concentrations over the different doses tested in dose-finding studies were far above the concentrations needed to provide full suppression of viral replication (maximum effect).
In summary, this study found no evidence that raltegravir accumulates in PBMCs. Despite once-daily instead of twice-daily dosing, mean raltegravir concentrations at the end of the dosing interval in plasma and PBMCs exceeded the reported protein-binding-adjusted IC95 and IC50 for wild-type viral strains, respectively, suggesting the possibility of once-daily dosing for raltegravir in antiretroviral-naïve HIV-infected patients.
Acknowledgments
We thank the patients and their caregivers for their participation in this study.
This study was funded by the Lluita contra la SIDA Foundation and by ISCIII-RETIC RD06/006. The research leading to these results has also received funding from Merck Sharp & Dohme Laboratories and from the European Community's Seventh Framework Programme (FP7/2007-2013) under the project Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN), grant agreement number 223131. M.V. is supported by FIS through grant CP04/00121 from the Spanish Health Department, in collaboration with the research institute of the Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. M.V. and M.J.B. are members of CIBERSAM.
We declare that we have no competing interests.
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Benech, H., F. Theodoro, A. Herbet, N. Page, D. Schlemmer, A. Pruvost, J. Grassi, and J. R. Deverre. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172-174. [DOI] [PubMed] [Google Scholar]
- 2.Croxtall, J. D., K. A. Lyseng-Williamson, and C. M. Perry. 2008. Raltegravir. Drugs 68:131-138. [DOI] [PubMed] [Google Scholar]
- 3.Else, L., V. Watson, J. Tjia, A. Hughes, M. Siccardi, S. Khoo, and D. Back. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878:1455-1465. [DOI] [PubMed]
- 4.Fayet-Mello, A., T. Buclin, C. Franc, S. Colombo, S. Cruchon, N. Guignard, J. Biollaz, A. Telenti, L. Decosterd, and M. Cavassini. 2010. Intracellular and plasma pharmacokinetics of raltegravir in HIV-infected patients, abstr. 614. Abstr. 17th Conf. Retrovir. Opportunistic Infect.
- 5.Grinsztejn, B., B. Y. Nguyen, C. Katlama, J. M. Gatell, A. Lazzarin, D. Vittecoq, C. J. Gonzalez, J. Chen, C. M. Harvey, and R. D. Isaacs. 2007. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet 369:1261-1269. [DOI] [PubMed] [Google Scholar]
- 6.Hazuda, D. J., P. Felock, M. Witmer, A. Wolfe, K. Stillmock, J. A. Grobler, A. Espeseth, L. Gabryelski, W. Schleif, C. Blau, and M. D. Miller. 2000. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287:646-650. [DOI] [PubMed] [Google Scholar]
- 7.Hicks, C., and R. M. Gulick. 2009. Raltegravir: the first HIV type 1 integrase inhibitor. Clin. Infect. Dis. 48:931-939. [DOI] [PubMed] [Google Scholar]
- 8.Iwamoto, M., L. A. Wenning, A. S. Petry, M. Laethem, M. De Smet, J. T. Kost, S. A. Merschman, K. M. Strohmaier, S. Ramael, K. C. Lasseter, J. A. Stone, K. M. Gottesdiener, and J. A. Wagner. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293-299. [DOI] [PubMed] [Google Scholar]
- 9.Lennox, J. L., E. Dejesus, D. S. Berger, A. Lazzarin, R. B. Pollard, J. V. Ramalho Madruga, J. Zhao, H. Wan, C. L. Gilbert, H. Teppler, A. J. Rodgers, R. J. Barnard, M. D. Miller, M. J. Dinubile, B. Y. Nguyen, R. Leavitt, and P. Sklar. 2010. Raltegravir versus efavirenz regimens in treatment-naive HIV-1-infected Patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J. Acquir. Immune Defic. Syndr. 55:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markowitz, M., J. O. Morales-Ramirez, B. Y. Nguyen, C. M. Kovacs, R. T. Steigbigel, D. A. Cooper, R. Liporace, R. Schwartz, R. Isaacs, L. R. Gilde, L. Wenning, J. Zhao, and H. Teppler. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir. Immune Defic. Syndr. 43:509-515. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz, M., B. Y. Nguyen, E. Gotuzzo, F. Mendo, W. Ratanasuwan, C. Kovacs, G. Prada, J. O. Morales-Ramirez, C. S. Crumpacker, R. D. Isaacs, H. Campbell, K. M. Strohmaier, H. Wan, R. M. Danovich, and H. Teppler. 2009. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J. Acquir. Immune Defic. Syndr. 52:350-356. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz, M., B. Y. Nguyen, E. Gotuzzo, F. Mendo, W. Ratanasuwan, C. Kovacs, G. Prada, J. O. Morales-Ramirez, C. S. Crumpacker, R. D. Isaacs, L. R. Gilde, H. Wan, M. D. Miller, L. A. Wenning, and H. Teppler. 2007. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46:125-133. [DOI] [PubMed] [Google Scholar]
- 12a.Miller, M., et al. 2008. Analysis of resistance to the HIV-1 integrase inhibitor raltegravir: results from the Benchmark 1 and 2, abstr. H-898. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 13.Moss, D., W. S. Kwan, N. Liptrott, M. Siccardi, D. Anstee, S. Khoo, D. Back, and A. Owen. 2010. Raltegravir is a substrate for the influx transporters OAT1 and PEPT1 and the efflux transporter Pgp but it is not transported by OATP1A2, OATP1B1, OATP1B3, OCT1, NTCO, or PEPT2, abstr. 613. Abstr. 17th Conf. Retrovir. Opportunistic Infect.
- 14.Murray, J. M., S. Emery, A. D. Kelleher, M. Law, J. Chen, D. J. Hazuda, B. Y. Nguyen, H. Teppler, and D. A. Cooper. 2007. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21:2315-2321. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson, C., S. Aboud, K. Karlen, B. Hejdeman, W. Urassa, and G. Biberfeld. 2008. Optimal blood mononuclear cell isolation procedures for gamma interferon enzyme-linked immunospot testing of healthy Swedish and Tanzanian subjects. Clin. Vaccine Immunol. 15:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 17.Poirier, J. M., P. Robidou, and P. Jaillon. 2008. Quantification of the HIV-integrase inhibitor raltegravir (MK-0518) in human plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 867:277-281. [DOI] [PubMed] [Google Scholar]
- 18.Ruitenberg, J. J., C. B. Mulder, V. C. Maino, A. L. Landay, and S. A. Ghanekar. 2006. VACUTAINER CPT and Ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steigbigel, R. T., D. A. Cooper, P. N. Kumar, J. E. Eron, M. Schechter, M. Markowitz, M. R. Loutfy, J. L. Lennox, J. M. Gatell, J. K. Rockstroh, C. Katlama, P. Yeni, A. Lazzarin, B. Clotet, J. Zhao, J. Chen, D. M. Ryan, R. R. Rhodes, J. A. Killar, L. R. Gilde, K. M. Strohmaier, A. R. Meibohm, M. D. Miller, D. J. Hazuda, M. L. Nessly, M. J. DiNubile, R. D. Isaacs, B. Y. Nguyen, and H. Teppler. 2008. Raltegravir with optimized background therapy for resistant HIV-1 infection. N. Engl. J. Med. 359:339-354. [DOI] [PubMed] [Google Scholar]
- 20.Summa, V., A. Petrocchi, F. Bonelli, B. Crescenzi, M. Donghi, M. Ferrara, F. Fiore, C. Gardelli, O. Gonzalez Paz, D. J. Hazuda, P. Jones, O. Kinzel, R. Laufer, E. Monteagudo, E. Muraglia, E. Nizi, F. Orvieto, P. Pace, G. Pescatore, R. Scarpelli, K. Stillmock, M. V. Witmer, and M. Rowley. 2008. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 51:5843-5855. [DOI] [PubMed] [Google Scholar]
- 21.ter Heine, R., J. W. Mulder, E. C. M. van Gorp, J. F. P. Wagenaar, J. H. Beijnen, and A. D. R. Huitema. 2010. Intracellular and plasma steady-state pharmacokinetics of raltegravir, darunavir, etravirine and ritonavir in heavily pre-treated HIV-infected patients Br. J. Clin. Pharmacol. 69:475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]