Abstract
Continuous infusion of vancomycin was evaluated against experimental endocarditis due to heterogeneous vancomycin-intermediate Staphylococcus aureus (hVISA) and VISA. Animals were infected with hVISA PC1 (vancomycin MIC, 2 mg/liter) or VISA PC3 (vancomycin MIC, 8 mg/liter) and treated for 5 days with constant serum levels of 20 or 40 mg/liter. Vancomycin continuous infusion was unsuccessful, as 20 mg/liter was barely active against PC1 (6 of 13 sterile vegetations) and 40 mg/liter failed against PC3 (2 of 9 sterile vegetations).
Vancomycin is still considered the agent of choice against infections caused by methicillin-resistant Staphylococcus aureus (MRSA). Treatment failures associated with the emergence of MRSA strains expressing reduced susceptibility to vancomycin, known as vancomycin-intermediate S. aureus (VISA) or heterogeneous VISA (hVISA), are of potential concern (6). Moreover, therapeutic failures have been observed in the treatment of infections due to MRSA with vancomycin MICs approaching 2 mg/liter (9, 15). To enhance the performance of vancomycin against such infections, some guidelines have recommended targeting trough levels of the drug ranging between 15 and 20 mg/liter (13). However, recent studies reported that even trough levels exceeding 15 mg/liter did not improve treatment efficacy against MRSA with a borderline vancomycin MIC of 2 mg/liter (16).
Continuous infusion may be the best approach to maximize the time-dependent activity of vancomycin, since serum levels of the drug can be maintained at some multiples of the MIC for the infecting organism during the entire dose interval (11, 12). Most isolates of hVISA and VISA have vancomycin MICs of 2 to 8 mg/liter, and vancomycin levels four to five times above the MIC can be reached in serum during continuous infusion of the drug.
Here we tested whether such an approach might be more efficacious than standard intermittent vancomycin administration against experimental endocarditis due to either of the well-described clinical isolates hVISA PC1 and VISA PC3 (14). The vancomycin-susceptible MRSA 217 clinical isolate was included as a control strain. MICs of vancomycin were determined by broth macrodilution according to the CLSI guidelines. The bactericidal activities of vancomycin were measured by time-kill curves with an inoculum of 107 CFU/ml and antibiotic concentrations similar to levels attained in rats during therapy.
Animal experiments were carried out according to Swiss regulations. The production of catheter-induced aortic vegetations and the installation of the infusion pump device for the delivery of vancomycin were performed as described previously (4, 5). Valve infection was induced 24 h after catheterization by intravenous (i.v.) challenge of the animals with 106 CFU of the test isolates. Groups of rats were treated with vancomycin by mimicking human treatment with either 1 g of the drug i.v. given every 12 h or by continuous infusion ensuring constant serum concentrations of 20 or 40 mg/liter. These concentrations were chosen because they maintained serum levels of at least four times the MIC for the infecting organisms and were considered desirable concentrations for treatment of patients with MRSA or VISA infections (8, 11). Therapy was started 12 h after bacterial challenge and lasted for 5 days. Each experiment included a control group of untreated animals. Control rats were killed at the onset of treatment, and treated rats were killed 8 h after the end of the last antibiotic dose. Aortic vegetations were removed and processed for colony counts as previously described (4). To detect a possible selection for vancomycin-resistant subpopulations in vivo, 0.1-ml samples from each vegetation homogenate from both control and treatment groups were plated directly onto agar plates containing 4 mg of vancomycin per liter for MRSA 217 and 8 and 32 mg of vancomycin per liter for hVISA PC1 and VISA PC3, respectively. These concentrations were equal to 4 times the MIC of the drug for each organism and do not tolerate bacterial growth, as shown by population analysis (14). The rates of valve infection of the various groups were compared by Fisher's exact test. The bacterial counts in vegetations were compared by unpaired t test or analysis of variance. Only animals that had received at least 48 h of treatment were taken into account in the final evaluation. For statistical comparisons, culture-negative vegetations were considered to contain 2 log10 CFU/g, the limit of detection. A P value of <0.05 was considered significant.
The MICs of vancomycin for MRSA 217, hVISA PC1, and VISA PC3 were 1, 2, and 8 mg/liter, respectively. In time-kill studies, vancomycin at concentrations similar to peak or steady-state serum levels produced a >3-log10-CFU/ml loss of viability by 24 h toward strain 217 but inflicted a <2-log10-CFU/ml decrease against strains PC1 and PC3.
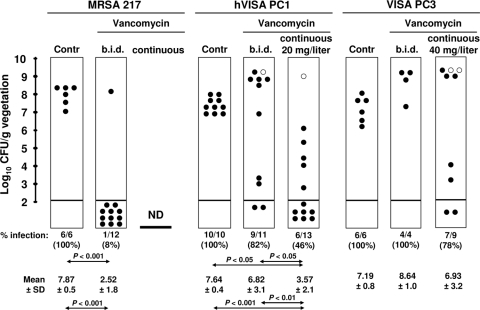
The results of a 5-day treatment of experimental endocarditis are presented in Fig. 1. Intermittent vancomycin administration (peak and trough levels of at least 50 and 10 mg/liter, respectively) (3) was very active against the vancomycin-susceptible MRSA 217. No vancomycin-resistant populations were selected. In contrast, this regimen failed against both hVISA PC1 and VISA PC3 organisms and selected for variants with further increased MICs. Against hVISA PC1, continuous infusion of vancomycin (28.4 ± 4.7 mg/liter) was significantly more effective than sequential treatment (P < 0.05) but yet could not sterilize all the valves, and 1 of 6 vegetation cultures contained variants that grew on plates containing 8 mg/liter of vancomycin at a frequency of 10−7. Moreover, continuous infusion of vancomycin (42.7 ± 4.3 mg/liter) was totally ineffective against VISA PC3 and selected further resistant variants of this organism in 2 of 7 infected valves, with a frequency of mutation of 10−7 as well. No bacteria resistant to higher levels of vancomycin were detected in vegetation from untreated controls. Mortality in animals infected with strain 217 after the intermittent regimen was 8%. Among rats infected with hVISA PC1 and VISA PC3, mortality rates were 38% and 50% after intermittent administration and 30% and 22% after continuous infusion therapy, respectively.
FIG. 1.
Results of 5 days of vancomycin treatment given either sequentially (b.i.d. [twice a day]) or by continuous infusion (continuous) against experimental endocarditis in rats infected with the vancomycin-susceptible MRSA 217, the hVISA PC1, or its VISA derivative PC3. Each dot in the figure represents the bacterial density in the vegetation of a single animal. Dots under the horizontal bars in the columns represent culture-negative vegetations. Open dots indicate the vegetations from which staphylococcal derivatives grew on plates containing vancomycin at concentrations (8 mg/liter for PC1 and 32 mg/liter for PC3) 4-fold above the original MIC. ND, not done.
The present study demonstrates that continuous infusion of vancomycin, at concentrations affording optimal constant serum levels of 10 times the MIC for the infecting organism, was more effective than standard sequential treatment but relatively not optimal against experimental endocarditis due to hVISA. In particular, it carried the risk of selecting a more resistant bacterial subpopulation in situ. Moreover, constant serum levels of 5 times greater than the MIC were unable to treat experimental endocarditis due to VISA and even selected for further resistance. Asseray et al. (1) also reported poor outcomes of steady-state vancomycin against VISA experimental endocarditis in rabbits. However, the treatment duration was limited to 48 h, and the study did not determine the risk of selection for further resistance. The failure of continuous infusion vancomycin in the present investigation extends this original observation to hVISA and especially to a more prolonged treatment period. Moreover, it shows that vancomycin failure may be attributed not only to poor bactericidal activity but also to the enrichment of less susceptible microbial subpopulations.
Thus, globally, these results do not support the theoretical arguments in favor of the use of continuous infusion vancomycin against hVISA and VISA infections. These data, together with the possible risk of nephrotoxicity associated with the maintenance of steady-state levels of ≥20 mg/liter (7, 10), suggest that there may be no clinical benefit to using continuous infusion of vancomycin to treat hVISA and VISA infections in humans. Vancomycin in combination with other agents (2) or alternative therapies with novel antistaphylococcal compounds should be considered for infections caused by S. aureus with reduced susceptibility to vancomycin.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Asseray, N., C. Jacqueline, V. Le Mabecque, E. Batard, D. Bugnon, G. Potel, and J. Caillon. 2005. Activity of glycopeptides against Staphylococcus aureus infection in a rabbit endocarditis model: MICs do not predict in vivo efficacy. Antimicrob. Agents Chemother. 49:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Entenza, J. M., Y. A. Que, J. Vouillamoz, M. P. Glauser, and P. Moreillon. 2001. Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance. Antimicrob. Agents Chemother. 45:3076-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fluckiger, U., P. Moreillon, J. Blaser, M. Bickle, M. P. Glauser, and P. Francioli. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heraief, E., M. P. Glauser, and L. R. Freedman. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howden, B. P., J. K. Davies, P. D. Johnson, T. P. Stinear, and M. L. Grayson. 2010. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 23:99-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ingram, P. R., D. C. Lye, P. A. Tambyah, W. P. Goh, V. H. Tam, and D. A. Fisher. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J. Antimicrob. Chemother. 62:168-171. [DOI] [PubMed] [Google Scholar]
- 8.Kitzis, M. D., and F. W. Goldstein. 2006. Monitoring of vancomycin serum levels for the treatment of staphylococcal infections. Clin. Microbiol. Infect. 12:92-95. [DOI] [PubMed] [Google Scholar]
- 9.Lodise, T. P., J. Graves, A. Evans, E. Graffunder, M. Helmecke, B. M. Lomaestro, and K. Stellrecht. 2008. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob. Agents Chemother. 52:3315-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodise, T. P., N. Patel, B. M. Lomaestro, K. A. Rodvold, and G. L. Drusano. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49:507-514. [DOI] [PubMed] [Google Scholar]
- 11.Pea, F., M. Furlanut, C. Negri, F. Pavan, M. Crapis, F. Cristini, and P. Viale. 2009. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob. Agents Chemother. 53:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybak, M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42(Suppl. 1):S35-S39. [DOI] [PubMed] [Google Scholar]
- 13.Rybak, M. J., B. M. Lomaestro, J. C. Rotscahfer, R. C. Moellering, W. A. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 49:325-327. [DOI] [PubMed] [Google Scholar]
- 14.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 15.Soriano, A., F. Marco, J. A. Martinez, E. Pisos, M. Almela, V. P. Dimova, D. Alamo, M. Ortega, J. Lopez, and J. Mensa. 2008. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 46:193-200. [DOI] [PubMed] [Google Scholar]
- 16.Yoon, Y. K., J. Y. Kim, D. W. Park, J. W. Sohn, and M. J. Kim. 2010. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J. Antimicrob. Chemother. 65:1015-1018. [DOI] [PubMed] [Google Scholar]