Abstract
Reports of potential drug-resistant strains of Plasmodium malariae in western Indonesia raise concerns that chloroquine resistance may be emerging in P. malariae and P. ovale. In order to assess this, in vivo and in vitro efficacy studies were conducted in patients with monoinfection in Papua, Indonesia. Consecutive patients with uncomplicated malaria due to P. ovale or P. malariae were enrolled in a prospective clinical trial, provided with supervised chloroquine treatment, and followed for 28 days. Blood from patients with P. malariae or P. ovale parasitemia greater than 1,000 per microliter underwent in vitro antimalarial drug susceptibility testing using a modified schizont maturation assay. Of the 57 evaluable patients in the clinical study (P. malariae, n = 46; P. ovale, n = 11), none had recurrence with the same species during follow-up. The mean parasite reduction ratio at 48 h was 86 (95% confidence interval [CI], 57 to 114) for P. malariae and 150 (95% CI, 54 to 245) for P. ovale (P = 0.18). One patient infected with P. malariae, with 93% of parasites at the trophozoite stage, was still parasitemic on day 4. In vitro drug susceptibility assays were carried out successfully for 40 isolates (34 infected with P. malariae and 6 with P. ovale). The P. malariae infections at trophozoite stages had significantly higher chloroquine 50% effective concentrations (EC50s) (median, 127.9 nM [range, 7.9 to 2,980]) than those initially exposed at the ring stage (median, 14.0 nM [range, 3.5 to 27.0]; P = 0.01). The EC50 for chloroquine in P. ovale was also higher in an isolate initially at the trophozoite stage (23.2 nM) than in the three isolates predominantly at ring stage (7.8 nM). Chloroquine retains adequate efficacy against P. ovale and P. malariae, but its marked stage specificity of action may account for reports of delayed parasite clearance times.
Plasmodium malariae and P. ovale exist over much of the world where malaria is endemic, causing an estimated 60 million clinical cases each year (12). Although they account for a small proportion of all malaria cases (<5%), their prevalence is likely to be underestimated, since they are frequently missed on routine microscopic blood film examination (4, 6, 11, 22). Clinical episodes of malariae and ovale malaria are usually associated with low peripheral parasitemias (<1,000 μl−1), with the fever classically displaying a periodicity of 3 days for P. malariae (quartan malaria) and 2 days for P. ovale (tertian malaria). Chronic, or recurring, parasitemia is more common following infection with P. malariae or P. ovale than with P. falciparum. In the case of P. ovale, this is attributable to the presence of liver stages (hypnozoites) similar to those found in P. vivax, resulting in relapsing infections, whereas P. malariae is thought to maintain prolonged subpatent parasitemia (27), which may cause clinical disease many years after the initial infection (20). Although generally regarded as benign, chronic P. malariae and P. ovale infections can result in anemia and, in the case of the former, an immune complex-mediated nephrotic syndrome.
In Papua, Indonesia, we have been conducting clinical and in vitro studies to ascertain the efficacy of standard antimalarials against P. falciparum and P. vivax. This region is unusual in harboring high levels of drug resistance in both of these species; treatment failure within 28 days exceeds 65% after chloroquine (CQ) monotherapy for P. vivax and 48% after chloroquine plus sulfadoxine plus pyrimethamine for P. falciparum (7, 16, 21). The high levels of chloroquine resistance in P. falciparum and P. vivax, coupled with previous observations of potential drug-resistant strains of P. malariae in western Indonesia (10), raised concerns that chloroquine resistance might also be emerging in P. malariae and P. ovale. The aim of the present study was to define the in vivo and in vitro efficacy of chloroquine in these species.
MATERIALS AND METHODS
Study site.
The study was carried out in Timika, Papua, Indonesia. The malaria epidemiology of this region has been reported previously (8). In brief, the forested lowland area has unstable malaria transmission associated with three mosquito vectors: Anopheles koliensis, Anopheles farauti, and Anopheles punctulatus (9, 14). The annual incidence of malaria is estimated to be 876 per 1,000 per year, with 90% of infections attributable to P. falciparum (55%) and P. vivax (45%) (8). Over a 5-year period (2004 to 2009), P. malariae accounted for 2,059 (2.9%) of 71,184 outpatient consultations for malaria at the local Rumah Sakit Mitra Masyarakat Hospital and 281 (1.5%) of 18,172 admissions with malaria (unpublished data). The corresponding figures for P. ovale were 72 and 6 cases.
The ethnic origin of the local population is diverse, with highland Papuans, lowland Papuans, and non-Papuans all resident in the region. In view of the high number of infections in nonimmune patients, local protocols recommend that all patients with patent parasitemia be given antimalarial therapy irrespective of whether they have symptoms.
Clinical study.
The clinical study was based on the 2003 World Health Organization (WHO) in vivo antimalarial drug sensitivity protocol (29) and was conducted in a rural outpatient clinic 10 km from the town center between July 2004 and August 2005. Consecutive patients with slide-confirmed malaria due to any species and fever or a history of fever during the preceding 48 h presenting at the clinic were enrolled in the study if they or their parents gave informed consent. Pregnant or lactating women, children under 10 kg body weight, and patients with signs of severity (30), a parasitemia of >4%, or concomitant disease requiring hospital admission were all excluded. Inclusion or exclusion in the clinical study was not based on the stage distribution of the parasite on the blood film.
Study procedures.
On admission, a standardized questionnaire was completed recording demographic information, details of symptoms and their duration, and the history of previous antimalarial medication. A full clinical examination was made, and the axillary temperature was measured using a digital electronic thermometer. Blood was taken for blood film, hematocrit, and white blood cell (WBC) count. Parasite counts were determined on Giemsa-stained thick films as the number of parasites per 200 WBCs. All slides were read by an expert microscopist with at least 10 years of experience. A thick smear was considered negative on initial review if no parasites were seen in 100 high-power fields. A thin smear was also examined to confirm the parasite species and was used for quantification if parasitemia was greater than 200 per 200 WBCs. All slides were cross-checked by another experienced microscopist. Upon cross-checking, the whole field was checked before a slide was considered negative.
Patients were examined daily thereafter until they became afebrile and aparasitemic. At each visit, a blood smear was taken and a symptom questionnaire was completed. Patients were then seen weekly, or on any other day when the patient was sick, for 4 weeks. At each clinic appointment, a full physical examination was performed, the symptom questionnaire was completed, and blood was taken to check the parasite count and hemoglobin level using a battery-operated portable photometer (HemoCue Hb201+). Blood spots on filter paper (Whatman 3MM Chromatography Paper) were also collected on day 0 and the day of failure.
Treatment.
Standard treatment courses of CQ (PT Bayer, Jakarta, Indonesia; 150 mg base/tablet; 25 mg/kg over 3 days) were administered for P. ovale or P. malariae infections. All drug administrations were supervised, and participants were observed for 30 to 60 min to exclude adverse reactions and to ensure the medication was not vomited. If vomiting occurred within 60 min, the whole dose was repeated once. If vomiting occurred again within 60 min, the patient was withdrawn from the study. If the axillary temperature was ≥38°C, paracetamol was given.
Recurrent parasitemias occurring during follow-up were re-treated with quinine (10 mg of salt/kg body weight/dose orally three times a day for 7 days) plus doxycycline (100 mg twice daily for 7 days) if the patient was more than 8 years old. Primaquine (0.5 mg of base/kg of body weight for 14 days) was administered to those individuals with P. ovale infection on day 28 of their participation in the study.
In vitro study.
Patients with symptomatic malaria presenting at the outpatient clinic of the local Rumah Sakit Mitra Masayarat (RSMM) were recruited into the study if singly infected with P. malariae or P. ovale with a parasitemia greater than 1,000 per microliter. After 2008, parasites for the in vitro assay were selected from those at majority ring stage. Patients treated with antimalarials in the previous 3 weeks were excluded from the study. Since patients were not enrolled in clinical studies, the therapeutic response to treatment could not be determined.
In vitro drug susceptibility assay.
The antimalarial susceptibilities of P. ovale and P. malariae isolates were measured using a protocol modified from the WHO microtest, as described previously (18). Venous blood (5 ml) was collected by venipuncture, and after removal of host white blood cells using a CF11 column, 2 ml of packed infected red blood cells (IRBC) were divided as follows: 1 ml was cryopreserved in glycerolyte, 200 μl was spotted onto filter paper, and 800 μl was used for the in vitro drug susceptibility assay. Two hundred microliters of a 2% hematocrit blood medium mixture (BMM) consisting of McCoy's 5A medium and 20% AB+ human serum was added to each well of predosed drug plates; the drug plates contained 11 serial concentrations (2-fold dilutions) of the antimalarials, with maximum concentrations of 5,910 nM for chloroquine, 557 nM for amodiaquine, 93 nM for artesunate, 338 nM for mefloquine, 87 nM for pyronaradine, and 769 nM for piperaquine. A candle jar was used to mature the parasites at 37.5°C for 15 to 56 h. Incubation was stopped when >40% of ring stage parasites had matured into mature schizonts in the drug-free control. Preliminary studies demonstrated that once the 40% schizont threshold had been reached, further incubation did not increase the final count.
Thick blood films made from each well were stained with 5% Giemsa solution for 30 min and examined microscopically. Differential counts of 200 asexual parasites in both the preincubation and test slides were classified into ring stage (ring-shaped trophozoites without pigment), mature trophozoites (single or double chromatin dots and hemazoin pigment visible), and schizonts. To ensure optimal maturity, ease of parasite identification, and reduction of parasite classification errors between microscopists, only schizonts with at least five well-defined chromatin dots were classified as schizonts at harvest. Free merozoites and gametocytes were not included in the count. The number of schizonts (≥5 chromatin dots visible) per 200 asexual-stage parasites was determined for each drug concentration and normalized to the drug-free control well. The dose-response data were analyzed using nonlinear regression analysis (WinNonLin 4.1; Pharsight Corporation), and the 50% inhibitory concentration (IC50) was derived using an inhibitory sigmoid Emax model. Only IC50 in vitro data from predicted curves where the maximum (Emax) and minimum (E0) were within 15% of 100 or 0, respectively, were used.
Confirmation of Plasmodium species.
A multiplex PCR was used to confirm Plasmodium species for pretreatment and recurrent isolates in the clinical study and isolates in the in vitro study prior to starting culture (13). For these isolates, genomic DNA from blood spots was extracted using a QIAamp DNA Mini Kit (Qiagen, Doncaster, Australia).
Statistical analysis.
Data were double entered and validated using EpiData 3.02 software (EpiData Association, Odense, Denmark), and analysis was performed using SPSS for Windows version 14 (SPSS Inc., Chicago, IL). The Mann-Whitney U test or Kruskal-Wallis method was used for nonparametric comparisons and Student's t test or one-way analysis of variance for parametric comparisons. For categorical variables, percentages and corresponding 95% confidence intervals were calculated using Wilson's method. Proportions were examined using χ2 with Yates' correction or by Fisher's exact test.
Treatment efficacy, as gauged by both the risk of recurrence and the rate of parasite clearance, was assessed using a modified intention-to-treat analysis. The evaluable population was defined as all patients in whom monoinfection by P. malariae and P. ovale could be confirmed. The risk of recurrent infection was derived by survival analysis, with the cumulative risk of failure calculated by the Kaplan-Meier product limit formula. Data were censored on the last day of follow-up for patients with protocol violations (e.g., incomplete treatment or lost to follow-up) or re-presenting with a peripheral parasitemia other than P. malariae and P. ovale.
Ethics.
The study was approved by the Ethics committee of the National Institute of Health Research and Development, the Indonesian Ministry of Health (Jakarta, Indonesia), and the Ethics Committee of Menzies School of Health Research (Darwin, Australia). Informed consent was obtained from all adult participants and from the parents of children. Informed consent was sought from all patients, or their parent/guardian, in their own language.
RESULTS
Clinical study.
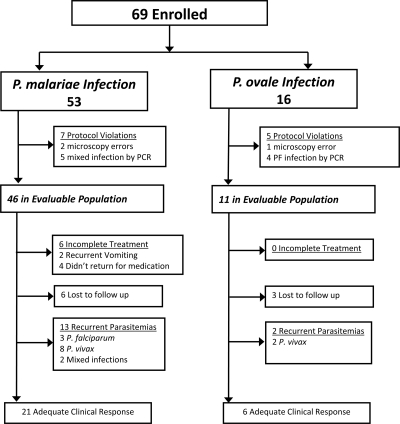
Between April 2004 and August 2005, 69 patients were enrolled in the clinical study. Microscopy cross-checking and PCR analysis revealed 12 protocol violations (7 in the P. malariae arm and 5 in the P. ovale arm); these patients were removed from further analysis (Fig. 1). The baseline characteristics of the remaining 57 patients in the evaluable population (P. malariae, n = 46; P. ovale, n = 11) are given in Table 1. Compared to patients infected with P. ovale, those infected with P. malariae were younger (P = 0.014) and had lower hemoglobin levels on enrollment (P < 0.001) (Table 1). Although all patients had a history of fever, only 15% (7/46) of P. malariae patients and 18% (2/11) of P. ovale patients had a recorded fever at enrollment. At presentation, patients with P. malariae or P. ovale infections had low parasitemias (median = 633 μl−1 and 645 μl−1, respectively), with asexual stages predominantly at the trophozoite stage; the median percentages of trophozoites were 99% (range, 66% to 100%) for P. malariae and 98% (range, 92% to 100%) for P. ovale.
FIG. 1.
Study profile of the clinical study.
TABLE 1.
Patient characteristics at baseline
| Characteristic of species at enrollment | Value |
|
|---|---|---|
| P. malariae | P. ovale | |
| No. in evaluable population | 46 | 11 |
| Geometric mean parasitemia (μl−1) (95% CI), range | 633 (459-863), 51-10,350 | 645 (247-1,686), 29-3,225 |
| Males [n (%)] | 29 (63) | 8 (73) |
| Age (yr) [median (range)] | 13.5 (2-38) | 28 (5-53) |
| <5 yr [n (%)] | 6 (13) | 0 (0) |
| 5-14 yr [n (%)] | 19 (41) | 2 (18) |
| >14 yr [n (%)] | 21 (46) | 9 (82) |
| Temp (°C) >37.5 [n (%)] | 7 (15) | 2 (18) |
| Hemoglobin (g/dl) [mean (SD)] | 9.5 (2.0) | 12.2 (3.1) |
| Anemia (Hb < 10 g/dl) [n (%)] | 31 (67) | 4 (36) |
| Gametocyte carriage on admission [n (%)] | 12 (26) | 5 (46) |
| Splenomegaly [n (%)] | 39 (85) | 8 (73) |
A 2-year-old female and a 3-year-old male in the P. malariae group had recurrent vomiting of chloroquine syrup on admission and were referred to the hospital for intravenous quinine. They made a full and unremarkable recovery. Four patients, all in the P. malariae arm, failed to return to the clinic for supervised therapy. The remaining 51 patients completed a full course of chloroquine, and none vomited their drug. The median dose of chloroquine administered was 26.1 (range, 20.6 to 33.3) mg/kg in the P. malariae group and 27.7 (range, 23.0 to 37.3) mg/kg in the P. ovale group.
Therapeutic response.
All 11 of the patients infected with P. ovale had defervesced within 24 h and were aparasitemic within 48 h. Six patients with P. malariae infection did not complete treatment, and blood films on day 2 were not available for an additional 3 patients. Of the remaining 37 patients, 7 (18.9%) were still parasitemic at 48 h, 1 of whom was febrile. Of these seven patients, one had cleared by day 3 and another five did not return for follow-up until day 7, when all were noted to be aparasitemic. The remaining patient, with an initial parasitemia of 675 μl−1 with 93% trophozoites, was still parasitemic on day 4 but was observed to be aparasitemic on day 11. The mean parasite reduction ratio at 48 h was 86 (95% confidence interval [CI], 57 to 114) for P. malariae and 150 (95% CI, 54 to 245) for P. ovale; P = 0.18.
In total, 6 patients infected with P. malariae (15%) and 3 infected with P. ovale (27%) did not complete 28 days of follow-up. In the P. malariae group, 13 patients had recurrent malaria (3 infected with P. falciparum, 8 infected with P. vivax, and 2 with mixed infections, all confirmed by PCR). Recurrent infection due to any species occurred a median of 21 (range, 14 to 28) days after treatment, with a day 28 cumulative risk of recurrence of 39% (95% CI, 22% to 56%). There were no recurrent infections with P. malariae, the cure rate being 100% (95% CI, 84.5% to 100%). In the P. ovale group, 2 patients had recurrent malaria due to P. vivax (at days 14 and 23), with an overall day 28 cumulative risk of recurrence of 23% (95% CI, 0% to 51%) and a P. ovale cure rate of 100% (95% CI, 61% to 100%).
Gametocyte carriage and anemia.
On admission, gametocytes were present in 26% (12/46) of patients infected with P. malariae and 46% (5/11) of patients infected with P. ovale (P = 0.16). None of the patients had P. malariae or P. ovale gametocytes during follow-up. On admission, 67% (31/46) of patients infected with P. malariae were anemic compared to 36% (4/11) of patients infected with P. ovale (P = 0.09). In those followed until day 28, these proportions had fallen to 22% (2/9) and 0% (0/2), respectively.
In vitro study.
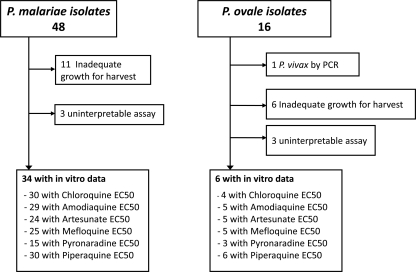
Between January 2005 and February 2008, 62 isolates from patients with single-species infections by either P. malariae (n = 46) or P. ovale (n = 16) were assessed for in vitro susceptibility (Fig. 2). The infecting species was confirmed by PCR in all but one isolate (initially processed as P. ovale, it was found to be P. vivax by molecular analysis). Susceptibility profiles were tested against chloroquine, amodiaquine, artesunate, mefloquine, pyronaridine, and piperaquine. The baseline characteristics of the isolates processed are presented in Table 2. Asexual parasitemias of both P. malariae and P. ovale infections revealed mixed stages in the peripheral blood. Whereas P. malariae isolates were mostly at trophozoite stages (median, 80% [range, 13% to 100%] trophozoites), P. ovale isolates in the in vitro study were mostly at the ring stage (median, 98% [2% to 100%] rings). Adequate growth for harvest was achieved in 77% (37/48) of P. malariae isolates and 60% (9/15) of P. ovale isolates. The time taken to reach the threshold for harvest was significantly longer for P. malariae isolates (median, 45 [range, 24 to 72] h) compared to P. ovale (median, 38 [range, 29 to 43] h); P = 0.05.
FIG. 2.
Study profile of the in vitro study.
TABLE 2.
Characteristics of isolates with successful in vitro susceptibility assays
| Baseline characteristic | Value |
|
|---|---|---|
| P. malariae | P. ovale | |
| Total no. of isolates reaching successful assay | 34 | 6 |
| Median (range) delay from venipuncture to start of culture (h) | 1.96 (0.75-4.08) | 1.88 (1.67-3.92) |
| Median (range) duration of assay (h) | 45 (24-72) | 38 (29-43) |
| Geometric mean (95% CI) parasitemia (no. of asexual parasites/μl) | 2,861 (2,244-3,648) | 2,736 (2,026-3,694) |
| Mean (95% CI) schizont count at harvest | 47 (42-52) | 39 (30-49) |
| Median initial % (range) of parasites at ring stage | 20 (0-87) | 98 (2-100) |
Our previous studies have highlighted the effects of infections with mixed asexual-stage infections on the derived drug susceptibility profile in P. vivax (18, 19). For this reason, the geometric mean IC50s for P. malariae and P. ovale are presented after stratifying them by whether infections were at majority ring or trophozoite stage (Table 3). P. malariae infections at trophozoite stages had chloroquine 50% effective concentrations (EC50s) (median, 127.9 [range, 7.9 to 2,980] nM) significantly higher than those initially exposed at ring stage (median, 14.0 [range, 3.5 to 27.0] nM; P = 0.01). This stage-specific activity was not apparent for any of the other drugs tested. The EC50 for chloroquine in P. ovale was also higher in an isolate initially at the trophozoite stage (23.2 nM) than in the three isolates predominantly at ring stage (7.8 nM).
TABLE 3.
In vitro susceptibility of each drug according to species tested
| Drug |
P. malariae |
P. ovale |
||||||
|---|---|---|---|---|---|---|---|---|
| Majority trophozoites |
Majority rings |
Majority trophozoites |
Majority rings |
|||||
| na | Median IC50b (range) | n | Median IC50 (range) | n | IC50 | n | Median IC50 (range) | |
| Chloroquine | 23 | 127.9 (7.9-2980) | 7 | 14.0c (3.5-27.0) | 1 | 23.2 | 3 | 7.8 (3.3-17.3) |
| Amodiaquine | 20 | 19.1 (2.5-96.4) | 9 | 21.7 (2.8-40.3) | 0 | 5 | 4.3 (1.0-12.8) | |
| Artesunate | 17 | 2.84 (0.15-15.8) | 7 | 1.77 (0.27-9.3) | 1 | 1.28 | 4 | 1.16 (0.17-2.85) |
| Mefloquine | 19 | 46.9 (9.8-336.9) | 6 | 36.8 (4.8-164.0) | 0 | 5 | 23.8 (6.2-49.9) | |
| Pyronaridine | 13 | 7.8 (0.1-150) | 2 | 4.1 (0.53-7.6) | 0 | 3 | 0.72 (0.17-1.39) | |
| Piperaquine | 22 | 38.4 (6.3-698) | 8 | 29.0 (6.6-35.9) | 1 | 7.57 | 5 | 10.2 (4.7-15.5) |
n, number of assays with acceptable IC50 data.
IC50s are given in nM concentrations.
P = 0.01, comparing IC50s in isolates with majority trophozoites to those with majority rings.
DISCUSSION
Drug-resistant strains of P. falciparum and P. vivax are now increasingly recognized throughout the world (1, 28), but few studies have addressed the clinical or in vitro efficacy of antimalarials against minor Plasmodium species. This is a likely consequence of their perceived importance, the lack of significant patient numbers available to be recruited into clinical trials, and an inability to sustain the parasites in continuous in vitro culture. In Papua, our clinical study, although with a limited sample size, found that all patients enrolled with either P. malariae or P. ovale infections responded to chloroquine therapy. Almost a third of patients had recurrent parasitemia within 28 days, but all of these were due to P. falciparum or P. vivax (confirmed by PCR), reflecting the known high levels of drug resistance in these species in this area (16). Infections due to low-grade drug-resistant isolates treated with a slowly eliminated antimalarial drug can lead to recrudescent infections as late as 63 days following initial treatment (25). The duration of follow-up in our study was limited to 28 days, and we cannot rule out the occurrence of late recrudescence. However, the lack of any recurrences within 28 days is reassuring and suggests that if chloroquine resistance is emerging, for the time being it has not reached a high level. Patients with ovale malaria responded rapidly to treatment, with all patients afebrile within 24 h and aparasitemic within 48 h. In contrast, the clinical response in patients with P. malariae infection was significantly slower, with almost 20% of patients still parasitemic by hour 48 and one patient remaining parasitemic on day 4. Similar observations have been reported from Madagascar, where a patient treated with chloroquine still had P. malariae detected by PCR on day 7, although it was subsequently cleared with no late recurrence (2). In P. falciparum and P. vivax infections, delayed parasite clearance is associated with a significantly greater risk of recurrence within 28 days and thus can be used as a useful marker of emerging parasite resistance (16, 26). However, the same may not be true of P. malariae. The slow early response of P. malariae to chloroquine was first documented by Young and Eyles in 1948 (31) and again by Collins and Jeffery in their retrospective analysis of archival data for patients treated with chloroquine between 1949 and 1963 (5). In the latter case series, 20% of patients were still parasitemic at day 7, a proportion that was apparently unrelated to the starting parasitemia. Although Maguire and colleagues raised concerns about the emergence of chloroquine-resistant P. malariae in Sumatra, Indonesia, in their clinical study in 2000 (10), this rested on observations of 2 patients out of 28 who were still parasitemic on day 8, along with a patient who rapidly cleared the initial infection but who had a subsequent recurrence on day 28. This could have been a reinfection.
The delay in the clearance of P. malariae following chloroquine may be attributable to factors other than parasite resistance. Clinical infections with P. falciparum are usually present in the peripheral circulation, with parasites predominantly at ring stage. In contrast, in P. vivax, P. malariae, and P. ovale infections, all stages of the parasite are present in the peripheral blood. The distribution of parasite stages varies between areas of differing endemicity, a factor that confounds the interpretation of in vitro susceptibility to chloroquine in both Thai and Indonesian isolates (18, 19). Chloroquine efficacy is significantly reduced against P. vivax trophozoites, and this is apparent in isolates from both chloroquine-sensitive (19) and -resistant populations (18), suggesting an intrinsic difference in stage-specific drug activity between species rather than one acquired due to chloroquine resistance.
The published literature of in vitro drug susceptibility in P. malariae and P. ovale is limited to no more than a few studies reporting a total of 20 isolates (3, 17, 23, 24). Overall, the derived chloroquine IC50s in our study were similar to those reported previously, although when analysis was restricted to parasites initially exposed to the drug at ring stage, our derived IC50s were a log order lower. Analysis of our P. malariae in vitro data revealed a significant confounding effect of the stage of the parasite, with isolates predominantly at ring stage having 9-fold-higher IC50s of chloroquine than parasites initially at trophozoite stage (median, 14 nM versus 128 nM). This difference was not observed for any of the other antimalarial drugs tested or for the P. ovale assay (although the number of isolates tested was low).
Our clinical and in vitro assessments of drug efficacy were not conducted simultaneously, so we are unable to correlate the in vitro and in vivo parasite responses directly. However, the absence of any P. malariae or P. ovale recurrences within 28 days in our clinical study, in conjunction with the in vitro data for isolates tested initially at ring stage (all had IC50s below 27 nM), suggests that in this region both species remain intrinsically susceptible to chloroquine. The patient infected with P. malariae who was still parasitemic on day 4 had a relatively high initial parasitemia on admission (1,184 μl−1, 93% of which were at the trophozoite stage). We estimate that the parasite reduction ratio at 48 h (defined as the parasitemia on admission divided by the parasitemia at day 2) is approximately 100 for P. malariae and 150 for P. ovale. These figures compare with estimates of 103 to 104 for chloroquine in sensitive isolates of P. falciparum and P. vivax (15, 26). A peripheral parasitemia of 1,000 μl−1 corresponds to a total biomass of about 1010. To reduce this to a level undetectable by microscopy (∼108 total parasite biomass) requires parasite exposure to drug for at least one life cycle. In the case of chloroquine and P. malariae, this needs to be longer if the initial parasite load is predominantly present at the innately resistant trophozoite stage. The life cycle of P. malariae is 72 h compared to 24 to 48 h for the other human Plasmodium species. In this context, clearance of P. malariae parasitemia within 4.5 days is to be expected rather than being indicative of declining drug efficacy. For clearance times longer than this, one would need to evoke other factors, such as a high parasitemia at presentation or partial drug absorption.
In conclusion, our studies show no evidence of declining chloroquine efficacy against P. ovale and P. malariae in Papua. The marked difference in the antimalarial activities of chloroquine against trophozoite and ring stage parasites in P. vivax, P. malariae, and perhaps P. ovale raises important questions regarding the pharmacodynamic antimalarial properties of chloroquine (19). Further studies are required to elucidate this, as well as the relevance of parasite staging for the clinical response to chloroquine of non-falciparum parasites.
Acknowledgments
We are grateful to the staff of PT Freeport Indonesia Public Health and Malaria Control Department, International SOS, and Lembaga Pengembangan Masyarakat Amungme Kamoro (LPMAK) for support and technical assistance. We also thank Maurits Obeseray, Rosmini, Buhari, Kim Piera, and Surinder Kaul for their support and technical assistance. Cross-checking of all slides was carried out by Ferryanto Chalfein (Timika) and Budi Prasetyorini (NIHRD, Jakarta). We thank the Australian Red Cross blood transfusion service for the supply of human sera.
The study was funded by the Wellcome Trust-NHRMC (Wellcome Trust ICRG GR071614MA-NHMRC ICRG ID 283321). N.M.A. is supported by an NHMRC Practitioner Fellowship. R.N.P. is funded by a Wellcome Trust Senior Research Fellowship in Clinical Science (091625).
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Baird, J. K. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 22:508-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnadas, C., A. Ratsimbasoa, H. Ranaivosoa, D. Ralaizandry, D. Raveloariseheno, V. Rabekotonorina, S. Picot, and D. Menard. 2007. Short report: prevalence and chloroquine sensitivity of Plasmodium malariae in Madagascar. Am. J. Trop. Med. Hyg. 77:1039-1042. [PubMed] [Google Scholar]
- 3.Basco, L. K., and J. Le Bras. 1994. Short-term in vitro culture of Plasmodium vivax and P. ovale for drug-susceptibility testing. Parasitol. Res. 80:262-264. [DOI] [PubMed] [Google Scholar]
- 4.Cavasini, M. T., W. L. Ribeiro, F. Kawamoto, and M. U. Ferreira. 2000. How prevalent is Plasmodium malariae in Rondonia, western Brazilian Amazon? Rev. Soc. Bras. Med. Trop. 33:489-492. [DOI] [PubMed] [Google Scholar]
- 5.Collins, W. E., and G. M. Jeffery. 2002. Extended clearance time after treatment of infections with Plasmodium malariae may not be indicative of resistance to chloroquine. Am. J. Trop. Med. Hyg. 67:406-410. [DOI] [PubMed] [Google Scholar]
- 6.Collins, W. E., and G. M. Jeffery. 2007. Plasmodium malariae: parasite and disease. Clin. Microbiol. Rev. 20:579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasugian, A. R., E. Tjitra, A. Ratcliff, H. Siswantoro, E. Kenangalem, R. M. Wuwung, H. L. Purba, K. A. Piera, F. Chalfien, J. Marfurt, P. M. Penttinen, B. Russell, N. M. Anstey, and R. N. Price. 2009. In vivo and in vitro efficacy of amodiaquine monotherapy for treatment of infection by chloroquine-resistant Plasmodium vivax. Antimicrob. Agents Chemother. 53:1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karyana, M., L. Burdarm, S. Yeung, E. Kenangalem, N. Wariker, R. Maristela, K. G. Umana, R. Vemuri, M. J. Okoseray, P. M. Penttinen, P. Ebsworth, P. Sugiarto, N. M. Anstey, E. Tjitra, and R. N. Price. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar. J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, V. H., S. Atmosoedjono, S. Aep, and C. D. Swaine. 1980. Vector studies and epidemiology of malaria in Irian Jaya, Indonesia. Southeast Asian J. Trop. Med. Public Health 11:341-347. [PubMed] [Google Scholar]
- 10.Maguire, J. D., I. W. Sumawinata, S. Masbar, B. Laksana, P. Prodjodipuro, I. Susanti, P. Sismadi, N. Mahmud, M. J. Bangs, and J. K. Baird. 2002. Chloroquine-resistant Plasmodium malariae in south Sumatra, Indonesia. Lancet 360:58-60. [DOI] [PubMed] [Google Scholar]
- 11.Mayxay, M., S. Pukrittayakamee, P. N. Newton, and N. J. White. 2004. Mixed-species malaria infections in humans. Trends Parasitol. 20:233-240. [DOI] [PubMed] [Google Scholar]
- 12.Mueller, I., P. A. Zimmerman, and J. C. Reeder. 2007. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol. 23:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padley, D., A. H. Moody, P. L. Chiodini, and J. Saldanha. 2003. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 97:131-137. [DOI] [PubMed] [Google Scholar]
- 14.Pribadi, W., I. Sutanto, S. Atmosoedjono, R. Rasidi, L. K. Surya, and L. Susanto. 1998. Malaria situation in several villages around Timika, south central Irian Jaya, Indonesia. Southeast Asian J. Trop. Med. Public Health 29:228-235. [PubMed] [Google Scholar]
- 15.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratcliff, A., H. Siswantoro, E. Kenangalem, M. Wuwung, A. Brockman, M. D. Edstein, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. S. Trop. Med. Hyg. 101:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringwald, P., J. Bickii, A. Same-Ekobo, and L. K. Basco. 1997. Pyronaridine for treatment of Plasmodium ovale and Plasmodium malariae infections. Antimicrob. Agents Chemother. 41:2317-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell, B., F. Chalfein, B. Prasetyorini, E. Kenangalem, K. Piera, R. Suwanarusk, A. Brockman, P. Prayoga, P. Sugiarto, Q. Cheng, E. Tjitra, N. M. Anstey, and R. N. Price. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharrock, W. W., R. Suwanarusk, U. Lek-Uthai, M. D. Edstein, V. Kosaisavee, T. Travers, A. Jaidee, K. Sriprawat, R. N. Price, F. Nosten, and B. Russell. 2008. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siala, E., M. Khalfaoui, A. Bouratbine, S. Hamdi, K. Hili, and K. Aoun. 2005. Relapse of Plasmodium malariae malaria 20 years after living in an endemic area. Presse Med. 34:371-372. [DOI] [PubMed] [Google Scholar]
- 21.Siswantoro, H., A. Ratcliff, E. Kenangalem, M. Wuwung, R. Maristela, R. Rumaseuw, F. Laihad, E. P. Ebsworth, N. M. Anstey, R. N. Price, and E. Tjitra. 2006. Efficacy of existing antimalarial drugs for uncomplicated malaria in Timika, Papua, Indonesia. Indonesian Med. J. 15:221-258. [Google Scholar]
- 22.Snounou, G., L. Pinheiro, A. Goncalves, L. Fonseca, F. Dias, K. N. Brown, and V. E. do Rosario. 1993. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans. R. Soc. Trop. Med. Hyg. 87:649-653. [DOI] [PubMed] [Google Scholar]
- 23.Tan-ariya, P., and S. Pasuralertsakul. 1994. First report of in vitro susceptibility of Plasmodium malariae Thai isolates to chloroquine. Southeast Asian J. Trop. Med. Public Health 25:784-787. [PubMed] [Google Scholar]
- 24.Tanomsing, N., M. Imwong, S. Pukrittayakamee, K. Chotivanich, S. Looareesuwan, M. Mayxay, C. Dolecek, T. T. Hien, V. E. do Rosario, A. P. Arez, P. Michon, G. Snounou, N. J. White, and N. P. Day. 2007. Genetic analysis of the dihydrofolate reductase-thymidylate synthase gene from geographically diverse isolates of Plasmodium malariae. Antimicrob. Agents Chemother. 51:3523-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, N. J. 2002. The assessment of antimalarial drug efficacy. Trends Parasitol. 18:458-464. [DOI] [PubMed] [Google Scholar]
- 26.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White, N. J. 2009. Malaria, p. 1201-1300. In G. C. Cook and A. I. Zumla (ed.), Manson's tropical diseases, 22nd ed. W. B. Saunders, Edinburgh, United Kingdom.
- 28.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush II, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 29.WHO. 2003. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. WHO, Geneva, Switzerland.
- 30.WHO. 2000. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 31.Young, M. D., and D. E. Eyles. 1948. The efficacy of chloroquine, quinacrine, quinine and totaquine in the treatment of Plasmodium malariae infections (quartan malaria). Am. J. Trop. Med. Hyg. 28:23-28. [DOI] [PubMed] [Google Scholar]