Abstract
Fifty-seven clinical isolates of Streptococcus pneumoniae were divided into four groups based on their susceptibilities to the fluoroquinolones ciprofloxacin and norfloxacin and the dyes ethidium bromide and acriflavine. Comparative reverse transcription-PCR was used to determine the level of expression of the genes patA and patB, which encode putative ABC transporters. Overexpression was observed in 14 of the 15 isolates that were resistant to both fluoroquinolones and dyes and in only 3 of 24 of those resistant to fluoroquinolones only. Isolates overexpressing patA and patB accumulated significantly less of the fluorescent dye Hoechst 33342 than wild-type isolates, suggesting that PatA and PatB are involved in efflux. Inactivation of patA and patB by in vitro mariner mutagenesis conferred hypersusceptibility to ethidium bromide and acriflavine in all isolates tested and lowered the MICs of ciprofloxacin in the patAB-overproducing and/or fluoroquinolone-resistant isolates. These data represent the first observation of overexpression of patA and patB in clinical isolates and show that PatA and PatB play a clinically relevant role in fluoroquinolone resistance.
Streptococcus pneumoniae is an important cause of community-acquired respiratory infections, including sinusitis, otitis media, and pneumonia, as well as serious invasive infections, such as septicemia and meningitis (9). Antibiotic resistance to β-lactams, macrolides, fluoroquinolones, and tetracycline is an increasing problem in many countries with S. pneumoniae infections (5, 13, 18, 29, 31). Fluoroquinolone resistance in particular is a cause for concern. Surveillance data supplied from the British Society for Antimicrobial Chemotherapy (BSAC) for 2007 to 2008 indicated that 95.6% of S. pneumoniae isolates from the United Kingdom show intermediate resistance to ciprofloxacin (0.12 μg/ml < MIC ≤ 2 μg/ml) and 4.4% of isolates are fully resistant to ciprofloxacin (MIC ≥ 4 μg/ml), while no isolates were susceptible according to the recommended breakpoint concentrations used. Fluoroquinolone resistance in S. pneumoniae is usually due to mutations in the genes encoding the target topoisomerase enzymes. Mutations frequently occur in parC, which encodes the A subunit of DNA topoisomerase IV, or gyrA, which encodes the A subunit of DNA gyrase (12). Less frequently, mutations can be found in the genes parE and gyrB, encoding the B subunits of these proteins (14, 23, 24). However, low-level resistance to fluoroquinolones can also be conferred by active efflux mediated by PmrA (6, 11) or PatA/PatB (10, 20, 21). Active efflux usually gives rise to smaller increases in the MIC of a fluoroquinolone than mutations affecting DNA topoisomerase IV and/or DNA gyrase. There have also been several studies whose results suggest that efflux pumps are a requirement for the selection of fluoroquinolone resistance in S. pneumoniae (6, 8, 16, 32). In addition, the overexpression of efflux pumps typically confers resistance to other antibacterial agents besides fluoroquinolones, such as some dyes (e.g., ethidium bromide), detergents (e.g., SDS), and disinfectants (e.g., cetrimide) (25). Genes encoding efflux pumps are typically chromosomally encoded, ensuring that mutations resulting in altered expression or function are retained in further generations in the absence of selective pressure or fitness cost.
Due to the lack of new antibacterial agents, there is considerable interest in potentiating the activity of current antibiotics, such as fluoroquinolones. Efflux pumps make attractive targets for drug discovery programs as inhibition confers multidrug susceptibility, reduces the frequency of selection of drug-resistant mutants, and potentiates the activity of existing drugs (19). S. pneumoniae contains a variety of different multidrug resistance (MDR) efflux pumps; in total, there are 25 transporters predicted to efflux antibiotics (http://www.membranetransport.org/index.html) (10, 11, 20, 21). Until recently, the only pneumococcal efflux pump implicated in fluoroquinolone efflux and resistance was PmrA (11). However, multidrug-resistant S. pneumoniae isolates carrying a nonfunctional pmrA gene have been described (7, 27). Marrer et al. (20, 21) identified overproduction of two putative ABC efflux transporters, PatA (SP2075) and PatB (SP2073), associated with fluoroquinolone resistance in a laboratory-selected ciprofloxacin-resistant mutant. In parallel, Robertson et al. (28) inactivated 13 genes encoding putative efflux pumps in S. pneumoniae strain R6 and found that the inactivation of patA and patB gave rise to hypersusceptibility to ciprofloxacin and norfloxacin, as well as to ethidium bromide and acriflavine. PatA and PatB also play a role in efflux inhibitor resistance in laboratory-selected reserpine-resistant mutants of S. pneumoniae (10). However, although several studies suggest that PatA and PatB play a role in fluoroquinolone resistance in laboratory strains of S. pneumoniae, there is little direct evidence to show whether PatA and PatB are overproduced in clinical isolates. In the present study, it is shown that patA and patB are overexpressed in a significant proportion of clinical isolates of S. pneumoniae. These isolates also accumulated less Hoechst 33342, a fluorescent dye which is a substrate of efflux pumps (30), and most were additionally resistant to the dyes ethidium bromide and acriflavine. This is the first report showing direct evidence of the involvement of PatA and PatB in fluoroquinolone resistance in clinical isolates of S. pneumoniae.
MATERIALS AND METHODS
Bacterial strains, storage, and growth.
S. pneumoniae M4 (NCTC 7465 type 1), S. pneumoniae M3 (NCTC 7466 type 2), S. pneumoniae R6 (3), and the well-characterized laboratory strains M169, M168, M22, M240, M246, M260, and M276 (10) were used throughout as control strains (Table 1). Fifty-seven clinical isolates were obtained from a variety of sources (27): 15 isolates were from MRL Pharmaceutical Services (14), 26 isolates were from the Lung Investigation Unit, University Hospital, Birmingham, United Kingdom (26), 14 clinical isolates were from the Centers for Disease Control and Prevention, Atlanta, GA (15), and 2 laboratory-selected mutants were from George Drusano, Albany, NY (16). All strains were grown in brain heart infusion broth (BHI; Oxoid, Basingstoke, United Kingdom) for 24 h at 37°C in 5% CO2. The identity of each species was confirmed by Gram stain, optochin sensitivity, and the presence of capsule, which was determined by using a Slidex Pneumo-Kit (BioMerieux, France).
TABLE 1.
Wild-type and control S. pneumoniae strains used in the present study
| S. pneumoniae strain | Comment | Reference |
|---|---|---|
| R6 | Unencapsulated | 3 |
| M4 | Wild-type (type 2) NCTC 7465 | |
| M3 | Wild-type (type 1) NCTC 7466 | |
| M168 | Spontaneous reserpine-resistant mutant of M4 | 10 |
| M169 | Spontaneous reserpine-resistant mutant of R6 | 10 |
| M22 | Spontaneous ciprofloxacin-resistant mutant of M4 | 26 |
| M240 | R6::patB magellan2 | 10 |
| M246 | R6::patA magellan2 | 10 |
| M260 | M22::patB magellan2 | This study |
| M276 | M22::patA magellan2 | This study |
Antibiotics and susceptibility determination.
All antibiotics, dyes, and efflux pump inhibitors were made up and used according to the manufacturer's instructions; they included ciprofloxacin, norfloxacin, levofloxacin, acriflavine, ethidium bromide, sodium orthovanadate, and reserpine (Sigma-Aldrich Company Ltd., Dorset, United Kingdom). The MIC of each antibiotic, dye, and efflux pump inhibitor for each strain was determined by using the standardized agar doubling dilution method according to the guidelines of BSAC (2). The effects of the efflux pump inhibitors reserpine (20 μg/ml) and sodium orthovanadate (50 μM) on the MICs of these agents were also measured. Synergy between reserpine or orthovanadate and a fluoroquinolone or a dye was defined as a reduction in the MIC of the agent of ≥2 dilutions in the presence of the inhibitor.
Growth kinetics.
The growth kinetics of all the S. pneumoniae strains used in this study were determined by monitoring optical density (read at 600 nm) every 10 min using a FLUOstar Optima (BMG Labtech, Aylesbury, United Kingdom). Samples were prepared by adding a 5% inoculum of S. pneumoniae culture pregrown to logarithmic phase (optical density at 600 nm [OD600] of 0.4, equivalent to 105 CFU/ml) to prewarmed BHI broth. Approximately 100 μl of sample for each strain was added to 3 separate wells on a microtiter tray and grown statically at 37°C for 24 h in aerobic conditions, with 5 s of shaking (300 rpm) before each reading. Samples from BHI broths were also removed and visualized microscopically in order to detect any gross changes to cell morphology. Generation times were calculated from the logarithmic phase of growth using Microsoft Excel. Differences in average generation time between groups were assessed for statistical significance using the nonpaired Student's t test.
Measurement of the expression of patA and patB.
To measure the levels of expression of patA and patB in parallel from a single mRNA preparation, comparative reverse transcription-PCR (c-RT-PCR) was combined with the rapid and high-throughput technique of denaturing high-pressure liquid chromatography (DHPLC) analysis of amplimers, as previously described by Garvey and Piddock (10). S. pneumoniae strains were grown on three separate occasions, and RNA was isolated from each of these three samples as described previously (10). To determine whether there was a significant difference in the expression of patA and patB between strains R6, M3, M4, and the 57 clinical isolates, mean peak areas were compared using the two-tailed Student's t test.
Accumulation of Hoechst 33342 with or without reserpine by S. pneumoniae.
The efflux activities of the clinical isolates, grown to mid-log phase in BHI broth, were compared with those of the laboratory strains R6, M3, and M4 by monitoring the uptake of Hoechst 33342 added to a final concentration of 2.5 μM, with measurements taken at excitation and emission wavelengths of 350 and 460 nm, respectively, over 30 min using a FLUOstar Optima (BMG Labtech). Differences in accumulation between strains were analyzed for statistical significance using Student's t test. In parallel, the accumulation of Hoechst 33342 with or without reserpine (20 μg/ml) was measured.
PCR and sequencing of patA and patB.
One set each of primers specific for patA and patB were designed to amplify these genes for sequencing (Table 2). The primer sets encompassed the entire coding sequence for patA and patB, as well as ∼200 bp upstream of each gene, covering the putative promoter regions. PCR was performed under the same conditions as described previously (10). The resulting amplimers were cleaned using a QIAquick PCR purification kit (Qiagen), and the products were eluted in 30 μl of UltraPure distilled water (Gibco), separated by 1% agarose gel electrophoresis (100 V for 35 min), and then quantified using GeneTools software (Syngene) and Hyperladder 1 (Bioline) as a quantification standard. The PCR sequencing reaction was performed using a BigDye terminator version 3.1 cycle sequencing kit (Applied Biosytems Ltd., United Kingdom) following the protocol outlined by the Functional Genomics Laboratory (School of Biosciences, University of Birmingham, Birmingham, United Kingdom). The sequences were read on an ABI Prism 3700 DNA analyzer, and the data analyzed using Chromas (chromatogram evaluation; Technelysium Pty. Ltd.) and GeneDoc tools (22) (DNA-protein translation).
TABLE 2.
Details of the DNA primers used in this investigation
| Gene amplified or primer set (purpose or description) | Forward primer | Reverse primer | Amplimer size (bp) |
|---|---|---|---|
| 16S rRNA | 5′-GAGAAGAACGAGTGTGAGAG-3′ | 5′-CTAACACCTAGCACTCATCG-3′ | 391 |
| patA (sequencing) | 5′-TCTTGCTCAGTCCATCATCGAATAT-3′ | 5′-CCGCTGTGGATTAGTTCATTTCC-3′ | 2,963 |
| patB (sequencing) | 5′-AGAATCCAGTCCAGCGAAAGCT-3′ | 5′-GAAAGAACGACCAGATGTTCCAAT-3′ | 2,959 |
| patA (expression) | 5′-ATGTTGTCCTCGCAGCCTAT-3′ | 5′-ACGAACCGATGAACAAGAGG-3′ | 212 |
| patB (expression) | 5′-TTGCTGGTTCGGCTGTACTT-3′ | 5′-AACTGCTGTCATCTGGCCTT-3′ | 330 |
| hexA (expression) | 5′-GAGAATGCTCGCTCAGGTAA-3′ | 5′-TCACATGAGGAGCTTCAGGA-3′ | 421 |
| 1 (patA forward primer + transposon primer) | 5′-TCTTGCTCAGTCCATCATCGAATAT-3′ | 5′-CCGGGGACTTATCAGCCAACC-3′ | 1,445 |
| 2 (patA reverse primer + transposon primer) | 5′-CCGCTGTGGATTAGTTCATTTCC-3′ | 5′-CCGGGGACTTATCAGCCAACC-3′ | 1,566 |
| 3 (patB forward primer + transposon primer) | 5′-AGAATCCAGTCCAGCGAAAGCT-3′ | 5′-CCGGGGACTTATCAGCCAACC-3′ | 1,487 |
| 4 (patB reverse primer + transposon primer) | 5′-GAAAGAACGACCAGATGTTCCAAT-3′ | 5′-CCGGGGACTTATCAGCCAACC-3′ | 1,520 |
In vitro mariner transposon mutagenesis of patA and patB.
Insertional inactivation of patA and patB was performed essentially as described by Garvey and Piddock (10). In brief, patA and patB PCR amplimers from R6 were used as targets for transposition and were inactivated by transfer of the magellan2 minitransposon catalyzed by the Himar1 transposase, as described previously (17). The transposition products were repaired (1) and transformed into target isolates as described below. Two test PCRs were used to confirm the insertion of the magellan2 transposon, as described previously (10).
Insertional inactivation of patA and patB in clinical isolates of S. pneumoniae.
Isolates were incubated at 37°C in 5% CO2 until an OD550 of 0.4 was reached (mid-logarithmic phase growth). The cultures were then diluted 1:20 in competence medium (Todd-Hewitt broth [THB; Oxoid, Basingstoke, United Kingdom], 1 mM calcium chloride [BDH, Poole, United Kingdom], 0.2% bovine serum albumin [Sigma-Aldrich Company Ltd., Poole, United Kingdom] and 100 ng/ml competence-stimulating peptide [CSP] 1 or 2 [Perbio Science United Kingdom Limited, Chester, United Kingdom]). Immediately after the addition of CSP 1 or 2, 1 μg of either the M246 PCR amplimer patA::magellan2 or the M240 PCR amplimer patB::magellan2 was added to 500 μl of the pneumococcal suspension and the reaction mixture incubated statically at 37°C in air for 150 min. In parallel, a duplicate mixture in which the DNA was replaced with an equivalent volume of sterile water or chromosomal DNA from a wild-type control strain, such as R6 (to provide a spontaneous mutation control) was incubated. After incubation, 100 μl of the transformation reaction mixture was inoculated onto Columbia blood agar (Oxoid, Basingstoke, United Kingdom) containing spectinomycin at 100 mg/liter (the antibiotic resistance cassette in magellan2 codes for spectinomycin resistance) and incubated overnight at 37°C in 5% CO2. Transformants growing on spectinomycin-containing agar were then subcultured onto fresh Columbia blood agar medium and incubated at 37°C in 5% CO2 overnight. Spontaneous mutations giving rise to spectinomycin resistance were detected by inoculating the transformation reaction mixture containing no DNA. Any colonies growing on the selective medium in the absence of donor DNA invalidated the transformation experiment.
RESULTS
Fifteen of 57 clinical isolates are resistant to both fluoroquinolones and dyes.
Three wild-type control strains were used throughout this study (10). M3 and M4 are NCTC type strains that produce a capsule and are susceptible to norfloxacin; the addition of reserpine had minimal effect upon susceptibility to fluoroquinolones or other agents. Strain R6 does not produce a capsule and is susceptible to norfloxacin. The wild-type strains of S. pneumoniae R6, M3, and M4 showed the typical susceptibility of this species to all agents tested (Table 3).
TABLE 3.
Susceptibility of all strains and isolates to fluoroquinolones and dyes
| Agent and efflux inhibitora | MIC (μg/ml) forb: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild-type strain |
FQDR isolates |
FQ-R isolates |
DR isolates |
S isolates |
|||||||
| M3 | M4 | R6 | Range | MIC90 | Range | MIC90 | Range | MIC90 | Range | MIC90 | |
| Cip | 1 | 1 | 0.5 | 2-64 | 16 | 1-64 | 8 | 0.25-0.25 | 0.25 | 0.25-0.5 | 0.25 |
| Cip + res | 0.5 | 0.5 | 0.5 | 0.5-32 | 4 | 0.5-64 | 4 | 0.5-0.5 | 0.5 | 0.25-1 | 0.5 |
| Cip + NaO | 1 | 1 | 0.1 | 0.12-64 | 8 | 0.12-32 | 2 | 0.5-0.5 | 0.5 | 0.12-1 | 0.5 |
| Nor | 4 | 8 | 4 | 16-64 | 32 | 16-32 | 32 | 4-4 | 4 | 2-4 | 4 |
| Nor + res | 2 | 4 | 2 | 1-16 | 8 | 2-16 | 8 | 1-1 | 1 | 1-2 | 1 |
| EtBr | 2 | 2 | 1 | 4-16 | 8 | 1-8 | 2 | 16-16 | 16 | 1-4 | 2 |
| EtBr + res | 0.5 | 0.3 | 0.3 | 0.12-0.5 | 0.5 | 0.12-4 | 0.5 | 0.5-0.5 | 0.5 | 0.06-0.5 | 0.25 |
| EtBr + NaO | 0.3 | 0.3 | 0.1 | 0.12-2 | 1 | 0.06-4 | 0.5 | 1-1 | 1 | 0.06-1 | 0.25 |
| Acr | 4 | 4 | 2 | 8-16 | 8 | 2-4 | 4 | 16-16 | 16 | 2-4 | 4 |
| Acr + res | 1 | 1 | 1 | 0.25-2 | 1 | 0.25-4 | 0.5 | 2-2 | 2 | 0.25-2 | 1 |
| Acr + NaO | 0.5 | 0.5 | 0.3 | 0.25-2 | 1 | 0.25-2 | 0.5 | 2-2 | 2 | 0.25-1 | 0.5 |
Cip, ciprofloxacin; Nor, norfloxacin; EtBr, ethidium bromide; Acr, acriflavine; res, reserpine; NaO, sodium orthovanadate.
FQDR, resistant to both fluoroquinolones and dyes (n = 15); FQ-R, resistant to fluoroquinolones alone (n = 24); DR, dye resistant (n = 2); S, susceptible to all agents (n = 16). MICs of each agent in the absence of an efflux inhibitor are highlighted in boldface.
The MICs of antibiotics for 46 of the clinical isolates have been described previously, detailing their phenotype and the occurrence of mutations in the quinolone resistance-determining regions (QRDRs) of the topoisomerase genes (27). In the present study, the susceptibility of all isolates to two fluoroquinolone antibiotics, ciprofloxacin and norfloxacin, and two dyes, ethidium bromide and acriflavine, was determined in parallel (Table 3). Isolates were defined as fluoroquinolone resistant (FQ-R) if they were resistant to either ciprofloxacin (MIC ≥ 2 μg/ml) or norfloxacin (MIC ≥ 16 μg/ml) according to the BSAC guidelines. BSAC breakpoint concentrations are not available for ethidium bromide and acriflavine, so isolates were defined as dye resistant (DR) if the MICs of both dyes were two or more dilutions higher than for the control strain R6 (MIC of ≥4 μg/ml for ethidium bromide and ≥8 μg/ml for acriflavine). Of the 57 clinical isolates in the study, 15 were resistant to both fluoroquinolones and dyes (FQDR), 24 were FQ-R only, two were DR only, and the remaining 16 were sensitive to all agents tested (S).
The MICs of these agents for these isolates were also measured in the presence of 20 μg/ml of the efflux pump inhibitor reserpine. The MICs of the dyes for all 15 FQDR isolates were reduced by reserpine, and the fluoroquinolone MICs were reduced by reserpine for 10 isolates. Of the 24 isolates that were FQ-R only, the MICs of dyes were reduced for 21 isolates, and fluoroquinolone MICs were reduced for 13. Reserpine reduced the MICs for the two isolates that were resistant to dyes only. Finally, the MICs of fluoroquinolones were reduced by reserpine in 8 of the 16 sensitive strains, and the MICs of dyes were reduced in 15. Similar reductions in MICs were observed with the addition of 50 μM sodium orthovanadate.
Of the 15 FQDR isolates, only one isolate did not possess mutations in the QRDRs of the topoisomerase genes parC, parE, gyrA, and gyrB. The remaining 14 isolates possessed mutations in one (n = 4), two (n = 4), or three (n = 6) of these genes. All 24 of the FQ-R isolates possessed mutations in one (n = 8), two (n = 11), or three (n = 5) QRDRs. Neither of the DR isolates contained mutations in these genes. One of the fluoroquinolone- and dye-susceptible isolates had a lysine-to-asparagine mutation in the parC gene. This mutation was also found in combination with other mutations in two FQ-R isolates and two FQDR isolates and in isolation in one FQDR isolate.
Nineteen of 57 clinical isolates overexpress patA and patB.
To determine whether overexpression of patA and/or patB was associated with a particular phenotype, the expression of patA and patB in all strains was measured by c-RT-PCR (Fig. 1). In total, 19 isolates expressed both patA and patB at levels significantly higher than those of the control strain R6. In all cases, patA and patB were overexpressed together, suggesting coregulation. Fourteen of the 15 FQDR isolates and both of the DR-only isolates overexpressed patA and patB, while only 3 of the 24 isolates that were FQ-R only did so. Overexpression of these two genes was not found in any of the fluoroquinolone- and dye-susceptible isolates.
FIG. 1.
Fold expression of patA (A) and patB (B) relative to that of R6, measured by c-RT-PCR. Crosses represent results for individual isolates, and solid lines represent the mean expression level for each group. Error bars show standard deviations. Wt, wild type; FQDR, isolates resistant to both fluoroquinolones and dyes (n = 15); FQ-R, isolates resistant to fluoroquinolones only (n = 24); DR, isolates resistant to dyes only (n = 2); S, isolates sensitive to all agents tested (n = 16). Asterisks denote groups of isolates for which mean levels of expression are significantly different from the results for the wild type (P < 0.01).
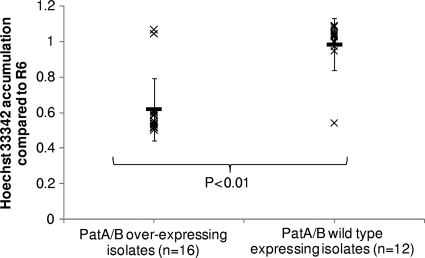
Isolates overexpressing patA and patB accumulate significantly less Hoechst 33342.
Overexpression of patA and patB has been associated with increased efflux (10). To confirm that the same was true for the clinical isolates in this study, the accumulation of the dye Hoechst 33342 was measured in 28 randomly chosen clinical isolates, including isolates from all groups. Out of 17 isolates overexpressing patA and patB, 15 showed significantly reduced accumulation of Hoechst 33342 in comparison to its accumulation in R6 (Fig. 2). In all isolates accumulating lower levels of Hoechst 33342, accumulation was increased in the presence of 20 μg/ml reserpine. Of the 11 isolates tested that did not overexpress patA and patB, all accumulated wild-type levels of Hoechst 33342 (Fig. 2).
FIG. 2.
Accumulation of Hoechst 33352 by representative isolates of each group compared to that by R6. Crosses represent results for individual isolates, and solid lines represent the mean accumulation of Hoechst by each group. Error bars show standard deviations.
Inactivation of patA and patB in the overexpressing clinical isolates confers loss of resistance.
To confirm whether increased expression of patA and/or patB conferred the FQDR phenotype, single mutants in which patA or patB was inactivated were constructed in 16 representative isolates that overexpressed patA and patB (consisting of 8 FQDR isolates, 2 DR isolates, and 1 FQ-R isolate) and 4 FQ-R isolates and one sensitive isolate that did not. When patA was inactivated, the magellan2 minitransposon had inserted between nucleotides 833 and 834 of patA, while when patB was inactivated, the minitransposon had inserted between nucleotides 1082 and 1083 of patB. The susceptibilities of the inactivated strains to ciprofloxacin, ethidium bromide, and acriflavine were determined and compared to those of the parental isolates (Table 4). Inactivation of either patA or patB conferred hypersusceptibility to ethidium bromide in 13 of the 15 drug-resistant isolates, while for one of the FQDR isolates and one of the FQ-R isolates, the hypersusceptibility phenotype was only observed when patA was inactivated. Ciprofloxacin resistance was reduced by 2 to 4 dilutions (4- to 8-fold) by inactivation of patA and patB in five of the eight FQDR isolates and four of the five FQ-R isolates, although in no case was the fluoroquinolone susceptibility returned to wild-type levels. For all but two of the inactivated strains, reserpine and sodium orthovanadate did not change the MIC of any agent by more than one dilution.
TABLE 4.
Susceptibility of isolates in which patA or patB has been inactivated to ciprofloxacin and ethidium bromidea
| Isolate | Group | Gene inactivated | MIC (μg/ml) of: |
|||||
|---|---|---|---|---|---|---|---|---|
| Cip | Cip + res | Cip + NaO | EtBr | EtBr + res | EtBr + NaO | |||
| R6 | Control | 0.5 | 0.5 | 0.25 | 1 | 0.25 | 0.12 | |
| M42 | FQDR | 64 | 32 | 16 | 8 | 0.5 | 1 | |
| M298 | patA | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M299 | patB | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M45 | FQDR | 4 | 1 | 1 | 4 | 0.5 | 1 | |
| M45A | patA | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | |
| M45B | patB | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | |
| M50 | FQDR | 64 | 32 | 32 | 4 | 0.5 | 1 | |
| M50A | patA | 4 | 4 | 4 | 0.5 | 0.5 | 0.5 | |
| M50B | patB | 4 | 4 | 4 | 0.5 | 0.5 | 0.5 | |
| M74 | FQDR | 64 | 32 | 64 | 4 | 0.5 | 1 | |
| M301 | patA | 8 | 8 | 8 | 0.5 | 0.5 | 0.25 | |
| M305 | patB | 8 | 8 | 8 | 0.5 | 0.5 | 0.25 | |
| M86 | FQDR | 2 | 0.5 | 0.5 | 4 | 0.5 | 0.5 | |
| M86A | patA | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| M86B | patB | 1 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| M87 | FQDR | 32 | 8 | 16 | 4 | 0.5 | 1 | |
| M87A | patA | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M87B | patB | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M296 | FQDR | 2 | 0.5 | 8 | 8 | 0.5 | 2 | |
| M304 | patA | 4 | 1 | 2 | 0.5 | 0.5 | 0.25 | |
| M310 | patB | 4 | 1 | 2 | 16 | 0.5 | 8 | |
| M297 | FQDR | 8 | 2 | 8 | 8 | 0.5 | 1 | |
| M311 | patA | 8 | 4 | 4 | 0.5 | 0.5 | 8 | |
| M311B | patB | 8 | 4 | 4 | 0.5 | 0.5 | 8 | |
| M79 | FQ-R | 32 | 32 | 16 | 2 | 0.5 | 0.5 | |
| M316 | patA | 8 | 8 | 8 | 0.25 | 0.5 | 0.5 | |
| M317 | patB | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M83 | FQ-R | 64 | 64 | 1 | 8 | 4 | 4 | |
| M318 | patA | 16 | 16 | 16 | 0.5 | 0.5 | 0.5 | |
| M319 | patB | 16 | 16 | 16 | 0.5 | 0.5 | 0.5 | |
| M97 | FQ-R | 8 | 4 | 8 | 4 | 0.5 | 1 | |
| M302 | patA | 4 | 4 | 4 | 0.5 | 0.5 | 0.5 | |
| M306 | patB | 4 | 4 | 4 | 0.25 | 0.5 | 0.5 | |
| M98 | FQ-R | 32 | 2 | 32 | 4 | 0.12 | 1 | |
| M314 | patA | 8 | 8 | 8 | 0.5 | 0.5 | 0.5 | |
| M307 | patB | 8 | 8 | 8 | 2 | 0.5 | 2 | |
| M100 | FQ-R | 8 | 2 | 1 | 4 | 0.25 | 1 | |
| M315 | patA | 2 | 1 | 0.12 | 0.25 | 0.12 | 0.25 | |
| M308 | patB | 2 | 1 | 0.12 | 0.25 | 0.25 | 0.5 | |
| M55 | DR | 0.25 | 0.5 | 0.5 | 16 | 0.5 | 1 | |
| M300 | patA | 2 | 2 | 2 | 0.5 | 0.5 | 0.5 | |
| M55B | patB | 2 | 2 | 2 | 0.5 | 0.5 | 0.5 | |
| M46 | DR | 0.25 | 0.5 | 0.5 | 16 | 0.5 | 1 | |
| M46A | patA | 0.12 | 0.12 | 0.12 | 0.5 | 0.5 | 0.5 | |
| M46B | patB | 0.12 | 0.12 | 0.12 | 0.5 | 0.5 | 0.5 | |
| M295 | S | 1 | 0.5 | 0.25 | 2 | 0.25 | 0.5 | |
| M303 | patA | 2 | 1 | 0.12 | 1 | 0.5 | 1 | |
| M309 | patB | 4 | 1 | 2 | 16 | 0.5 | 8 | |
Abbreviations are as defined for Table 3. MICs of each agent for the parent strains in which patA and patB are active are highlighted in boldface.
Susceptibility to levofloxacin is not affected by lack of or overexpression of the PatAB transporter in these isolates.
For a representative subset of isolates (consisting of five FQDR isolates, three FQR isolates, one DR isolate, and three S isolates) and their corresponding mutants in which patA or patB have been inactivated, the MICs of levofloxacin were determined as described above in the presence or absence of 20 μg/ml reserpine or 50 μM sodium orthovanadate. According to the BSAC guidelines, all of the FQDR and FQR isolates were resistant to levofloxacin (MIC > 2 μg/ml), whereas all DR and S isolates were susceptible (MIC ≤ 2 μg/ml). These MICs were not reduced by more than one dilution by either of the efflux inhibitors or by the inactivation of patA or patB (data not shown).
No mutations were found in patA or patB or the upstream region of the MDR clinical isolates.
The protein-coding regions of patA and patB from 21 randomly selected clinical isolates were sequenced to rule out the possibility that any increases in PatA and PatB activity could be due to a functional change in the transporter protein itself. In all isolates tested, the nucleotide sequences of patA and patB were identical to those of the wild-type strains R6, M3, and M4.
It was hypothesized that the overexpression of patA and patB could be due to a nucleotide change in the patAB promoter region that increases the activity of the promoter. To check this, a 200-bp region upstream of the start site of patA was sequenced from the same set of 21 isolates. Again, no differences in sequence compared to that of the wild type were found in any of the isolates tested.
No differences in growth rate were observed between the clinical isolates and control strains.
To determine whether the overexpression of patA and patB confers a fitness cost on the clinical isolates that also carry topoisomerase mutations, the growth kinetics of a randomly chosen selection of isolates (consisting of six isolates that overexpressed patA and patB and eight that did not) were determined by measuring the optical density of cultures over time. The wild-type strain R6 was used as a control. The average generation time of R6 under the conditions used was 55 min. Generation times ranged from 29 min to 74 min for the isolates that did not overexpress patA and patB and from 35 to 100 min for the isolates that did (data not shown). There was no statistically significant difference in growth rates between the two groups, indicating that overproducing PatA and PatB confers no gross fitness costs on the clinical isolates under the conditions tested.
DISCUSSION
Overexpression of PatA and PatB has been previously shown to be responsible for multidrug resistance phenotypes of laboratory-selected ciprofloxacin- and efflux pump inhibitor-resistant mutants (10, 21). However, to date, this resistance mechanism has not been shown to be relevant in a clinical setting. Here, we report that in a set of 57 clinical isolates, 14 out of 15 isolates that were resistant to both fluoroquinolones and dyes overexpressed patA and patB. In addition, 3 of 24 isolates that were resistant to fluoroquinolones only and 2 isolates that were resistant to dyes only also overexpressed patA and patB. This suggests that overexpression of patA and patB occurs in a clinical setting and, in most cases, appears to confer a phenotype similar to that observed in the laboratory mutants. In all cases, patA and patB were overexpressed together, suggesting that they are likely to be coregulated.
As observed in laboratory mutants, overexpression of patA and patB in these clinical isolates confers an efflux phenotype, demonstrated by the decreased accumulation of Hoechst 33342 observed in isolates with increased expression of patA and patB. The majority of isolates overexpressing patA and patB showed reduced susceptibility to both fluoroquinolones and dyes, which was partially (fluoroquinolones) or fully (dyes) reversible by the addition of an efflux inhibitor. This is consistent with an increase in efflux conferred by a multidrug efflux pump.
The inactivation of patA conferred hypersusceptibility to ethidium bromide and acriflavine in all strains tested, regardless of whether patA and patB were overexpressed. This suggests that the PatA and PatB transporters are required for low-level, intrinsic resistance to these agents, even when expressed at wild-type levels. The MICs of ciprofloxacin in 9 of 13 isolates resistant to fluoroquinolones were reduced by 2 or more dilutions when patA was inactivated. The MICs were not reduced to the wild-type levels observed for R6, presumably due to the coexisting presence of mutations in the QRDRs of the fluoroquinolone-resistant isolates. The fact that this decrease in the ciprofloxacin MIC was not observed in all fluoroquinolone-resistant isolates may indicate that the relative contribution of QRDR mutations and efflux to ciprofloxacin resistance varies between different genetic backgrounds. For some isolates, the inactivation of patB alone did not result in increased sensitivity to dyes. This could suggest that PatA is capable of providing some efflux activity when expressed alone. In a previous study on reserpine-selected mutants overexpressing patA and patB (10), the inactivation of patB reversed the multidrug-resistant phenotype but was unable to restore reserpine sensitivity. To resolve this, more work is needed to precisely define the roles of PatA and PatB in the formation of the efflux pump.
The inactivation of patA or patB or the addition of efflux inhibitors did not increase susceptibility to levofloxacin in any isolate tested. This result is consistent with those of previous reports suggesting that levofloxacin, as a hydrophobic fluoroquinolone, is poorly transported by the PatA and PatB transporters compared to their transport of the hydrophilic fluoroquinolones ciprofloxacin and norfloxacin (4).
Analysis of the DNA sequences of the QRDRs of parC, parE, gyrA, and gyrB of 37 of the 39 fluoroquinolone-resistant isolates in this study revealed that 36 isolates contain mutations in one (n = 11), two (n = 14), or three (n = 11) genes. While the origins of these isolates are not fully known, it is clear that many must have been exposed to a fluoroquinolone for a prolonged period or repeatedly for these mutations to have accumulated, as it is unlikely that such isolates could have emerged after a single exposure. To obtain a similar mutant in the laboratory would require at least three exposures to a fluoroquinolone. However, one isolate that overexpressed patA and patB possesses no QRDR mutations. Another isolate has only one (lysine-to-asparagine) mutation in parC, which is also found in one of the susceptible isolates, suggesting that it is not involved in conferring fluoroquinolone resistance. These isolates are resistant to fluoroquinolones, which suggests that, although overexpression of patA and patB is observed in conjunction with QRDR mutations, it is also able to confer resistance to fluoroquinolones independently in clinical isolates.
Previous work showed that laboratory-selected strains that overexpress patA and patB grow at a similar rate to their parental strains. Similarly, in this study, no significant differences in growth rate were observed between isolates overexpressing and not overexpressing patA and patB. This suggests that this overexpression does not confer a fitness cost on the organism under the conditions used.
This study provides evidence to show that overexpression of patA and patB is found in clinical isolates and, as in laboratory mutants, leads to reduced susceptibility to the hydrophilic fluoroquinolones ciprofloxacin and norfloxacin, as well as other agents. Overexpression of the transporter did not affect growth under laboratory conditions, suggesting that this resistance mechanism may carry a low fitness cost and so may be easily selected in a clinical setting.
Acknowledgments
This work was supported by a Bristol Myers Squibb Unrestricted Grant in Infectious Diseases to L.J.V.P. A.J.B. is supported by an MRC DTG to the University of Birmingham.
Footnotes
Published ahead of print on 11 October 2010.
REFERENCES
- 1.Akerley, B. J., E. J. Rubin, A. Camilli, D. J. Lampe, H. M. Robertson, and J. J. Mekalanos. 1998. Systematic identification of essential genes by in vitro mariner mutagenesis. Proc. Natl. Acad. Sci. U. S. A. 95:8927-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, J. M. 2001. The development of the BSAC standardized method of disc diffusion testing. J. Antimicrob. Chemother. 48(Suppl. 1):29-42. [DOI] [PubMed] [Google Scholar]
- 3.Avery, O. T., C. M. Macleod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avrain, L., M. Garvey, N. Mesaros, Y. Glupczynski, M. P. Mingeot-Leclercq, L. J. Piddock, P. M. Tulkens, R. Vanhoof, and F. Van Bambeke. 2007. Selection of quinolone resistance in Streptococcus pneumoniae exposed in vitro to subinhibitory drug concentrations. J. Antimicrob. Chemother. 60:965-972. [DOI] [PubMed] [Google Scholar]
- 5.Baquero, F., J. A. Garcia-Rodriguez, J. Garcia de Lomas, and L. Aguilar. 1999. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996-1997) multicenter surveillance study. The Spanish Surveillance Group for Respiratory Pathogens. Antimicrob. Agents Chemother. 43:357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranova, N. N., and A. A. Neyfakh. 1997. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenwald, N. P., P. Appelbaum, T. Davies, and M. J. Gill. 2003. Evidence for efflux pumps, other than PmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Clin. Microbiol. Infect. 9:140-143. [DOI] [PubMed] [Google Scholar]
- 8.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bridy-Pappas, A. E., M. B. Margolis, K. J. Center, and D. J. Isaacman. 2005. Streptococcus pneumoniae: description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacotherapy 25:1193-1212. [DOI] [PubMed] [Google Scholar]
- 10.Garvey, M. I., and L. J. Piddock. 2008. The efflux pump inhibitor reserpine selects multidrug-resistant Streptococcus pneumoniae strains that overexpress the ABC transporters PatA and PatB. Antimicrob. Agents Chemother. 52:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janoir, C., V. Zeller, M. D. Kitzis, N. J. Moreau, and L. Gutmann. 1996. High-level fluoroquinolone resistance in Streptococcus pneumoniae requires mutations in parC and gyrA. Antimicrob. Agents Chemother. 40:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins, S. G., S. D. Brown, and D. J. Farrell. 2008. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US years 1-4. Ann. Clin. Microbiol. Antimicrob. 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, M. E., D. F. Sahm, N. Martin, S. Scheuring, P. Heisig, C. Thornsberry, K. Kohrer, and F. J. Schmitz. 2000. Prevalence of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997-1998 respiratory season. Antimicrob. Agents Chemother. 44:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorgensen, J. H., L. M. Weigel, M. J. Ferraro, J. M. Swenson, and F. C. Tenover. 1999. Activities of newer fluoroquinolones against Streptococcus pneumoniae clinical isolates including those with mutations in the gyrA, parC, and parE loci. Antimicrob. Agents Chemother. 43:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jumbe, N. L., A. Louie, M. H. Miller, W. Liu, M. R. Deziel, V. H. Tam, R. Bachhawat, and G. L. Drusano. 2006. Quinolone efflux pumps play a central role in emergence of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 50:310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lampe, D. J., M. E. Churchill, and H. M. Robertson. 1996. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO J. 15:5470-5479. [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 19.Lomovskaya, O., and K. A. Bostian. 2006. Practical applications and feasibility of efflux pump inhibitors in the clinic—a vision for applied use. Biochem. Pharmacol. 71:910-918. [DOI] [PubMed] [Google Scholar]
- 20.Marrer, E., A. T. Satoh, M. M. Johnson, L. J. Piddock, and M. G. Page. 2006. Global transcriptome analysis of the responses of a fluoroquinolone-resistant Streptococcus pneumoniae mutant and its parent to ciprofloxacin. Antimicrob. Agents Chemother. 50:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrer, E., K. Schad, A. T. Satoh, M. G. Page, M. M. Johnson, and L. J. Piddock. 2006. Involvement of the putative ATP-dependent efflux proteins PatA and PatB in fluoroquinolone resistance of a multidrug-resistant mutant of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 50:685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas, K. B., H. B. Nicholas, Jr., and D. W. Deerfield II. 1997. GeneDoc: analysis and visualization of genetic variation. EMBnet.NEWS 4:1-4. [Google Scholar]
- 23.Pan, X. S., J. Ambler, S. Mehtar, and L. M. Fisher. 1996. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:2321-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan, X. S., and L. M. Fisher. 1996. Cloning and characterization of the parC and parE genes of Streptococcus pneumoniae encoding DNA topoisomerase IV: role in fluoroquinolone resistance. J. Bacteriol. 178:4060-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piddock, L. J. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 26.Piddock, L. J., M. Johnson, V. Ricci, and S. L. Hill. 1998. Activities of new fluoroquinolones against fluoroquinolone-resistant pathogens of the lower respiratory tract. Antimicrob. Agents Chemother. 42:2956-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piddock, L. J., M. M. Johnson, S. Simjee, and L. Pumbwe. 2002. Expression of efflux pump gene pmrA in fluoroquinolone-resistant and -susceptible clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson, G. T., T. B. Doyle, and A. S. Lynch. 2005. Use of an efflux-deficient Streptococcus pneumoniae strain panel to identify ABC-class multidrug transporters involved in intrinsic resistance to antimicrobial agents. Antimicrob. Agents Chemother. 49:4781-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz, F. J., A. C. Fluit, M. Luckefahr, B. Engler, B. Hofmann, J. Verhoef, H. P. Heinz, U. Hadding, and M. E. Jones. 1998. The effect of reserpine, an inhibitor of multidrug efflux pumps, on the in-vitro activities of ciprofloxacin, sparfloxacin and moxifloxacin against clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 42:807-810. [DOI] [PubMed] [Google Scholar]
- 30.Webber, M. A., L. P. Randall, S. Cooles, M. J. Woodward, and L. J. Piddock. 2008. Triclosan resistance in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 62:83-91. [DOI] [PubMed] [Google Scholar]
- 31.Woodford, N., and D. M. Livermore. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl. 1):S4-S16. [DOI] [PubMed] [Google Scholar]
- 32.Zeller, V., C. Janoir, M. D. Kitzis, L. Gutmann, and N. J. Moreau. 1997. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1973-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]