Abstract
Antimicrobial pharmacokinetic-pharmacodynamic (PK/PD) science and clinical trial simulations have not been adequately applied to the design of doses and dose schedules of antituberculosis regimens because many researchers are skeptical about their clinical applicability. We compared findings of preclinical PK/PD studies of current first-line antituberculosis drugs to findings from several clinical publications that included microbiologic outcome and pharmacokinetic data or had a dose-scheduling design. Without exception, the antimicrobial PK/PD parameters linked to optimal effect were similar in preclinical models and in tuberculosis patients. Thus, exposure-effect relationships derived in the preclinical models can be used in the design of optimal antituberculosis doses, by incorporating population pharmacokinetics of the drugs and MIC distributions in Monte Carlo simulations. When this has been performed, doses and dose schedules of rifampin, isoniazid, pyrazinamide, and moxifloxacin with the potential to shorten antituberculosis therapy have been identified. In addition, different susceptibility breakpoints than those in current use have been identified. These steps outline a more rational approach than that of current methods for designing regimens and predicting outcome so that both new and older antituberculosis agents can shorten therapy duration.
Advances in the treatment of tuberculosis (TB) have suffered from a lack of scientific imagination. A century ago, TB research was the true cutting edge of medical innovation (65, 90, 97). Unfortunately, while scientific knowledge for the therapy of most diseases virtually exploded, we and others in the TB world still use dated paradigms and language. In the meantime, the pandemic shows no signs of subsiding. In 2008, there were 8.9 to 9.9 million incident cases of TB; prevalence was 9.6 to 13.3 million cases, and 1.8 million people died from TB (107). “Short-course chemotherapy” is actually long. Long-term mortality, morbidity, relapse, and resistance emergence are still problems even after “successful” therapy (20, 77, 78, 93). Indeed, while it often said that the therapy has a 95% success rate under directly observed therapy (DOTS) programs, a look at high-TB-burden countries with good DOTS programs reveals cure rates for pulmonary TB of only 34 to 76%, and those are under study conditions (1, 103). Recently, the efficacy of DOTS itself has been challenged (14, 103). Clearly, the traditional approaches have not succeeded in eradicating TB. It is time to take a different road.
The advent of anti-TB chemotherapy in the 1940s was a miracle; we have all benefited from that work of scientific “giants.” Since then, important developments have occurred in other areas of science which have the potential to further catapult anti-TB therapeutics. The first of these is the application of molecular methods and high-throughput screening to identify promising therapeutic molecules. This has led to the discovery of a variety of anti-TB molecules. A second important development is antimicrobial pharmacokinetic-pharmacodynamic (PK/PD) science, founded on the work of Eagle and Craig (5, 21, 22, 39). Third has been population pharmacokinetic (PK) analysis, founded on the work of Sheiner (94, 95). Population PK analysis became possible because of the advent of electronic computers, starting with the Atanasoff-Berry model of 1939. Further work in the field led to the ENIAC, which was utilized to establish and test a new mathematical technique, the Monte Carlo method (69, 70). Its initial utility was shown by application to the study of fissile material. Starting around 1986, computer-aided clinical trial simulations were applied to clinical dosing regimens (12, 58, 100). Greater knowledge of concentrations associated with optimal efficacy, and the application of population PKs, eventually enabled full clinical trial simulations that depend on the Monte Carlo methods. In the field of infectious diseases, Drusano et al. utilized these methods to identify the optimal clinical doses and susceptibility breakpoints of antimicrobial agents by integrating exposures derived in preclinical PK/PD models and the distribution of MICs in clinical isolates (38). Each of these developments has a direct role in developing new paradigms for the treatment of TB.
There have been concerns by researchers as to the applicability to TB patients of antimicrobial PK/PD parameters derived in preclinical models. In particular, preclinical PK/PD models such as small rodents and hollow-fiber systems have met with skepticism (71, 73). It has also been stated that the existence of the three metabolic populations of Mycobacterium tuberculosis is more important in determining bacterial response to drugs than are PK/PD relationships (71). Dose selection has tended to ignore PK/PD methods and to rely on more traditional approaches. When the PK/PD methods are applied, this is often peripheral to the clinical development of the drug and rarely for the derivation of optimal doses and dose schedules. Since the treatment of TB relies on a multidrug regimen, the drugs enter combination without rational optimization, compounding the errors.
To get to the root of discontent and perceived discordance with anti-TB PK/PD science, we performed a Medline search for PK/PD work that included adequate PK, dose-effect, and dose-scheduling studies. First-line drugs examined included rifamycins (rifampin or rifapentine), isoniazid, pyrazinamide, and ethambutol. Moxifloxacin, which is currently being tested for a role as a first-line agent (17), was also included. There were 11 antimicrobial PK/PD studies in which these drugs had been studied (45, 50-55, 60, 61, 92, 98). Next, clinical studies performed with anti-TB monotherapy and dual therapy, in which PKs were documented or a dose-scheduling study design was employed, were sought throughout the literature. The drug concentrations published in these clinical studies were modeled by us using population PK analysis in the computer programs ADAPT II and ADAPT 5 (25, 26), when data were presented in such a way that this could be performed. Since the drug concentration that is associated with killing of pathogens in pulmonary infections is best reflected by that in epithelial lining fluid (ELF) (22, 23, 48, 55), the exposures achieved in the epithelium were utilized for this minireview.
PYRAZINAMIDE
The hollow-fiber system (HFS) is a model in which bacteria and viruses grow and are exposed to dynamic drug concentrations that mimic human PKs (10, 11). We have adapted the model to examine PK/PD parameters against various metabolic populations of M. tuberculosis. Pyrazinamide PK/PD studies were performed in the HFS (55), which revealed kill rates and the time to emergence of resistance that were then compared to those encountered in the sputum of TB patients treated with monotherapy in the classic clinical studies of Yeager et al. and Jindani et al. (62, 63, 109). The results shown in Table 1 demonstrate that time to start of microbial kill and kill rates were similar between the PK/PD model and patients. In addition, resistance emergence occurred between 2 and 3 weeks in the HFS, similar to the timing of the “escape” phenomenon in patients (109).
TABLE 1.
Pyrazinamide kill rates in sputum of tuberculosis patients versus those in the hollow-fiber systema
| Time | Kill rate in system |
|
|---|---|---|
| Hollow fiber | Patient sputum | |
| Day 0-4 (log10 CFU/ml) | −0.1 | −0.1 ± 0.2 |
| Day 4-14 (log10 CFU/ml) | 0.09-0.1 | 0.12 ± 0.05 |
Time to emergence of resistance was 2 to 3 weeks for patients and hollow-fiber systems.
The pyrazinamide PK/PD parameter linked to microbial kill in the HFS was the 0- to 24-h area under the concentration-time curve (AUC) to MIC (AUC0-24/MIC) ratio (55). The parameter linked to pyrazinamide resistance was the percentage of time that the concentration persisted above MIC (%TMIC), with optimal resistance suppression associated with a %TMIC of >67% of the dosing interval (55). To establish the veracity of this in the clinic, we reanalyzed the results of a clinical study performed in East Africa four decades ago (13). All patients were treated with 1 g of intramuscular streptomycin daily for 6 days of the week. In one group of patients, pyrazinamide was administered as 0.5 g given three times daily (t.i.d.), in the second group as a single 1.5-g dose (QD), and in a third group administered as a 3-g dose three times weekly (t.i.w.). Patients were examined each month for positive sputum cultures and for the emergence of pyrazinamide resistance. Pyrazinamide concentrations were also measured in subgroups of these patients (40). We performed a population PK analysis and then compared the rate of patients with sputum conversion between groups with different pyrazinamide exposures. Penetration of the drug into epithelial lining fluid (ELF) was taken as 17.8-fold that in serum, based on studies elsewhere (18). The modal MIC was taken as 50 mg/liter, based on this and another study (88). Table 2 demonstrates that there were no differences in sputum conversion after 2 months (P = 0.57) or at 6 months (P = 0.15) or either moderate to considerable radiological improvement (P = 0.26) or any radiological improvement (P = 0.64). Thus, clinical outcomes were most closely associated with the AUC. However, since the point of combination therapy is to reduce the emergence of resistance to the companion drug, it is not possible to draw PK/PD conclusions on resistance, since all patients also received streptomycin.
TABLE 2.
Relationship between pyrazinamide exposure and outcome in tuberculosis patients
| Parameter (measurement) | Result for dosing regimen |
||
|---|---|---|---|
| 500 mg three times a day | 1,500 mg daily | 3,000 mg alternate days | |
| Serum AUC0-168 (mg·h/liter) | 2,189 | 2,267 | 2,163 |
| ELF AUC0-24/MICa | 111 | 115 | 110 |
| Serum Cmax (mg/liter) | 20.2 | 33.8 | 67.5 |
| ELF Cmax/MICa | 7.2 | 12.0 | 24 |
| Sputum conversion (no. converted/total no. [%]) | |||
| 2-month time point | 30/65 (46) | 34/70 (49) | 39/71 (55) |
| End of 6 months | 28/66 (42) | 32/72 (44) | 42/73 (58) |
| Any radiological improvement (no. showing improvement/total no. [%]) | 32/63 (51) | 41/70 (59) | 40/70 (57) |
| Pyrazinamide resistance (no. resistant/total no. [%]) | 21/63 (33) | 20/65 (31) | 17/68 (25) |
| Streptomycin resistance (no. resistant/total no. [%]) | 39/65 (60) | 38/69 (55) | 29/72 (40) |
Several pyrazinamide population PK studies have been performed (106, 111). Variability in pyrazinamide systemic clearance (SCL) and volume of distribution (Vd) were driven by patient weight and gender (40, 106). Based on these PK parameter estimates and covariates and the AUC/MIC associated with maximal kill in the HFS, as well as MIC distribution in clinical isolates, Monte Carlo simulations were performed (55). The standard dose of 15 to 30 mg/kg achieved an exposure associated with optimal bacterial kill in ≤53% of 10,000 simulated patients, with the best performance achieved by doses ≥60 mg/kg. In order to suppress drug resistance, doses of ≥3 g would have to be administered daily. These high doses predicted to be more optimal in clinical trial simulations lead to the obvious concern of toxicity. We examined the role of high doses in causing arthropathy and hepatoxicity in patients, based on a toxicodynamic analysis as well as a meta-analysis of 29 prospective controlled clinical studies (76). Arthropathy was clearly associated with administration of pyrazinamide and was best associated with the amount of time that serum concentration persisted above 5 mg/liter. We speculate that pyrazinoic acid (POA) saturates xanthine oxidase at a relatively low concentration, and the longer the concentration persists above this threshold the more urate is deposited in joints, but once therapy stops the concentrations quickly fall below threshold and deposition stops. Fortunately, the arthropathy is usually clinically inconsequential. On the other hand, the incidence of hepatotoxcity was no different whether regimens consisted of pyrazinamide or not, and when doses of pyrazinamide were examined, daily doses of 30 mg/kg, 40 mg/kg, or 60 mg/kg did not result in statistically significant increases in hepatotoxicity when the drug was administered for only 2 months (76). Obviously, this final conclusion is controversial, and further work on pyrazinamide toxicity is still needed. Nevertheless, the results mean that the higher doses predicted to be more effective by Monte Carlo simulations should be examined for both faster sputum conversion and shorter-duration anti-TB therapy. We acknowledge that since hepatotoxicity may be fatal, caution will be needed even in conducting such clinical trials.
ISONIAZID
Prior to the current PK/PD era, Dickinson et al. injected tubercle bacilli into thigh muscles of guinea pigs and then treated them with isoniazid doses of 4, 8, 16, or 32 mg/kg every 8 days, as a single dose, or every 4 days, 2 days, or 1 day (30). The only measure of disease burden of the several they employed that we could subject to current PK/PD interpretation is spleen bacillary CFU, which demonstrated a clear dose-response relationship between the doses but demonstrated no differences in CFU/spleen within each dose regardless of dosing schedule. There was no resistance emergence. These results are consistent with an AUC/MIC-driven effect. We used (or interpreted) their published PK and CFU/spleen in an inhibitory sigmoid maximal effect (Emax) model. We identified the 50% effective concentration (EC50) as an AUC0-24/MIC ratio of 82 ± 21 and a Hill coefficient of 1.6 ± 1.3 (r2 = 0.99). However, the study had obviously not been designed with PK/PD considerations in mind, having been performed in an earlier period. The PK/PD properties of isoniazid were actually first examined in a murine model of TB by Jayaram et al. (61). Dose-scheduling studies demonstrated that isoniazid microbial kill was linked to the AUC0-24/MIC ratio (r2 = 0.83). A second study utilized the HFS and confirmed that microbial kill was indeed AUC0-24/MIC linked (r2 = 0.97) (54). The HFS study further demonstrated that both AUC/MIC and Cmax/MIC were linked to resistance suppression (54). Comparison of the inhibitory sigmoid Emax curves from these preclinical studies to a dose-effect early bactericidal activity (EBA) study of TB patients (32), reveals results shown in Table 3, virtually similar across the three systems, as well as to the guinea pig study discussed above. The EBA of a drug is the daily rate of sputum bacillary load decline in patients during the first 2 days of therapy (33). Indeed, the effect of both dose and acetylation status (and therefore SCL) on EBA has been explicitly demonstrated in patients by Donald et al. (31, 32, 34). Since AUC0-24 is proportional to dose/SCL, this further confirms the link between AUC and clinical effect. These results point to remarkable concordance between preclinical PK/PD models and pharmacologic events in early therapeutic events in TB patients.
TABLE 3.
Isoniazid inhibitory sigmoid Emax curve in the hollow-fiber system, mice, and humans
| Inhibitory sigmoid Emax model parameter | Result in system |
||
|---|---|---|---|
| Mouse | Hollow fiber | Patient sputum | |
| EC50 (AUC0-24/MIC) ratio | 63 | 62 ± 28 | —a |
| Hill coefficient (slope) | 1.0 | 0.9 ± 0.4 | 1.0 ± 0.4 |
| Emax for EBAb (log10 CFU/ml per day) | —c | 0.9 ± 0.2 | 0.6 ± 0.1 |
| r2 | 0.83 | 0.97 | 0.95 |
Reported as mg/kg/day.
EBA, early bactericidal activity.
Calculated as 0.2 log10 CFU/g of mouse lung.
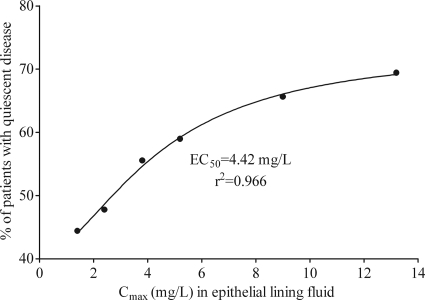
In the 1960s, several monotherapy doses of isoniazid were administered to TB patients in Chennai for up to a year (43, 44, 91). Monthly rates of sputum conversion and resistance emergence, as well as serum isoniazid concentrations, were measured by the investigators. We performed population PK analysis of these concentrations, taking into account a penetration ELF/serum ratio of 2 (19, 64), and then related the drug exposures to the proportion of patients with quiescent disease at the end of 12 months. Since this was monotherapy, there was considerable resistance emergence, so the outcomes at 12 months should be viewed as a composite measure of microbial kill and resistance emergence. %TMIC is difficult to calculate from the study design, since it will depend on the concentration-time profile and the MIC of the isolate infecting each patient. Nevertheless, for 200 mg twice daily (b.i.d.) versus 400 mg once daily (QD) for each acetylation status, the average %TMIC will be greater with b.i.d. dosing (the more fractionated regimen) than with the once-daily regimen. Thus, the response rate should be greater with the b.i.d. regimen than the QD regimen at each time point if the effect is %TMIC linked. However, the proportion of patients with negative sputum was 25/34 (74%) versus 23/39 (59%) for slow acetylators (P = 0.22) and 21/32 (66%) versus 15/27 (56%) for fast acetylators (P = 0.59), with QD versus b.i.d. dosing, respectively. Indeed, while this result is not statistically significant, more patients actually did better with QD dosing, which implies the opposite of the %TMIC link. Based on the sigmoid Emax model of AUC0-24 versus the proportion of patients with successful therapy at 12 months, the EC50 was 31.26 ± 36.37 mg·h/liter (r2 = 0.49). It should be noted that the EC50 in this case is expressed as AUC0-24, not as an AUC0-24/MIC ratio. When Cmax and response were examined in a sigmoid Emax model, the relationship between Cmax and the proportion of patients with good response was as shown in Fig. 1. To reiterate, final response status in this study should be viewed as a composite of both bacterial kill and the emergence of drug resistance. In terms of resistance, the proportion of patients with resistance at each of four observation periods was examined for 200 mg b.i.d. versus 400 mg QD, and the b.i.d. dosing schedule, associated with a higher %TMIC, was associated with a lower proportion of patients with resistance only one of eight times. Thus, %TMIC was likely not linked to resistance emergence, leaving AUC/MIC and Cmax/MIC as the remaining possibilities. This would also be consistent with preclinical PK/PD models. This suggests, for monotherapy, that PK/PD indices associated with microbial kill in preclinical models are the same as in EBA studies and are also the same as in long-duration TB treatment.
FIG. 1.
Relationship between isoniazid Cmax in epithelial lining fluid and proportion of patients with response at 12 months.
There were two other interesting studies which employed dose fractionation techniques but did not measure drug concentrations (59, 85). Nevertheless, these studies are instructive. The first study was of patients with noncavitary TB treated for 6 months with isoniazid either 300 mg QD (n = 109) or 100 mg t.i.d. (n = 99) (59). Times to sputum conversion were virtually identical in either dose schedule (59). In the second study, isoniazid dosing schedules were as above, but both groups received 4 g of Para-aminosalicylic-acid t.i.d. (85). Toxicity rates, radiological improvement rates, and time to sputum conversion were also virtually identical. These studies confirm that it is the total isoniazid daily dose, and not the dosing schedule, which drives microbiologic and other clinical outcomes. Therefore, AUC drove clinical effect. The second study also means that the PK/PD parameter associated with optimal isoniazid microbial kill is the same in long-term combination therapy studies as in monotherapy studies.
Clinical trial simulations were performed to identify the effect of the standard dose of 300 mg in patients from several areas in the world. This study was to benchmark the accuracy of use of preclinical PK/PD exposures in Monte Carlo simulations of TB patients (54). Isoniazid SCL is genetically determined via single-nucleotide polymorphisms (SNP) of the n-acetyltransferase 2 (NAT-2) gene (41). Using the relationship between AUC/MIC and bacterial kill from HFS studies, and population pharmacokinetic parameters of isoniazid from Peloquin et al. (82), as well as the frequency of NAT-2 SNPs in patients in Cape Town, Hong Kong, and Chennai, the EBA expected to be achieved in 10,000 TB patients treated with isoniazid 300 mg daily was identified (2, 25, 34, 89). The results were then compared to those observed in clinical trials (96). Results in Table 4 demonstrate that in silico clinical trials were able to predict regional EBAs accurately. Thus, this pathway provides a rational method to be used in further anti-TB regimen design.
TABLE 4.
In silico clinical trial predictions versus observed isoniazid early bactericidal activity
| Location of patient population | Estimated EBA (95% CI)a |
|
|---|---|---|
| Predicted | Found in clinical trials | |
| Hong Kong | 0.40 (0.23-0.69) | 0.37 (0.16-0.58) |
| Cape Town | 0.60 (0.38-0.74) | 0.65 (0.43-0.87) |
| Chennai | 0.90 (0.40-0.93) | 0.94 (0.45-1.43) |
EBA, early bactericidal activity (log10 CFU/ml per day). CI, confidence interval.
RIFAMYCINS
Two studies from the era prior to modern PK/PD studies offer interesting insights. Mitchison and Dickinson treated guinea pigs (72), while Verbist treated mice that had TB with rifampin for up to 6 weeks (102). There was microbiologic and survival superiority in animals treated with once-weekly rifampin compared to the same cumulative dose divided and given daily. With current understanding, this would be most consistent with a Cmax/MIC-driven effect. The first-ever published study employing current PK/PD methods for anti-TB drugs was in 2003 by the group led by Balasubramanian (60). In a 6-day murine TB study, they demonstrated that rifampin microbial kill was linked to the AUC0-24/MIC ratio (r2 = 0.95), closely followed by Cmax/MIC (r2 = 0.86). We performed HFS studies and also demonstrated that rifampin microbial kill was indeed AUC0-24/MIC linked (51). Furthermore, the HFS also demonstrated that rifampin resistance suppression (r2 = 0.84) and postantibiotic effect (r2 = 0.96) were most closely linked to the Cmax/MIC ratio, with optimal suppression of resistance at a Cmax/MIC of 175. In fact, all four studies are essentially saying the same thing: the longer-duration animal studies from circa 1970 are talking about a composite measure of effect consisting of microbial kill, postantibiotic effect, and resistance suppression over a long-enough period that allows enough rounds of replication by the slowly growing M. tuberculosis, which unmasks many of the Cmax/MIC-linked effects identified by HFS studies (51). This is consistent with a clinical study from Poland in which patients with isoniazid-resistant TB were treated with 600 mg of rifampin and 25 mg/kg of ethambutol daily for 12 weeks, followed by either rifampin 600 mg plus 50 mg/kg ethambutol twice a week or rifampin 1,200 mg plus ethambutol 50 mg/kg for up to 2 years (6). Thus, during the long continuation phase both groups received a 1,200-mg rifampin dose weekly and therefore had similar AUCs (AUC = dose/SCL), but the once-weekly regimen had twice the Cmax and a lower %TMIC. Relapse occurred in 5/74 patients treated with 600 mg twice weekly but only 1/168 with the once-weekly regimen (P = 0.02), despite the fact that the once-weekly treated patients actually received less ethambutol. Thus, the PK/PD parameters identified in preclinical models have relevance not only during the continuation phase of therapy but to relapse.
Cognizant of the PK/PD studies, Nuermberger and colleagues ignored rifapentine's mouse half-life of 14 to 18 h (8, 9) and instead of dosing once a week, as the half-life would dictate, treated mice with either twice-weekly, thrice-weekly, five-times-weekly, or daily rifapentine, in combination with moxifloxacin and pyrazinamide (86). They measured drug concentrations, which they related to time to sterilizing effect. Table 5 is adapted from their work. The %TMIC was ≥90% in all rifapentine regimens and 60% in the standard regimen and was therefore above the optimal exposures of 60% in all regimens. As can be seen in Table 5, as AUC0-168/MIC and Cmax/MIC increased, duration of therapy needed to cure the mice without relapse decreased, so therapy could be shortened to 3 months or less by optimizing the PK/PD parameters. These fascinating results provide dramatic evidence that PK/PD-optimized regimens have the potential to shorten therapy duration.
TABLE 5.
Relationship between rifapentine exposure, duration of therapy, and relapse in mice (adapted from reference 86 with permission)
| Free drug exposure |
%TMIC after monthsa |
||||
|---|---|---|---|---|---|
| AUC0-168/MIC | Cmax/MIC | 2 | 3 | 4 | 6 |
| 311b | 6.6b | ND | ND | 90 | 0 |
| 547 | 8.7 | ND | 10 | 0 | |
| 674 | 7.5 | 60 | 5 | ||
| 730 | 11.6 | 95 | 20 | ||
| 803 | 10.2 | 95 | 0 | ||
| 899 | 10.0 | 35 | 0 | ||
| 1,129 | 10.3 | 20 | |||
ND, not determined.
Rifampin rather than rifapentine was used.
The most cogent call for higher doses of rifampin in the PK/PD era first came from Peloquin, almost a decade ago (79). Recently, Goutelle et al. (48) performed 10,000 patient clinical trial simulations to test the adequacy of rifampin doses of either 600 mg or 1,200 mg daily. These doses were examined for the ability to achieve an AUC0-24/MIC of ≥665 in ELF of TB patients based on optimal rifampin microbial kill in murine PK/PD studies (60) or the Cmax/MIC of ≥175 associated with resistance suppression in HFS studies (51). The study revealed that 600 mg achieved a Cmax/MIC of ≥175 in only 36% and an AUC0-24/MIC of ≥665 in only 55% of TB patients. However, the dose of 1,200 mg performed better. Thus, the best step forward would be to reexamine the maximum tolerated doses of rifamycins administered as daily doses. Recently, Diacon et al. examined the effect of increasing rifampin doses and exposures on EBA in South African patients (29). Their results, shown in Table 6, demonstrate that as the AUC0-24 increased, EBA also increased. However, clearly the AUC0-24 and Cmax covaried. This not only confirms the clinical trial simulations but also demonstrates that even at double the standard dose, the dose is still on the steep portion of the dose-response curve, so that even higher doses would result in faster bacterial kill. Thus, if patients could tolerate it, increasing rifamycin doses and administering them daily could offer a possibility for reducing the duration of therapy.
TABLE 6.
Relationship between rifampin exposure and early bactericidal activity in patients
| Dose (mg/kg) | Cmax (mg/liter) | AUC (mg·h/liter) | EBA (log10 CFU/ml per day) |
|---|---|---|---|
| 3 | 2.53 | 13.1 | 0.03 |
| 6 | 3.19 | 24.5 | 0.12 |
| 12 | 13.0 | 100.0 | 0.22 |
| 20 | 14.0 | 171.0 | 0.44 |
FLUOROQUINOLONES
Some of the very pivotal studies that established the clinical relevance of general PK/PD science were with fluoroquinolones; these agents' microbial kill in many bacteria is linked to AUC/MIC (5, 37, 42). Microbial kill of M. tuberculosis by these compounds was linked to the AUC0-24/MIC ratio in murine TB (92) likely because of a long postantibiotic effect (PAE) (45). The earliest fluoroquinolone PK/PD work for the treatment of TB was actually performed in the HFS (50, 52). Moxifloxacin dose-effect studies and a ciprofloxacin study demonstrated that some AUC0-24/MIC exposures associated with excellent microbial kill maximally amplified the size of the resistant subpopulation (50, 52). For quinolone monotherapy, effective kill was terminated in 10 to 14 days by resistance emergence in HFS. The time frame was consistent with a case report that had just been published (47). In other words, based on the HFS, with currently recommended doses as monotherapy there is a biphasic effect, starting with a good response (microbial kill) followed by “relapse.” The biphasic response in TB patients inadvertently treated with quinolones, as well as the emergence of resistance with at least 10 to 13 days of fluoroquinolone exposure, has since been demonstrated in large clinical studies (28, 36). Thus, clearly, preclinical PK/PD models were predictive of events later encountered in patients. The ciprofloxacin PK/PD study explicitly pointed out the potential consequence of using ciprofloxacin and ofloxacin in MDR-TB at standard doses when they are often effectively monotherapy given the low efficacy of other second-line drugs: resistance would emerge rapidly (50). The danger predicted by the preclinical PK/PD models became reality a few years later with the emergence of extremely drug-resistant TB (XDR-TB), given the poor efficacy of accompanying second-line drugs (14, 84).
The HFS studies also established that at higher exposures, such as a free drug AUC0-24/MIC ratio of >53 (total drug, 106), resistance could be minimized (52). However, one murine TB study reached somewhat a contrary conclusion when mice exposed to a continuous oral diet of moxifloxacin that achieved an AUC0-24/MIC of >106 by day 56 still developed moxifloxacin-resistant M. tuberculosis (46). However, the authors pointed out that the mice had a poor oral intake of the drug in the beginning, and quinolone resistance could have developed early under subtherapeutic exposures. Another explanation could be that the HFS study was for a short duration of time while the mouse study was for 2 months and the exposure identified in the HFS as suppressing resistance would fail to suppress resistance in longer-term studies. It is known, for example, that resistance emergence is a function of therapy duration (54, 99). In another study, mice that achieved a total moxifloxacin AUC0-24/MIC of 54.8 (free drug, 27.4) developed drug resistance while mice that received exposures far above the ratio of 106 did not develop moxifloxacin resistance (3).
Moxifloxacin doses of 400, 600, and 800 mg each day were examined for the ability to achieve or exceed the free-drug AUC0-24/MIC of ≥53, associated with suppression of resistance in HFS in Monte Carlo simulations (52). These doses were able to achieve this in 59%, 86%, and 93% of virtual patients, respectively. In a recent clinical study in Brazil, Peloquin et al. administered 400 mg of moxifloxacin to nine TB patients, performed PK studies, and established a moxifloxacin MIC for each patient's M. tuberculosis isolate (81). Their data reveals that an AUC0-24/MIC of ≥53 was achieved in 66% of the TB patients, very close to the 59% predicted by the clinical trial simulations. Thus, the pathway that starts with preclinical PK/PD models, which is then translated using clinical trial simulations, has good clinical predictive power. The simulations predict that daily doses of 800 mg may be more optimal. However, the tolerability of this dose is unknown. Nevertheless, examining the safety of these higher doses is made more pressing by the finding that such likely companion drugs as rifampin reduce the moxifloxacin AUC0-24 by about one-third (105), so even the intended exposures for 400 mg a day are often not achieved.
ETHAMBUTOL
In 1968, Dickinson et al. studied the effect of spacing out ethambutol doses of 640, 160, 320, and 80 mg/kg every 8 days either as a single dose every 8 days or as equally divided doses every 4 days, 3 days, and 1 day in guinea pig TB (30). After 6 weeks of treatment with ethambutol, the authors reported that the less frequently administered doses were associated with a lower spleen bacillary burden. This would nowadays be interpreted as a Cmax/MIC-linked effect. Our examination of their data within each dose group revealed no consistent progressive decrease in CFU/spleen with increase in dosing interval, so we could also read an AUC/MIC-linked effect into that study. Ethambutol PK/PD studies using current standards were recently performed in HFS (98). In a dose-effect study, the relationship between exposure and EBA revealed a maximal effect (Emax) of 0.22 (95% CI, 0.14-0.29) log10 CFU/ml/day; after 2 days the maximal kill rates fell to 0.04 to 0.10 log10 CFU/ml/day (98). In TB patients, ethambutol is also dose dependent, with a maximal EBA of 0.26 ± 0.12 log10 CFU/ml/day, followed by 0.16 ± 0.09 log10 CFU/ml/day between days 2 and 14 (62, 63). Microbial kill in the HFS was linked to AUC0-24/ MIC, although in some experiments Cmax/MIC could also have explained the kill. In addition, this differs from studies with intracellular M. avium, which demonstrated a link to Cmax/MIC in the HFS (27).
In terms of resistance, treatment with ethambutol monotherapy for up to 7 days also abolished the isoniazid effect (98). Emergence of dual ethambutol and isoniazid resistance was due to induction of efflux pumps common to both drugs, which was linked to %TMIC. In vitro studies by Colangeli et al. have demonstrated induction of ethambutol and isoniazid resistance by exposure to isoniazid alone (16). The PK/PD results may explain, in part, why ethambutol resistance in clinical isolates of M. tuberculosis is often accompanied by isoniazid resistance (56, 68, 75).
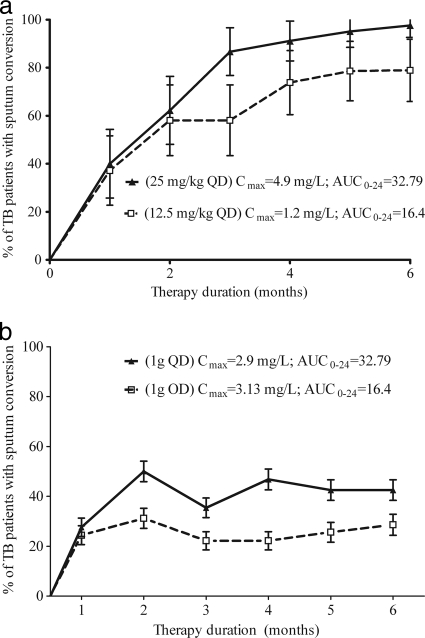
In one study, Japanese patients were treated with two different doses of ethambutol administered either concomitantly with isoniazid or as monotherapy (35). Unfortunately, in that study, our attempts at population PK analysis led to rather imprecise estimates of SCL, very different from PK parameters in recent population PK publications (80, 110); thus, estimates of AUC are less precise. With that limitation in mind, the relationships between outcome and PK/PD exposure are shown in Fig. 2. The role of %TMIC could not be determined given that in addition to the imprecision of estimating SCL, the MICs were also unknown. The sputum conversion rates seem more consistent with an AUC-driven effect, given that in Fig. 2 B there are virtually similar Cmaxs, but different outcomes. However, clearly definite conclusions could not be drawn, and further examination of the correlations between preclinical PK/PD studies, population PK, and clinical outcome is still needed.
FIG. 2.
Relationship between ethambutol exposure and sputum conversion in patients. (a) Initial treatment with 12.5 mg/kg versus 25 mg/kg. (b) Retreatment with 1 g.
In Chennai (India), TB patients were treated with one of four regimens, two amenable to dose-scheduling analysis: ethambutol 45 mg/kg and isoniazid 15 mg/kg given twice weekly (E2H2) versus ethambutol 90 mg/kg once a week with isoniazid 15 mg/kg twice weekly (E1H2) (7). The proportions of patients with good microbiological response at 1 year were 88/102 for the E2H2 group and 96/101 for the E1H2 group (P = 0.052); if subsequent relapse was also taken into consideration, patients with a good bacteriological outcome at the end of the study would be 78/102 in the E2H2 group and 82/101 for E1H2 (P = 0.49). In a subgroup of the patients who were found to have isoniazid resistance prior to the start of treatment (and thus got ethambutol as the only effective agent during the second phase of therapy), 6/11 E2H2-treated versus 4/10 E1H2-treated patients had a good response by the 1-year time point (P = 0.67). Thus, in these two groups that received identical isoniazid regimens but two different dosing schedules for ethambutol, outcomes were similar, consistent with an AUC/MIC-driven effect.
IDENTIFICATION OF NEW SUSCEPTIBILITY BREAKPOINTS
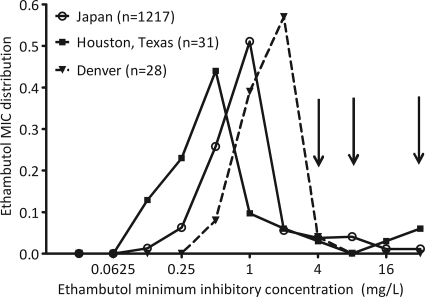
As for other infectious-disease organisms, the susceptibility breakpoints for M. tuberculosis were established based on epidemiologic cutoff values. Based on this philosophy, resistance breakpoint is the critical concentration that inhibits the growth of 95% of wild-type isolates of bacteria from patients who have not been exposed to the drug (15). This was an important breakthrough in the standardization of susceptibility testing and still forms the backbone of M. tuberculosis susceptibility testing all over the world. From our vantage point in time, however, that approach has some limitations. This approach somehow assumes, de facto, that the MIC distribution pattern of wild-type isolates in species will be the same around the globe. Studies of anti-TB drug MIC distribution from around the world are very scanty and, when available, involve small numbers. Even with these limitations, however, a selection of ethambutol susceptibility patterns obtained using Middlebrook broth-based methods from three different places and times (57, 104, 108), when compiled, look like those in Fig. 3. This is no surprise when looked at from the point of view of evolution: M. tuberculosis has continued to evolve in patients in different locales around the world, and part of that is reflected by the variability of MIC distributions. In Fig. 3, the 95% cutoff point would lead to three different breakpoints. Which locale's wild-type MIC distribution should be used for breakpoints used all over the world? It has become clear for many bacteria that while epidemiologic cutoff values perform well in detecting changes in resistance patterns in each locale at an epidemiologic level, their predictive power is limited when it comes to individual patient response to therapy (4, 74, 101).
FIG. 3.
Distribution of ethambutol MICs from three different places.
The ability of any antibiotic to kill bacteria in a majority of patients will also depend on the drug exposures achieved at site of infection based on penetration ability, as well as the between-patient variability in concentrations achieved. In other words, there is no “average” patient, so drug concentrations assumed to be achieved in the average patient have little clinical meaning from patient to patient. After administration of the highest tolerable dose, bacterial isolates with MICs that cannot be achieved or exceeded by the drug concentrations in most patients (usually ≥90%), given pharmacokinetic variability, are defined as resistant to the drug since they will not be killed in an acceptable proportion of patients. Using Monte Carlo simulations based on population pharmacokinetics, and the PK/PD exposures associated with near-maximal kill of M. tuberculosis at the site of infection, we identified new susceptibility breakpoints for isoniazid, rifampin, and pyrazinamide, while for ethambutol and moxifloxacin the breakpoints remained unchanged (Table 7) (49). The results suggest that the prevalence of MDR and XDR TB might be higher than currently assumed.
TABLE 7.
Current versus proposed susceptibility breakpoints in Middlebrook medium
| Drug | Breakpoint (mg/liter) |
|
|---|---|---|
| Current | Proposed | |
| Ethambutol | 5 | 4 |
| Isoniazid | 0.2/1.0 | 0.03/0.125 |
| Moxifloxacin | 1.0 | 1.0 |
| Pyrazinamide | 100 | 50 |
| Rifampin | 1 | 0.0625 |
It could be argued that current anti-TB drug susceptibility breakpoints have done well in the clinical setting, so there is little need to change them. However, as discussed earlier, in many places cure rates in “drug-susceptible” TB are 34 to 76% despite DOTS. Second, breakpoints for other agents, such as cephalosporins (to name but one class), that “worked well” for decades in the past against Enterobacteriaceae have been revised downwards based on methods similar to the ones we employed, and the newer breakpoints have been found to perform even better in terms of predicting clinical success and failure (4, 74). Thus, arguments of past performance are not sufficient against an improvement of accuracy. A better approach would be to test the accuracy of the newly proposed breakpoints against that of the older ones in prospective clinical studies.
LIMITATIONS
As with all methods used in science, there are some limitations to the methods utilized in the field. First, most antimicrobial PK/PD studies performed so far have examined one strain of M. tuberculosis, either M. tuberculosis H37Rv or H37Ra. While the PK/PD parameter linked to effect will not change across strains, inclusion of more clinical strains leads to a more robust and precise estimate of exposures associated with optimal effect (67). Second, for some anti-TB agents, the only reliable population PK estimates are from studies of healthy volunteers. Often, the disease process itself as well as common comorbid conditions such as AIDS will alter antibiotic PK estimates (37, 83). Thus, more population PK data from TB patients are needed. Third, in the case of the penetration of anti-TB drugs into ELF, some studies have utilized only one or two ELF sampling time points. The penetration of drug from serum to ELF is a function of time, and the concentration-time profile of antibiotic in blood and ELF may vary substantially in a strongly nonlinear fashion based on lag, i.e., hysteresis (66, 87). Thus, examination of concentrations at a single time point in ELF (or any other PK compartment for that matter), as was the case for the published data we used for Monte Carlo simulations, may introduce imprecision. Another problem is the very small number of clinical isolates used in determining MIC distributions. Indeed, one of the greatest deficits in the TB world is the lack of good studies of MIC distributions of a large number of isolates from different geographic areas. This, too, impacts on an optimal dose determination which takes into account the MIC distribution. A closely related problem is the technical difficulty in performing pyrazinamide MICs, so that even when enough isolates are tested, different pyrazinamide MIC distributions may result when the same isolates are tested. In this respect, our use of terms such as “modal MIC” of this drug (see, for example, Table 2) needs to be viewed with this limitation in mind. However, these limitations represent more areas that should be prioritized for further research, so that even more precise forecasting can be performed than is currently done.
A SCIENTIFIC PATHWAY INTO THE FUTURE
There are several overall lessons from currently available literature. First, preclinical antimicrobial PK/PD data have great clinical relevance to the treatment of TB. Thus, as new drugs are created, it would be advantageous to have them undergo rigorous PK/PD studies. Each of the drug exposures must be optimized to maximize microbial kill and chances of suppressing resistance to self. Second, doses must be chosen based on rational reasons that take into account PK variability as well as the variability in MICs from place to place. To state the obvious, a drug concentration is relevant only if it can kill the particular strain of M. tuberculosis infecting that particular patient. Dosing based on “average concentrations” achieved in the “average” patient or naïve pooled PKs should be abandoned to history, as is the practice of just considering PKs without taking into account bacterial response to concentration-time profiles. Indeed, dosing practices so derived may be partly to blame for the problem of MDR-TB and XDR-TB. Monte Carlo simulations for dose selection offer that possibility for applying PK/PD to optimize efficacy of anti-TB compounds. Clinical trial simulations also offer the possibility of examination of toxicity and also determination of the duration of therapy. We freely acknowledge that even the pathway we propose is not a panacea and that inevitably better methods will be fashioned as science continues to grow. Nevertheless, the methods are at least a better and more rational oracle to listen to as we fashion shorter-duration chemotherapy and are more effective than the current standards.
Acknowledgments
We are supported by an NIH Director New Innovator Award (NIGM/NIH 1 DP2 OD001886) and NIAID/NIH R01AI079497.
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Abdool Karim, S. S., K. Naidoo, A. Grobler, N. Padayatchi, C. Baxter, A. Gray, T. Gengiah, G. Nair, S. Bamber, A. Singh, M. Khan, J. Pienaar, W. El-Sadr, G. Friedland, and K. Q. Abdool. 2010. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 362:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, C. H., C. J. Werely, T. C. Victor, E. G. Hoal, G. Rossouw, and P. D. van Helden. 2003. Allele frequencies for glutathione S-transferase and N-acetyltransferase 2 differ in African population groups and may be associated with oesophageal cancer or tuberculosis incidence. Clin. Chem. Lab. Med. 41:600-605. [DOI] [PubMed] [Google Scholar]
- 3.Almeida, D., E. Nuermberger, S. Tyagi, W. R. Bishai, and J. Grosset. 2007. In vivo validation of the mutant selection window hypothesis with moxifloxacin in a murine model of tuberculosis. Antimicrob. Agents Chemother. 51:4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrose, P. G. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 26:129-134. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. 1976. A comparative study of daily followed by twice- or once-weekly regimens of ethambutol and rifampicin in the retreatment of patients with pulmonary tuberculosis: second report. Tubercle 57:105-113. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. 1981. Ethambutol plus isoniazid for the treatment of pulmonary tuberculosis-a controlled trial of our regimens. Tubercle 62:13-29. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. 2008. Rifapentine. Tuberculosis (Edinb.) 88:155-158. [DOI] [PubMed] [Google Scholar]
- 9.Assandri, A., B. Ratti, and T. Cristina. 1984. Pharmacokinetics of rifapentine, a new long lasting rifamycin, in the rat, the mouse and the rabbit. J. Antibiot. (Tokyo) 37:1066-1075. [DOI] [PubMed] [Google Scholar]
- 10.Bilello, J. A., G. Bauer, M. N. Dudley, G. A. Cole, and G. L. Drusano. 1994. Effect of 2′,3′-didehydro-3′-deoxythymidine in an in vitro hollow-fiber pharmacodynamic model system correlates with results of dose-ranging clinical studies. Antimicrob. Agents Chemother. 38:1386-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131-137. [DOI] [PubMed] [Google Scholar]
- 12.Bonate, P. L. 2000. Clinical trial simulation in drug development. Pharm. Res. 17:252-256. [DOI] [PubMed] [Google Scholar]
- 13.British Medical Research Council. 1969. A controlled comparison of four regimens of streptomycin plus pyrazinamide in the retreatment of pulmonary tuberculosis. Tubercle 50:81-114. [DOI] [PubMed] [Google Scholar]
- 14.Calver, A. D., A. A. Falmer, M. Murray, O. J. Strauss, E. M. Streicher, M. Hanekom, T. Liversage, M. Masibi, P. D. van Helden, R. M. Warren, and T. C. Victor. 2010. Emergence of increased resistance and extensively drug-resistant tuberculosis despite treatment adherence, South Africa. Emerg. Infect. Dis. 16:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canetti, G., S. Froman, J. Grosset, P. Hauduroy, M. Langerova, H. T. Mahler, G. Meissner, D. A. Mitchison, and L. Sula. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. World Health Organ. 29:565-578. [PMC free article] [PubMed] [Google Scholar]
- 16.Colangeli, R., D. Helb, S. Sridharan, J. Sun, M. Varma-Basil, M. H. Hazbon, R. Harbacheuski, N. J. Megjugorac, W. R. Jacobs, Jr., A. Holzenburg, J. C. Sacchettini, and D. Alland. 2005. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Mol. Microbiol. 55:1829-1840. [DOI] [PubMed] [Google Scholar]
- 17.Conde, M. B., A. Efron, C. Loredo, G. R. De Souza, N. P. Graca, M. C. Cezar, M. Ram, M. A. Chaudhary, W. R. Bishai, A. L. Kritski, and R. E. Chaisson. 2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373:1183-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, S. Duncan, E. McKenna, and E. Zurlinden. 2002. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob. Agents Chemother. 46:2358-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox, H., Y. Kebede, S. Allamuratova, G. Ismailov, Z. Davletmuratova, G. Byrnes, C. Stone, S. Niemann, S. Rusch-Gerdes, L. Blok, and D. Doshetov. 2006. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med. 3:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 22.Craig, W. A. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-19. In C. H. Nightangle, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and practice. Informa Healthcare U. S. A., Inc., New York, NY.
- 23.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 24.Dandara, C., C. M. Masimirembwa, A. Magimba, S. Kaaya, J. Sayi, D. K. Sommers, J. R. Snyman, and J. A. Hasler. 2003. Arylamine N-acetyltransferase (NAT2) genotypes in Africans: the identification of a new allele with nucleotide changes 481C>T and 590G>A. Pharmacogenetics 13:55-58. [DOI] [PubMed] [Google Scholar]
- 25.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA.
- 26.D'Argenio, D. Z., A. Schumitzky, and X. Wang. 2009. ADAPT 5 User's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA.
- 27.Deshpande, D., S. Srivastava, C. Meek, R. Leff, and T. Gumbo. 2010. Ethambutol optimal clinical dose and susceptibility breakpoint identification by use of a novel pharmacokinetic-pharmacodynamic model of disseminated intracellular Mycobacterium avium. Antimicrob. Agents Chemother. 54:1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devasia, R. A., A. Blackman, T. Gebretsadik, M. Griffin, A. Shintani, C. May, T. Smith, N. Hooper, F. Maruri, J. Warkentin, E. Mitchel, and T. R. Sterling. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am. J. Respir. Crit. Care Med. 180:365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diacon, A. H., R. F. Patientia, A. Venter, P. D. van Helden, P. J. Smith, H. McIlleron, J. S. Maritz, and P. R. Donald. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51:2994-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson, J. M., G. A. Ellard, and D. A. Mitchison. 1968. Suitability of isoniazid and ethambutol for intermittent administration in the treatment of tuberculosis. Tubercle 49:351-366. [DOI] [PubMed] [Google Scholar]
- 31.Donald, P. R., D. P. Parkin, H. I. Seifart, H. S. Schaaf, P. D. van Helden, C. J. Werely, F. A. Sirgel, A. Venter, and J. S. Maritz. 2007. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur. J. Clin. Pharmacol. 63:633-639. [DOI] [PubMed] [Google Scholar]
- 32.Donald, P. R., F. A. Sirgel, F. J. Botha, H. I. Seifart, D. P. Parkin, M. L. Vandenplas, B. W. Van de Wal, J. S. Maritz, and D. A. Mitchison. 1997. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 156:895-900. [DOI] [PubMed] [Google Scholar]
- 33.Donald, P. R., F. A. Sirgel, A. Venter, D. P. Parkin, H. I. Seifart, B. W. Van de Wal, J. S. Maritz, and P. B. Fourie. 2003. Early bactericidal activity of antituberculosis agents. Expert Rev. Anti Infect. Ther. 1:141-155. [DOI] [PubMed] [Google Scholar]
- 34.Donald, P. R., F. A. Sirgel, A. Venter, D. P. Parkin, H. I. Seifart, B. W. Van de Wal, C. Werely, P. D. van Helden, and J. S. Maritz. 2004. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin. Infect. Dis. 39:1425-1430. [DOI] [PubMed] [Google Scholar]
- 35.Donomae, I., and K. Yamamoto. 1966. Clinical evaluation of ethambutol in pulmonary tuberculosis. Ann. N.Y. Acad. Sci. 135:849-881. [DOI] [PubMed] [Google Scholar]
- 36.Dooley, K. E., J. Golub, F. S. Goes, W. G. Merz, and T. R. Sterling. 2002. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin. Infect. Dis. 34:1607-1612. [DOI] [PubMed] [Google Scholar]
- 37.Drusano, G. L., S. L. Preston, C. Fowler, M. Corrado, B. Weisinger, and J. Kahn. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 38.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eagle, H., R. Fleischman, and A. D. Musselman. 1950. Effect of schedule of administration on the therapeutic efficacy of penicillin; importance of the aggregate time penicillin remains at effectively bactericidal levels. Am. J. Med. 9:280-299. [DOI] [PubMed] [Google Scholar]
- 40.Ellard, G. A. 1969. Absorption, metabolism and excretion of pyrazinamide in man. Tubercle 50:144-158. [DOI] [PubMed] [Google Scholar]
- 41.Evans, D. A., K. A. Manley, and V. A. McKusick. 1960. Genetic control of isoniazid metabolism in man. Br. Med. J. 2:485-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gangadharam, P. R., A. L. Bhatia, S. Radhakrishna, and J. B. Selkon. 1961. Rate of inactivation of isoniazid in South Indian patients with pulmonary tuberculosis. Bull. World Health Organ. 25:765-777. [PMC free article] [PubMed] [Google Scholar]
- 44.Gangadharam, P. R., S. Devadatta, W. Fox, C. N. Nair, and J. B. Selkon. 1961. Rate of inactivation of isoniazid in South Indian patients with pulmonary tuberculosis. 3. Serum concentrations of isoniazid produced by three regimens of isoniazid alone and one of isoniazid plus PAS. Bull. World Health Organ. 25:793-806. [PMC free article] [PubMed] [Google Scholar]
- 45.Ginsburg, A. S., J. Lee, S. C. Woolwine, J. H. Grosset, F. M. Hamzeh, and W. R. Bishai. 2005. Modeling in vivo pharmacokinetics and pharmacodynamics of moxifloxacin therapy for Mycobacterium tuberculosis infection by using a novel cartridge system. Antimicrob. Agents Chemother. 49:853-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginsburg, A. S., R. Sun, H. Calamita, C. P. Scott, W. R. Bishai, and J. H. Grosset. 2005. Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model. Antimicrob. Agents Chemother. 49:3977-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginsburg, A. S., S. C. Woolwine, N. Hooper, W. H. Benjamin, Jr., W. R. Bishai, S. E. Dorman, and T. R. Sterling. 2003. The rapid development of fluoroquinolone resistance in M. tuberculosis. N. Engl. J. Med. 349:1977-1978. [DOI] [PubMed] [Google Scholar]
- 48.Goutelle, S., L. Bourguignon, P. H. Maire, G. M. Van, J. E. Conte, Jr., and R. W. Jelliffe. 2009. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob. Agents Chemother. 53:2974-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gumbo, T. 2010. New susceptibility breakpoints for first-line antituberculosis drugs based on antimicrobial pharmacokinetic/pharmacodynamic science and population pharmacokinetic variability. Antimicrob. Agents Chemother. 54:1484-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gumbo, T., A. Louie, M. R. Deziel, and G. L. Drusano. 2005. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob. Agents Chemother. 49:3178-3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gumbo, T., A. Louie, M. R. Deziel, W. Liu, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 53.Gumbo, T., A. Louie, W. Liu, P. G. Ambrose, S. M. Bhavnani, D. Brown, and G. L. Drusano. 2007. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J. Infect. Dis. 195:194-201. [DOI] [PubMed] [Google Scholar]
- 54.Gumbo, T., A. Louie, W. Liu, D. Brown, P. G. Ambrose, S. M. Bhavnani, and G. L. Drusano. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gumbo, T., C. S. Siyambalapitiyage Dona, C. Meek, and R. Leff. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta, P., G. P. Jadaun, R. Das, U. D. Gupta, K. Srivastava, A. Chauhan, V. D. Sharma, D. S. Chauhan, and V. M. Katoch. 2006. Simultaneous ethambutol and isoniazid resistance in clinical isolates of Mycobacterium tuberculosis. Indian J. Med. Res. 123:125-130. [PubMed] [Google Scholar]
- 57.Heifets, L. B., M. D. Iseman, and P. J. Lindholm-Levy. 1986. Ethambutol MICs and MBCs for Mycobacterium avium complex and Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 30:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holford, N. H., H. C. Kimko, J. P. Monteleone, and C. C. Peck. 2000. Simulation of clinical trials. Annu. Rev. Pharmacol. Toxicol. 40:209-234. [DOI] [PubMed] [Google Scholar]
- 59.Hyde, L. 1967. Comparison of single and divided daily doses of isoniazid in original treatment of minimal and noncavitary moderately advanced pulmonary tuberculosis. XVIII. A report of the Veterans Administration-Armed Forces Cooperative Study on the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 96:204-208. [DOI] [PubMed] [Google Scholar]
- 60.Jayaram, R., S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharat, R. K. Shandil, E. Kantharaj, and V. Balasubramanian. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jayaram, R., R. K. Shandil, S. Gaonkar, P. Kaur, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, E. Kantharaj, and V. Balasubramanian. 2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 63.Jindani, A., C. J. Dore, and D. A. Mitchison. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 64.Katiyar, S. K., S. Bihari, and S. Prakash. 2008. Low-dose inhaled versus standard dose oral form of anti-tubercular drugs: concentrations in bronchial epithelial lining fluid, alveolar macrophage and serum. J. Postgrad. Med. 54:245-246. [DOI] [PubMed] [Google Scholar]
- 65.Koch, R. 1882. Die atiologie der tuberkulose. Berliner Klinischen Wocheschr. 15:221-230. [Google Scholar]
- 66.Lodise, T. P., Jr., M. Gotfried, S. Barriere, and G. L. Drusano. 2008. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 52:2300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Macgowan, A. P., R. Reynolds, A. R. Noel, and K. E. Bowker. 2009. Bacterial strain-to-strain variation in pharmacodynamic index magnitude, a hitherto unconsidered factor in establishing antibiotic clinical breakpoints. Antimicrob. Agents Chemother. 53:5181-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madison, B., B. Robinson-Dunn, I. George, W. Gross, H. Lipman, B. Metchock, A. Sloutsky, G. Washabaugh, G. Mazurek, and J. Ridderhof. 2002. Multicenter evaluation of ethambutol susceptibility testing of Mycobacterium tuberculosis by agar proportion and radiometric methods. J. Clin. Microbiol. 40:3976-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metropolis, N. 1987. The beginning of the Monte Carlo method. Los Alamos Sci. Special Issue:125-130. [Google Scholar]
- 70.Metropolis, N., and S. Ulam. 1949. The Monte Carlo method. J. Am. Stat. Assoc. 44:335-341. [DOI] [PubMed] [Google Scholar]
- 71.Mitchison, D. A. 2004. Antimicrobial therapy of tuberculosis: justification for currently recommended treatment regimens. Semin. Respir. Crit. Care Med. 25:307-315. [DOI] [PubMed] [Google Scholar]
- 72.Mitchison, D. A., and J. M. Dickinson. 1971. Laboratory aspects of intermittent drug therapy. Postgrad. Med. J. 47:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchison, D. A., A. Jindani, G. R. Davies, and F. Sirgel. 2007. Isoniazid activity is terminated by bacterial persistence. J. Infect. Dis. 195:1871-1872. [DOI] [PubMed] [Google Scholar]
- 74.Mouton, J. W., P. G. Ambrose, G. Kahlmeter, M. Wikler, and W. A. Craig. 2007. Applying pharmacodynamics for susceptibility breakpoint selection, p. 21-44. In C. H. Nightangle, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and practice. Informa Healthcare U. S. A., Inc., New York, NY.
- 75.Parsons, L. M., M. Salfinger, A. Clobridge, J. Dormandy, L. Mirabello, V. L. Polletta, A. Sanic, O. Sinyavskiy, S. C. Larsen, J. Driscoll, G. Zickas, and H. W. Taber. 2005. Phenotypic and molecular characterization of Mycobacterium tuberculosis isolates resistant to both isoniazid and ethambutol. Antimicrob. Agents Chemother. 49:2218-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pasipanodya, J. G., and T. Gumbo. 2010. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob. Agents Chemother. 54:2847-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pasipanodya, J. G., S. J. McNabb, P. Hilsenrath, S. Bae, K. Lykens, E. Vecino, G. Munguia, T. L. Miller, G. Drewyer, and S. E. Weis. 2010. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health 10:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pasipanodya, J. G., T. L. Miller, M. Vecino, G. Munguia, R. Garmon, S. Bae, G. Drewyer, and S. E. Weis. 2007. Pulmonary impairment after tuberculosis. Chest 131:1817-1824. [DOI] [PubMed] [Google Scholar]
- 79.Peloquin, C. 2003. What is the “right” dose of rifampin? Int. J. Tuberc. Lung Dis. 7:3-5. [PubMed] [Google Scholar]
- 80.Peloquin, C. A., A. E. Bulpitt, G. S. Jaresko, R. W. Jelliffe, J. M. Childs, and D. E. Nix. 1999. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob. Agents Chemother. 43:568-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peloquin, C. A., D. J. Hadad, L. P. Molino, M. Palaci, W. H. Boom, R. Dietze, and J. L. Johnson. 2008. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peloquin, C. A., G. S. Jaresko, C. L. Yong, A. C. Keung, A. E. Bulpitt, and R. W. Jelliffe. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perlman, D. C., Y. Segal, S. Rosenkranz, P. M. Rainey, R. P. Remmel, N. Salomon, R. Hafner, and C. A. Peloquin. 2005. The clinical pharmacokinetics of rifampin and ethambutol in HIV-infected persons with tuberculosis. Clin. Infect. Dis. 41:1638-1647. [DOI] [PubMed] [Google Scholar]
- 84.Pillay, M., and A. W. Sturm. 2007. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin. Infect. Dis. 45:1409-1414. [DOI] [PubMed] [Google Scholar]
- 85.Reisner, D. 1966. Comparison of single and divided daily dosages of isoniazid and PAS in the treatment of pulmonary tuberculosis. XV. A report of the Veterans Administration-Armed Forces Cooperative Study on the chemotherapy of tuberculosis. Am. Rev. Respir. Dis. 94:849-857. [DOI] [PubMed] [Google Scholar]
- 86.Rosenthal, I. M., M. Zhang, K. N. Williams, C. A. Peloquin, S. Tyagi, A. A. Vernon, W. R. Bishai, R. E. Chaisson, J. H. Grosset, and E. L. Nuermberger. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubino, C. M., L. Ma, S. M. Bhavnani, J. Korth-Bradley, J. Speth, E. Ellis-Grosse, K. R. Rodvold, P. G. Ambrose, and G. L. Drusano. 2007. Evaluation of tigecycline penetration into colon wall tissue and epithelial lining fluid using a population pharmacokinetic model and Monte Carlo simulation. Antimicrob. Agents Chemother. 51:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salfinger, M., and L. B. Heifets. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarma, G. R., S. Kailasam, M. Datta, G. K. Loganathan, F. Rahman, and A. S. Narayana. 1990. Classification of children as slow or rapid acetylators based on concentrations of isoniazid in saliva following oral administration of body-weight and surface-area-related dosages of the drug. Indian Pediatr. 27:134-142. [PubMed] [Google Scholar]
- 90.Schatz, A., E. Bugie, and S. A. Waksman. 1944. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc. Soc. Exp. Biol. Med. 55:66-69. [DOI] [PubMed] [Google Scholar]
- 91.Selkon, J. B., W. Fox, P. R. Gangadharam, K. Ramachandran, C. V. Ramakrishnan, and S. Velu. 1961. Rate of inactivation of isoniazid in South Indian patients with pulmonary tuberculosis. 2. Clinical implications in the treatment of pulmonary tuberculosis with isoniazid either alone or in combination with PAS. Bull. World Health Organ. 25:779-792. [PMC free article] [PubMed] [Google Scholar]
- 92.Shandil, R. K., R. Jayaram, P. Kaur, S. Gaonkar, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, and V. Balasubramanian. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaw, J. E., J. G. Pasipanodya, and T. Gumbo. 2010. Meningeal tuberculosis: high long-term mortality despite standard therapy. Medicine 89:189-195. [DOI] [PubMed] [Google Scholar]
- 94.Sheiner, L. B., B. Rosenberg, and V. V. Marathe. 1977. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J. Pharmacokinet. Biopharm. 5:445-479. [DOI] [PubMed] [Google Scholar]
- 95.Sheiner, L. B., B. Rosenberg, and K. L. Melmon. 1972. Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput. Biomed. Res. 5:411-459. [DOI] [PubMed] [Google Scholar]
- 96.Sirgel, F. A., P. R. Donald, J. Odhiambo, W. Githui, K. C. Umapathy, C. N. Paramasivan, C. M. Tam, K. M. Kam, C. W. Lam, K. M. Sole, and D. A. Mitchison. 2000. A multicentre study of the early bactericidal activity of anti-tuberculosis drugs. J. Antimicrob. Chemother. 45:859-870. [DOI] [PubMed] [Google Scholar]
- 97.Smith, D. G., and S. A. Waksman. 1947. Tuberculostatic and tuberculocidal properties of streptomycin. J. Bacteriol. 54:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srivastava, S., S. Musuka, C. Sherman, C. Meek, R. Leff, and T. Gumbo. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J. Infect. Dis. 201:1225-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tam, V. H., A. Louie, T. R. Fritsche, M. Deziel, W. Liu, D. L. Brown, L. Deshpande, R. Leary, R. N. Jones, and G. L. Drusano. 2007. Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J. Infect. Dis. 195:1818-1827. [DOI] [PubMed] [Google Scholar]
- 100.Tiefenbrunn, A. J., R. A. Graor, A. K. Robison, F. V. Lucas, A. Hotchkiss, and B. E. Sobel. 1986. Pharmacodynamics of tissue-type plasminogen activator characterized by computer-assisted simulation. Circulation 73:1291-1299. [DOI] [PubMed] [Google Scholar]
- 101.Turnidge, J., and D. L. Paterson. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin. Microbiol. Rev. 20:391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verbist, L. 1969. Rifampicin activity “in vitro” and in established tuberculosis in mice. Acta Tuberc. Pneumol. Belg. 60:397-412. [PubMed] [Google Scholar]
- 103.Volmink, J., and P. Garner. 2007. Directly observed therapy for treating tuberculosis. Cochrane Database Syst. Rev. CD003343. [DOI] [PubMed]
- 104.Wanger, A., and K. Mills. 1996. Testing of Mycobacterium tuberculosis susceptibility to ethambutol, isoniazid, rifampin, and streptomycin by using Etest. J. Clin. Microbiol. 34:1672-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiner, M., W. Burman, C. C. Luo, C. A. Peloquin, M. Engle, S. Goldberg, V. Agarwal, and A. Vernon. 2007. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob. Agents Chemother. 51:2861-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilkins, J. J., G. Langdon, H. McIlleron, G. C. Pillai, P. J. Smith, and U. S. Simonsson. 2006. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur. J. Clin. Pharmacol. 62:727-735. [DOI] [PubMed] [Google Scholar]
- 107.World Health Organization. 2009. Global tuberculosis control: a short update of the 2009 report. WHO/HTM/TB/2009.426. World Health Organization, Geneva, Switzerland.
- 108.Yamane, N., S. Ichiyama, S. Kawahara, Y. Iinuma, H. Saitoh, M. Shimojima, H. Udagawa, and I. Nakasone. 1999. Multicenter evaluation of broth microdilution test, BrothMIC MTB, to determine minimum inhibitory concentrations (MICs) of antimicrobial agents for Mycobacterium tuberculosis-Evaluation of interlaboratory precision and interpretive compatibility with agar proportion method. Jpn. J. Clin. Pathol. 47:754-766. [PubMed] [Google Scholar]
- 109.Yeager, R. L., W. G. Munroe, and F. I. Dessau. 1952. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 65:523-546. [PubMed] [Google Scholar]
- 110.Zhu, M., W. J. Burman, J. R. Starke, J. J. Stambaugh, P. Steiner, A. E. Bulpitt, D. Ashkin, B. Auclair, S. E. Berning, R. W. Jelliffe, G. S. Jaresko, and C. A. Peloquin. 2004. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int. J. Tuberc. Lung Dis. 8:1360-1367. [PubMed] [Google Scholar]
- 111.Zhu, M., J. R. Starke, W. J. Burman, P. Steiner, J. J. Stambaugh, D. Ashkin, A. E. Bulpitt, S. E. Berning, and C. A. Peloquin. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686-695. [DOI] [PubMed] [Google Scholar]