Abstract
Susceptibility to several β-lactams and β-lactamase production was investigated in a collection of 20 strains of Pseudomonas otitidis, a new Pseudomonas species that has been recently recognized in association with otic infections in humans. All strains appeared to be susceptible to piperacillin, cefotaxime, ceftazidime, and aztreonam, while resistance or decreased susceptibility to carbapenems was occasionally observed. All strains were found to express metallo-β-lactamase (MBL) activity and to carry a new subclass B3 MBL gene, named blaPOM, that appeared to be highly conserved in this species. P. otitidis, therefore, is the first example of a pathogenic Pseudomonas species endowed with a resident MBL. The POM-1 protein from P. otitidis type strain MCC10330 exhibits the closest similarity (60 to 64%) to the L1 MBL of Stenotrophomonas maltophilia. Expression in Escherichia coli and Pseudomonas aeruginosa revealed that, similar to L1 and other subclass B3 MBLs, POM-1 confers decreased susceptibility or resistance to carbapenems, penicillins, and cephalosporins but not to aztreonam. Expression of the POM MBL in P. otitidis is apparently constitutive and, in most strains, does not confer a carbapenem-resistant phenotype. However, a strong inoculum size effect was observed for carbapenem MICs, and carbapenem-resistant mutants could be readily selected upon exposure to imipenem, suggesting that carbapenem-based regimens should be considered with caution for P. otitidis infections.
Pseudomonas otitidis is a new Pseudomonas species that has recently been recognized in association with otic infections in humans, including acute otitis externa, acute otitis media, and chronic suppurative otitis media (2). Genotypically and phenotypically, P. otitidis is closely related to Pseudomonas aeruginosa (2), and this similarity likely accounts for the belated identification of this new pathogenic species within the Pseudomonas genus.
The susceptibility of P. otitidis was previously investigated with several antimicrobial agents, including aminoglycosides, fluoroquinolones, macrolides, β-lactams, tetracycline, chloramphenicol, and polymyxin B, and overall, the behavior of this species appeared to be similar to that of P. aeruginosa (2). However, of β-lactams, only piperacillin was tested, while no information is available on the susceptibility of this species to antipseudomonal cephalosporins, aztreonam, and carbapenems.
In this work, we investigated the susceptibility of P. otitidis to several β-lactams and the production of β-lactamase activity by this species. Results revealed that P. otitidis strains constitutively produce a novel subclass B3 metallo-β-lactamase (MBL), that was named POM (after P. otitidis metallo-β-lactamase), which is active on carbapenems and other β-lactams.
MATERIALS AND METHODS
Bacterial strains.
The P. otitidis strains investigated in this work included 19 strains from the collection previously described by Clark et al. (2) and a clinical isolate from South Korea (isolate YMC-Po/06) cultured from the purulent discharge of a patient suffering from chronic suppurative otitis media (Table 1). Identification of the latter isolate was carried out by 16S rRNA gene sequencing (7) and biochemical profiling (2). Escherichia coli strain MC1061 and Pseudomonas aeruginosa strain PAO1 were used as hosts for recombinant plasmids.
TABLE 1.
P. otitidis strains investigated in this work, MBL activity measured in crude extracts, and MICs of various β-lactams
| Source of strain or MIC parametera | Strain | MBL productionb | MIC (μg/ml) of β-lactamc: |
|||||
|---|---|---|---|---|---|---|---|---|
| IMP | MEM | PIP | AZT | CTX | CAZ | |||
| CU | MCC04446 | 46.0 | 0.75 | 3 | 1.5 | 6 | 6 | 6 |
| CU | MCC04511 | 28.7 | >32 | >32 | 4 | 6 | 4 | 2 |
| CU | MCC04517 | 37.8 | 3 | 6 | 3 | 4 | 2 | 6 |
| CSOM | MCC09159 | 44.7 | 2 | −12 | 0.75 | 2 | 1.5 | 4 |
| AOE | MCC10330T | 42.3 | 3 | 3 | 4 | 4 | 2 | 6 |
| AOE | MCC10429 | 58.1 | 2 | 2 | 6 | 4 | 6 | 8 |
| AOE | MCC10744 | 66.7 | 1 | 2 | 4 | 6 | 4 | 3 |
| AOE | MCC11140 | 42.4 | 1.5 | 1 | 4 | 6 | 3 | 6 |
| AOE | MCC11061 | 28.5 | 1 | 2 | 4 | 8 | 3 | 4 |
| AOE | MCC11338 | 41.1 | 1 | 3 | 1.5 | 2 | 2 | 4 |
| AOE | MCC11683 | 30.3 | 1 | 1.5 | 0.75 | 6 | 4 | 4 |
| AOE | MCC12065 | 38.8 | 0.5 | 4 | 1.5 | 6 | 2 | 4 |
| AOE | MCC12178 | 36.2 | 1.5 | 2 | 4 | 8 | 3 | 3 |
| AOM | MCC40150 | 17.9 | 1 | 2 | 1.5 | 3 | 1.5 | 3 |
| AOM | MCC40159 | 16.6 | 0.75 | 6 | 4 | 8 | 4 | 3 |
| AOM | MCC51196 | 15.1 | 0.75 | 0.25 | 1 | 6 | 3 | 3 |
| AOMT | MCC61379 | 97.2 | 2 | 16 | 3 | 6 | 4 | 4 |
| AOMT | MCC61485 | 16.1 | 2 | 2 | 2 | 6 | 2 | 3 |
| AOMT | MCC61433 | 31.5 | 2 | 24 | 3 | 4 | 4 | 4 |
| CSOM | YMC-Po/06 | 81.7 | >32 | >32 | 2 | 4 | 3 | 4 |
| MIC range | 0.50->32 | 0.25->32 | 0.75-6 | 2-8 | 1.5-6 | 2-8 | ||
| MIC50 | 1.5 | 3 | 3 | 6 | 3 | 4 | ||
| MIC90 | 3 | 24 | 4 | 8 | 4 | 6 | ||
CU, corneal ulcer; CSOM, chronic suppurative otitis media; AOE, acute otitis externa; AOM, acute otitis media; AOMT, otitis media with otorrhoea drainage through tympanostomy tube.
MBL production is shown in nanomoles of imipenem hydrolyzed per minute per milligram of protein. Data are the mean values of measurements for three replicate samples (the standard deviation was always <10%); in all cases, the activity was inhibited by at least 80% in the presence of EDTA. Carbapenemase activity measured with the negative control (P. aeruginosa ATCC 27853) yielded a value of <10 nmol of imipenem hydrolyzed/min/mg of protein.
The MICs were determined by Etest. IMP, imipenem; MEM, meropenem; PIP, piperacillin; AZT, aztreonam; CTX, cefotaxime; CAZ, ceftazidime.
Antimicrobial susceptibility testing.
MICs were determined by Etest (bioMérieux SA, Marcy l'Etoile, France) according to the manufacturer's instructions or by an agar dilution technique in Mueller-Hinton (MH) agar (3). The latter method was used to evaluate the inoculum size effect, using inocula of 105 and 107 CFU per spot, respectively. Results were recorded after incubation at 37°C for 18 to 20 h. P. aeruginosa ATCC 27853 was used for quality control of susceptibility testing.
In vitro selection of carbapenem-resistant mutants.
Approximately 109 CFU of P. otitidis strain MCC10330T were plated on MH agar containing a gradient concentration of imipenem (from 0 to 5 μg/ml) and incubated overnight at 37°C. Colonies grown in the presence of the antibiotic were subcultured in antibiotic-free MH agar and subjected to reidentification by 16S rRNA gene sequencing and biochemical profiling (2, 7).
β-Lactamase assays.
Production of carbapenemase activity in crude extracts was assayed spectrophotometrically as described previously (9) in buffer containing 50 mM HEPES plus 50 μM ZnSO4 at pH 7.5 and 30°C, with 150 μM imipenem as the substrate. Inhibition by EDTA was tested by measuring carbapenemase activity in the presence of 5 mM EDTA after preincubation of the sample for 20 min at 30°C with the same EDTA concentration. Isoelectric focusing (IEF) analysis was performed as described previously (5). β-Lactamase induction experiments were carried out with exponentially growing cells in MH broth, using imipenem to induce β-lactamase. β-Lactamase activity was determined in crude extracts, using imipenem and nitrocefin as substrates as described previously (9).
DNA analysis and manipulation techniques.
Basic recombinant DNA methodology was performed essentially as described by Sambrook et al. (10). Genomic DNA was extracted from P. otitidis strains as described previously (9). The genomic library from P. otitidis MCC10330T was constructed in the E. coli pACYC184 plasmid vector as described previously (9). The shuttle vector pPME6001 (1) was used as the subcloning vector. The presence of the blaPOM-1-like genes in P. otitidis isolates was investigated by PCR. Amplification reactions were carried out in a 50-μl volume, with the Pomseq/F and Pomseq/R forward and reverse primers, respectively (Table 2) (25 pM each), 125 μM (each) deoxynucleoside triphosphates (dNTPs), and 50 ng of template DNA. GoTaq (Promega, Madison, WI) was used according to the manufacturer's instructions. The following cycling conditions were used: 5 min at 96°C, 30 cycles (1 cycle consists of 45 s at 95°C, 30 s at 56°C, and 30 s at 72°C), and 7 min at 72°C. P. otitidis MCC10330T and P. aeruginosa ATCC 27853 were used as the positive control and negative control, respectively. Amplification reactions with primers PomOrf/F and POM948/R (Table 2) were also performed with some strains to determine the sequences of blaPOM genes. The following cycling conditions were used: 5 min at 96°C, 30 cycles (1 cycle consists of 45 s at 95°C, 30 s at 51°C, and 45 s at 72°C), and 7 min at 72°C. Sequencing was carried out on both strands at an external sequencing facility (Macrogen, Seoul, South Korea) using custom primers.
TABLE 2.
Oligonucleotide primers used in this work
| Primer | Sequence (5′→3′) | Locationa |
|---|---|---|
| Pom-seq/F (primer 1) | CTGCACAGCCACGCCCAC | +298/+315 |
| Pom-seq/R (primer 2) | GTCATGCCGCCCAGCTCC | +509/+492 |
| POTSEQ/F (primer 3) | ACGTCGCTGATGCTCAG | −265/−249 |
| Pom948/R (primer 4) | CCTGCGTCATCAGAGACCTC | −879/−870 |
| C5ft/F (primer 5) | TGTCCTTGATGTGGTTGTGG | −1091/−1071 |
| CT1Prom/R (primer 6) | GGGGGGTCAGGGTACGCA | +20/+2 |
Database search and sequence analysis.
Database search was performed using BLAST software (version 2.2.23) available at the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi). DNA and protein sequence alignments were performed with ClustalW software (version 2) available at the EBI web server (http://www.ebi.ac.uk/tools/clustalw2), that was also used to create phylogenetic trees. Signal peptide cleavage site was predicted using SignalP (version 3.0). Putative promoter sequences were detected using Bprom software (Softberry, Inc., Mount Kisco, NY).
Nucleotide sequence accession numbers.
The nucleotide sequences reported in this paper have been submitted to the GenBank/EMBL sequence database and assigned accession no. EU315252 (for the blaPOM-1 gene from P. otitidis MCC10330T) and GU002288 to GU002298 for partial blaPOM gene sequences from other P. otitidis strains.
RESULTS AND DISCUSSION
β-Lactam susceptibility and β-lactamase production of P. otitidis strains.
The susceptibility of 20 P. otitidis strains to various β-lactams, including piperacillin, cephalosporins, carbapenems, and aztreonam, was determined by Etest. MICs of piperacillin, aztreonam, cefotaxime, and ceftazidime were quite homogeneous, with a 4- to 8-fold variability at maximum between different strains. Higher variability was observed with carbapenems, with a few strains showing high-level carbapenem MICs (Table 1). Using the CLSI breakpoints for P. aeruginosa (4), all P. otitidis strains were susceptible to piperacillin, cefotaxime, ceftazidime, and aztreonam, while two strains (10%) were resistant to imipenem and 7 strains (35%) were intermediate or resistant to meropenem. Using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints for P. aeruginosa (http://www.eucast.org), all P. otitidis strains were susceptible to piperacillin and ceftazidime and intermediate to aztreonam, while two strains (10%) were resistant to imipenem and 11 strains (55%) were intermediate or resistant to meropenem. Imipenem MICs were usually lower than those of meropenem, with a few exceptions (Table 1). The two imipenem-resistant strains exhibited high-level MIC values (>32 μg/ml) for both imipenem and meropenem (Table 1).
Analytical IEF, carried out with five strains, including the type strain (MCC10330T), two additional carbapenem-susceptible strains (MCC12065 and MCC61485) and the two high-level carbapenem-resistant strains (MCC04511 and YMC-Po/06) revealed in all cases the presence of a single β-lactamase band with a pI of around 6 to 6.2 (data not shown).
Analysis of crude extracts from exponential-phase cultures revealed the presence of carbapenemase activity in all P. otitidis strains (Table 1). The specific activity was variable within an approximately 5-fold range, and in all cases, the activity was inhibited (≥80%) by EDTA. No clear relationship was observed between the amount of carbapenemase activity and carbapenem susceptibility (Table 1).
Following exposure to subinhibitory concentrations of imipenem (0.25× MIC), the MBL activity measured at 2 h after induction was not significantly different from that observed under basal conditions either with MCC10330T or with YMC-Po/06 (data not shown), showing that production of the MBL activity was independent of β-lactam exposure. Activity against nitrocefin was also unaffected by exposure to imipenem (data not shown).
According to these data, the behavior of P. otitidis to β-lactams and the β-lactamase profile of P. otitidis appeared to be different from those of P. aeruginosa. Unlike P. aeruginosa, P. otitidis constitutively produces an MBL while it apparently lacks additional inducible β-lactamase genes.
Cloning and characterization of the MBL-encoding gene from P. otitidis MCC10330T.
A clone producing MBL activity was isolated from a genomic library of the P. otitidis type strain, constructed in the E. coli plasmid vector pACYC184 and transformed in the E. coli strain MC1061, after replica plating of the library onto LB medium containing imipenem (1 μg/ml).
The MBL-producing clone, named CT-1, carried a DNA insert of about 6 kb. Sequencing of the insert revealed the presence of an 859-bp open reading frame (ORF) encoding a protein similar to MBLs of subclass B3 (Fig. 1) that was named POM-1 (after P. otitidis metallo-β-lactamase). The ORF starts with a GTG codon and is preceded by a recognizable ribosomal binding site and by a putative promoter region.
FIG. 1.
Tree showing the similarity of the POM-1 protein (GenBank/EMBL accession no. EU315252) with other known subclass B3 MBLs. The tree has been constructed using the Clustal2 program (http://www.ebi.ac.uk/tools/clustalw2). Other MBLs (Swiss-Prot accession nos.) are FEZ-1 from Fluoribacter gormanii (Q9K578), GOB-1 from Elizabethkingia meningoseptica (Q9RB00), CAR-1 from Erwinia carotovora (ECA2849), L1 from S. maltophilia (Q9RBQ3), THIN-B from Janthinobacterium lividum (Q9AEF9), CAU-1 from Caulobacter crescentus (Q8KKG1), and BJP-1 from Bradyrhizobium japonicum (Q89GW5).
The POM-1 enzyme exhibits the closest similarity (60 to 64% amino acid identity) to the several known variants of the L1 enzyme of S. maltophilia and a lower similarity to other enzymes of subclass B3 (Fig. 1), suggesting a closer ancestry with the L1 enzyme. BLAST search also showed that POM-1 was almost identical to a protein fragment (amino acids [aa] 23 to 308) deduced from the translation of the genome of Culex quinquefasciatus (the southern house mosquito), suggesting the occurrence of bacterial contamination in that genome project (GenBank no. AAWU00000000).
Genetic context of the blaPOM-1 gene.
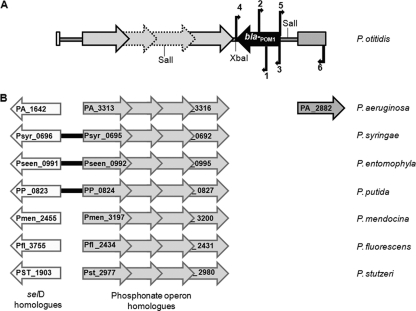
Sequencing of the regions flanking the cloned blaPOM-1 gene revealed the following: (i) upstream of blaPOM-1, the presence of the 5′ moiety of an ORF encoding a putative sensor-histidine kinase homologous to the P. aeruginosa PA_2882 protein; (ii) downstream of blaPOM-1, an operon for phosphonate utilization and the 5′ end of a selD homologue, which are highly conserved among different Pseudomonas species (Fig. 2). Altogether, the region downstream of blaPOM-1 exhibited colinearity with the genomes of Pseudomonas syringae, Pseudomonas entomophila, and Pseudomonas putida at least until the selD gene, and with the genomes of P. aeruginosa, Pseudomonas mendocina, Pseudomonas fluorescens, and Pseudomonas stutzeri until the phosphonate operon (Fig. 2). On the other hand, the region upstream of blaPOM-1 did not show any significant homology to other Pseudomonas genomes except for P. aeruginosa in which, however, the gene for the PA_2882 homologue is not linked with the phosphonate operon (Fig. 2).
FIG. 2.
(A) Genetic context of blaPOM-1 and (B) homologies of flanking regions with the genomes of other Pseudomonas species. The location of primers used for PCR amplification (Table 2) is shown by numbered arrows: 1, Pom-seq/F; 2, Pom-seq/R; 3, POTSEQ/F; 4, POM948/R; 5, CT1ft/F; 6, CT1Prom/R. The NCBI accession numbers for the Pseudomonas genome sequences are as follows: NC_002516.2 for P. aeruginosa PAO1, NC_007005.1 for P. syringae pv. syringae B728a, NC_008027.1 for P. entomophila L48, NC_002947.3 for P. putida KT22440, NC_009439.1 for P. mendocina ymp, NC_007492.2 for P. fluorescens Pf-01, and NC_009434.1 for P. stutzeri A1501. Colinearity of genes/operons is indicated by thick black lines; where no such lines are drawn, the homologues are present but located apart from each other on the chromosome.
Finding of blaPOM-1 within a similar genetic context in the absence of homologues in any other Pseudomonas genome suggests that the gene was acquired by horizontal transfer, followed by recombination into the chromosome downstream of the conserved phosphonate operon, after divergence of P. otitidis from the other species.
Prevalence and conservation of blaPOM-like MBL genes in P. otitidis strains.
Since MBL production was a constant feature of P. otitidis, we investigated the presence of blaPOM-related sequences in the genomic DNAs of the 20 P. otitidis strains. By PCR amplification of an internal fragment, using the Pomseq/F and Pomseq/R primers (Table 2 and Fig. 2), an amplicon of the expected size (212 bp) was obtained from all tested strains (data not shown), suggesting that blaPOM-1-like genes are actually resident in this species.
To investigate the variability of blaPOM-1-like genes carried by different strains, the coding region was amplified using primers POTSEQ/F and Pom984/R (Table 2 and Fig. 2) from the genomic DNAs of 10 randomly selected P. otitidis strains from the United States and from the South Korean strain YMC-Po/06. Amplicons of the expected size (1,143 bp) were obtained from all tested strains. Amplicon sequencing allowed comparison of most of the coding sequences (except for a 45-bp region at the 3′ end) and revealed an overall high degree of sequence conservation, with a nucleotide homology ranging from 97.0 to 99.1% and an amino acid homology ranging from 97.0 to 100% for the aligned region (Fig. 3). All key residues known to be involved in metal binding in subclass B3 enzymes (His/Gln116, His118, His196, Asp120, His121, and His263), in the BBL numbering scheme (6) were conserved (Fig. 3). In one case (strain MCC51196), a three-amino-acid deletion in the putative leader peptide was observed (Fig. 3). Interestingly, this strain was the one with the lowest meropenem MIC, although carbapenemase activity was detectable in the crude extract (Table 1). This could reflect some impairment in the secretion process of this enzyme variant.
FIG. 3.
Sequence alignment of the POM-1-like proteins from different P. otitidis strains. The conserved residues known to be involved in metal binding are indicated by gray shading. Identical residues (*), strongly similar residues (:), and weakly similar residues (.) are indicated below the sequences. Gaps introduced to optimize alignment are indicated by dashes.
Functional characterization of POM-1.
A 2,213-bp XbaI fragment, including the blaPOM-1 gene and flanking regions, was subcloned from pCT-1 into the shuttle plasmid pPME6001 to obtain the recombinant plasmid pPom6/11. This plasmid was used to transform E. coli MC1061 and P. aeruginosa PAO1 to investigate the impact of POM-1 production on β-lactam resistance. MBL production was detected in both transformants, although the specific activity was much higher in P. aeruginosa PAO1(pPom6/11) (Table 3). Expression of POM-1 in E. coli and P. aeruginosa affected the susceptibility of the bacterial hosts to penicillins, cephalosporins, and carbapenems, while aztreonam MICs were not affected, revealing a broad substrate specificity. The impact on MIC values was most evident with ampicillin in E. coli and with carbapenems in P. aeruginosa (Table 3). The different impact observed with E. coli and P. aeruginosa could reflect the different expression level and/or different outer membrane permeability and/or a different contribution of efflux systems in the two bacterial hosts. Investigation of these issues and biochemical characterization of the POM-1 enzyme will be the subject of future studies.
TABLE 3.
MICs and carbapenemase activity conferred by blaPOM-1 gene carriage in E. coli MC1061 and in P. aeruginosa PAO1
| Strain | Relevant characteristics | MIC (μg/ml) of antimicrobial agenta: |
Carbapen. act.b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | PIP | LOT | FOX | CTX | CAZ | IMP | MEM | ERT | AZT | |||
| MC1061(pPME6001) | E. coli host with empty vector | 4 | 2 | 4 | 2 | 0.047 | 0.38 | 0.25 | 0.047 | 0.012 | 0.25 | <10 |
| MC1061(pPom6/11) | E. coli host carrying the cloned blaPOM-1 gene | >256 | 16 | 32 | 4 | 0.125 | 1 | 1 | 0.75 | 0.25 | 0.25 | 16 ± 2 |
| PAO1(pPME6001) | P. aeruginosa host with empty vector | ND | 3 | ND | ND | ND | 0.75 | 1 | 0.38 | ND | 1.5 | <10 |
| PAO1(pPom6/11) | P. aeruginosa host carrying the cloned blaPOM-1 gene | ND | 12 | ND | ND | ND | 2 | >32 | 32 | ND | 1.5 | 230 ± 18 |
AMP, ampicillin; PIP, piperacillin; LOT, cephalothin; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; IMP, imipenem; MEM, meropenem; ERT, ertapenem; AZT, aztreonam; ND, not determined.
Carbapen. act., carbapenemase activity. Carbapenemase activity is shown in nanomoles of imipenem hydrolyzed per minute per milligram of protein. A value of <10 indicates that no significant activity was detected in the extract.
Effect of inoculum size on carbapenem susceptibility of P. otitidis and selection of carbapenem-resistant mutants.
Carbapenem susceptibility of the P. otitidis type strain was remarkably affected by the inoculum size. The imipenem and meropenem MICs increased by 16-fold (from 2 to 32 μg/ml) and 64-fold (from 2 to 128 μg/ml), respectively, when the inoculum size was increased from 105 to 107 CFU. This behavior was consistent with the production of carbapenemase activity and was not observed with P. aeruginosa PAO-1 whose carbapenem MICs increased by only 1- to 4-fold by a similar increase of inoculum size.
By plating a large inoculum (109 CFU) of P. otitidis MCC10330T on a plate containing a gradient concentration (0 to 5 μg/ml) of imipenem, colonies growing in the presence of the highest antibiotic concentrations were reproducibly obtained. Analysis of three of these colonies, selected at random from three independent experiments, revealed carbapenem MICs of >32 μg/ml, while the susceptibility to other β-lactams (including piperacillin, ceftazidime, cefotaxime, and aztreonam) was unchanged. The high-level carbapenem-resistant phenotype was stable upon subculturing in the absence of selective pressure and was not related to modification of the carbapenemase activity or mutations in the blaPOM-1 gene sequence (data not shown). Analogous to other species (e.g., P. aeruginosa), a mutation leading to an outer membrane permeability defect and/or to upregulation of an efflux system could be the most likely cause of the resistance observed in this case as for MCC04511 and YMC-Po/06 isolates. Investigation of this issue will be the subject of future studies.
Concluding remarks.
Investigation of the β-lactamase profile of P. otitidis carried out in this work revealed a unique pattern which consists of constitutive production of a subclass B3 MBL (named POM-1), while other β-lactamases (e.g., AmpC-type β-lactamases) that are resident in P. aeruginosa, P. putida, and P. fluorescens (NCBI completed microbial genomes accessed on 28 July 2010) are apparently lacking. As such, P. otitidis is the first example of a pathogenic Pseudomonas species endowed with a resident MBL.
The fact that several P. otitidis strains were not categorized as carbapenem resistant in conventional susceptibility testing despite the carbapenemase activity of the POM-1 enzyme is not entirely surprising. In fact, a similar phenomenon is well documented with carbapenemase-producing members of the family Enterobacteriaceae (8) and could reflect a relatively high outer membrane permeability to carbapenems in wild-type P. otitidis strains. However, the strong inoculum size effect observed with these drugs and the ease with which resistant mutants could be selected upon carbapenem exposure suggest that the use of carbapenem-based regimens should be considered with caution for treating P. otitidis infections.
Acknowledgments
The skillful technical contribution of Gianluca Lentini to some experimental work is acknowledged.
This work was partially supported by grant 223031 from the European FP7 TROCAR project.
Footnotes
Published ahead of print on 8 November 2010.
REFERENCES
- 1.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. U. S. A. 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark, L. L., J. J. Dajc, C. H. McLean, J. G. Bartell, and D. W. Stroman. 2006. Pseudomonas otitidis sp. nov., isolated from patients with otic infections. Int. J. Syst. Evol. Microbiol. 56:709-714. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A8. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing. Supplement M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Docquier, J. D., F. Pantanella, F. Giuliani, M. C. Thaller, G. Amicosante, M. Galleni, J. M. Frere, K. Bush, and G. M. Rossolini. 2002. CAU-1, a subclass B3 metallo-β-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 46:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garau, G., I. García-Sáez, C. Bebrone, C. Anne, P. Mercuri, M. Galleni, J. M. Frère, and O. Dideberg. 2004. Update of the standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 48:2347-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Inc., New York, NY.
- 8.Miriagou, V., G. Cornaglia, M. Edelstein, I. Galani, C. G. Giske, M. Gniadkowski, E. Malamou-Lada, L. Martinez-Martinez, F. Navarro, P. Nordmann, L. Peixe, S. Pournaras, G. M. Rossolini, A. Tsakris, A. Vatopoulos, and R. Canton. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112-122. [DOI] [PubMed] [Google Scholar]
- 9.Rossolini, G. M., M. A. Condemi, F. Pantanella, J. D. Docquier, G. Amicosante, and M. C. Thaller. 2001. Metallo-β-lactamase producers in environmental microbiota: new molecular class B enzyme in Janthinobacterium lividum. Antimicrob. Agents Chemother. 45:837-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, New York, NY.