Abstract
Clearance of apoptotic neutrophils is a central feature of the resolution of inflammation. Findings indicate that immuno-modulation and induction of neutrophil apoptosis by macrolide antibiotics generate anti-inflammatory benefits via mechanisms that remain obscure. Tulathromycin (TUL), a new antimicrobial agent for bovine respiratory disease, offers superior clinical efficacy for reasons not fully understood. The aim of this study was to identify the immuno-modulating effects of tulathromycin and, in this process, to establish tulathromycin as a new model for characterizing the novel anti-inflammatory properties of antibiotics. Bronchoalveolar lavage specimens were collected from Holstein calves 3 and 24 h postinfection, challenged intratracheally with live Mannheimia haemolytica (2 × 107 CFU), and treated with vehicle or tulathromycin (2.5 mg/kg body weight). Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining and enzyme-linked immunosorbent assay (ELISA) revealed that tulathromycin treatment significantly increased leukocyte apoptosis and reduced levels of proinflammatory leukotriene B4 in M. haemolytica-challenged calves. In vitro, tulathromycin concentration dependently induced apoptosis in freshly isolated bovine neutrophils from healthy steers in a capase-3-dependent manner but failed to induce apoptosis in bovine fibroblasts, epithelial cells, and endothelial cells, as well as freshly isolated bovine blood monocytes and monocyte-derived macrophages. The proapoptotic effects of TUL were also, in part, drug specific; equimolar concentrations of penicillin G, oxytetracycline, and ceftiofur failed to cause apoptosis in bovine neutrophils. In addition, tulathromycin significantly reduced levels of phosphorylated IκBα, nuclear translocation of NF-κB p65, and mRNA levels of proinflammatory interleukin-8 in lipopolysaccharide (LPS)-stimulated bovine neutrophils. The findings illustrate novel mechanisms through which tulathromycin confers anti-inflammatory benefits.
Excessive extravasation of neutrophils and their uncontrolled death by necrosis at the site of inflammation result in the local release of proteolytic enzymes, reactive oxygen species, and proinflammatory mediators, which exacerbate inflammatory responses and cause self-amplifying tissue injury (18). This inflammatory milieu is characterized by the accumulation of various mediators, including the potent neutrophil chemoattractants CXCL8 (also known as interleukin-8) and leukotriene B4 (LTB4), an arachidonic acid metabolite synthesized via the 5-lipoxygenase pathway (6, 11). High concentrations of these products in inflamed tissues have become markers of self-perpetuating inflammatory disease (6, 19, 47).
Under homeostatic conditions, neutrophils are inactivated and removed from the site of inflammation by apoptosis (programmed cell death) (10, 49, 52, 57). During apoptosis, the destruction of intracellular organelles occurs while cell plasma membrane integrity is preserved, hence preventing the release of proinflammatory and histolytic compounds into the surrounding milieu. Ultimately, caspase-3 catalyzes the specific cleavage of many key cellular proteins and nuclear DNA, leading to cell death (13). Caspase-3 also inhibits the release of prosurvival and proinflammatory mediators by cleaving the transcription factor NF-κB (4, 24, 28, 37, 51). In resting cells, NF-κB is located in the cytoplasm as an inactive complex through interactions with inhibitory IκB proteins. Upon stimulation, IκBs are rapidly phosphorylated and degraded. This in turn releases NF-κB, allowing its translocation into the nucleus, where it binds to specific DNA sites and induces the expression of target genes responsible for both inflammation and cell survival (30). Reports have demonstrated that inhibition of NF-κB in granulocytes accelerates apoptosis in a caspase-3-dependent manner (55).
Apoptotic cells are recognized and removed from the site of inflammation by phagocytes (16, 40, 42). This process triggers the secretion of anti-inflammatory mediators, such as transforming growth factor β (TGF-β) and interleukin-10 (IL-10) (15, 45). Conversely, should this phagocytic process or neutrophil apoptosis be inhibited, severe inflammation will ensue (17, 44, 45). Therefore, studies of the regulatory mechanisms of neutrophil apoptosis and their consequences for the resolution of inflammation represent topics of intensive research activity.
Consistent with recent observations that found that cyclin-dependent kinase (CDK) inhibitors as well as the veterinary macrolide tilmicosin enhance the resolution of inflammation by inducing neutrophil apoptosis, the present study is based on the hypothesis that antibiotics that promote inflammatory cell apoptosis may have significant therapeutic benefits in infectious diseases in which host inflammation is central to pathogenesis (5, 39). The mechanisms and molecular signals that promote neutrophil apoptosis offer promising research grounds from which to develop new anti-inflammatory drugs.
Bacterial pneumonia, in humans, cattle, or swine, is a prime example in which both bacterial virulence factors and host inflammatory responses participate in disease pathogenesis, a process that ultimately leads to respiratory failure and death. This is indeed the case in bovine respiratory disease caused by Mannheimia haemolytica, (9, 19, 46, 54). M. haemolytica secretes a leukotoxin that opens transmembrane pores in leukocytes (12, 46), which in turn activates NF-κB signaling, and stimulates the release of various proinflammatory mediators, including high concentrations of CXCL8 and LTB4 (5, 21). This process overwhelms the homeostatic apoptotic removal of inflammatory cells, which accumulate and die by necrosis at the site of inflammation (5). Therefore, this model offers an ideal opportunity to investigate the mechanisms and benefits of antibiotic-induced neutrophil apoptosis.
Macrolide antibiotics are known to modulate immune cell functions in addition to their antimicrobial function. This class of antibiotics is known to influence the recruitment and infiltration of neutrophils (23, 26, 31) and to alter their ability to secrete histotoxic compounds (23) and proinflammatory cytokines (31, 50, 53). Recent findings also indicate that some macrolides may induce cellular death by apoptosis (3, 5, 7, 8, 26) and block NF-κB signaling in T lymphocytes, macrophages, or epithelial cells (27, 36, 56). However, the precise mechanisms underlying the anti-inflammatory and proapoptotic activities of macrolides in neutrophils remain unclear. Of particular interest, research is warranted to determine whether these processes involve a modulation of NF-κB, a pivotal transcription factor during inflammation.
Tulathromycin belongs to a new class of triamilide macrolides; its 15-membered ring comprised of 3 polar amine groups distinguishes it from other macrolides (14). This semisynthetic derivative of erythromycin is used for the treatment and prevention of respiratory disease in cattle and swine. The therapeutic success of tulathromycin is partially attributed to its pharmacodynamic concentration in appropriate tissues and low inhibitory concentrations against various bacterial pathogens (14). Additionally, tulathromycin has a very high affinity for uptake within neutrophils and, to a lesser extent, macrophages, which helps target the delivery of the drug to infected tissues (14). Compared to other antibiotics used in the treatment and prevention of bovine respiratory disease, tulathromycin has shown superior clinical efficacy and clears the infection and inflammation for reasons that remain not completely understood (14, 35, 38, 48). We hypothesized that tulathromycin offers a powerful model system to uncover novel mechanisms through which induction of neutrophil apoptosis may confer anti-inflammatory benefits to an antibiotic.
Using complementary models of experimental bovine respiratory disease in calves in vivo and cell systems in vitro, this study investigates the immuno-modulating and proapoptotic mechanisms of tulathromycin in bovine neutrophils. Together, the results illustrate for the first time that tulathromycin induces neutrophil apoptosis and modulates proinflammatory signaling. In a broader sense, findings from this study reveal mechanisms through which antibiotic-induced neutrophil apoptosis and inhibition of NF-κB signaling reduce the production of mediators that are responsible for severe inflammation in a variety of disorders, including bacterial pneumonia.
MATERIALS AND METHODS
Animals.
Male healthy Holstein calves, 2 to 3 weeks old and weighing 47 to 53 kg, were used in all experiments. After 7 days of acclimation, calves were randomly assigned to 1 of 3 groups: (i) control calves given 10 ml endotoxin-free Hanks' balanced salt solution vehicle (HBSS; with NaHCO3 and without phenol red, calcium chloride, or magnesium sulfate) intratracheally, (ii) infected untreated calves challenged intratracheally with 2 × 107 CFU live M. haemolytica in 10 ml HBSS in combination with a subcutaneous injection of 25% propylene glycol vehicle, or (iii) infected treated calves given a subcutaneous injection of tulathromycin (Draxxin; Pfizer Animal Health, Kalamazoo, MI) using the recommended dose, 2.5 mg/kg body weight, at the time of intratracheal infection. Calves were housed at the animal facilities of the University of Calgary (Spy Hill location), fed antibiotic-free milk replacer 2 times a day, and given access to water ad libitum. Photoperiods were 12:12 h, and temperature was 20 ± 3°C with 40% humidity. Care and experimental practices were conducted under the standards of the Canadian Council on Animal Care and approved by the University of Calgary Life and Environmental Science Animal Care Committee.
Bacteria.
Mannheimia haemolytica biotype A serotype 1 (strain B122), isolated from a steer that died from pneumonic pasteurellosis, was used for infection, as previously described (32). Bacteria were grown overnight on Columbia blood agar plates at 37°C. Flasks of sterile Columbia broth were inoculated with three bacterial colonies and incubated overnight at 37°C in a shaking incubator. Inoculants were prepared at a bacterial concentration of 2 × 108 CFU M. haemolytica//ml, as estimated by MacFarland nephelometry, and confirmed by CFU enumeration on Columbia blood agar. The bacterial suspension was then resuspended in endotoxin-free HBSS to a final concentration of 2 × 107 CFU prior to inoculation.
Bacterial challenge in vivo.
Following local lidocane anesthesia (2% HCl and epinephrine injection; Biomeda-MTC, Animal Health Inc.), a sterile trochar was inserted through a percutaneous incision into the trachea of each calf, a sterile 1.7-mm catheter (Kendall Sovereign, Mansfield MA) was threaded through the trochar with the catheter tip extending to the tracheal bifurcation, and 2 × 107 CFU live M. haemolytica in 10 ml HBSS was injected through the catheter into the lungs, as previously described (8). Control and untreated calves received 10 ml HBSS vehicle only. The catheter and trochar were removed, and the incision was sealed using tissue adhesive (Vet-Bond; 3 M Animal Care Products, St. Paul, MN). Rectal temperatures, respiratory rate, and heart rate were measured to ensure that all calves were healthy prior to infection. There was no significant change in these measures at 3 and 24 h postinfection in any of the experimental groups.
Bronchoalveolar lavage.
Three and 24 h postinoculation, bronchoalveolar lavage (BAL) fluid samples were obtained by 3 sequential washings with 20 ml of endotoxin-free sterile HBSS, as described previously (8). BAL fluid (100 μl) was centrifuged for 10 min at 1,000 × rpm onto a microscope slide, using a Shandon Cytospin 4 cytocentrifuge (Thermo Electron Corporation, Pittsburgh, PA). Cells were fixed with either DiffQuick stain (Baxter Healthcare Corp., Miami, FL) or 4% paraformaldehyde in pyrogen-free phosphate-buffered saline (PBS) solution (pH 7.2, 0.15 M NaCl) for cell identification and apoptosis detection, respectively. Infiltration of neutrophils was calculated for each sample as the percentage of neutrophils in total leukocytes in the BAL fluid. The remaining BAL fluid was centrifuged for 10 min at 1,500 × g. A portion of the BAL fluid supernatant was serially diluted, plated onto Columbia blood agar, and incubated overnight at 37°C for enumeration of M. haemolytica organisms. The remainder of the supernatants were snap-frozen in liquid nitrogen and stored at −80°C for further analysis. The pellet was resuspended in HBSS, cell counts were determined using a hemocytometer, and equal numbers of leukocytes were used for detection of apoptosis, as described below.
Neutrophil purification for studies in vitro.
Purified neutrophil preparations were obtained from peripheral blood drawn from the jugular veins of healthy Holstein cows into BD Vacutainers containing 1.5 ml anticoagulant acid citrate dextrose (ACD solution A; Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ), as previously described (7, 8, 26). Briefly, the blood was pooled and spun at 1,200 × g for 20 min at 4°C. The plasma and buffy coat were removed, and the remaining cells were washed with 20 ml HBSS and spun again at 1,200 × g for 10 min at 4°C. Contaminating erythrocytes were eliminated with 20 ml of cold filter-sterilized hypotonic lysis solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4), and isotonicity was restored by adding 10 ml of cold 3× hypertonic restoring solution (10.6 mM Na2HPO4, 2.7 mM NaH2PO4, 462 mM NaCl). The lysing procedure was repeated two more times. After centrifugation at 1,200 × g, the cell pellet was resuspended in HBSS containing 10% heat-inactivated fetal bovine serum (HI-FBS; Sigma) to optimize the cell environment. Using a hemocytometer, neutrophil viability was determined, based on the percentage of cells that excluded 0.1% trypan blue (Flow Laboratories Inc., McLean, VA). Differential cell counts were performed on cytospin preparations stained with DiffQuick (Baxter Healthcare Corp., Miami, FL) to assess neutrophil purity. Neutrophil populations used were >90% pure and >90% viable throughout the study.
Monocyte isolation, macrophage differentiation, and cell lines used in the study.
Isolation of circulating bovine monocytes was performed via differential centrifugation at 1,200 × g for 20 min in a Beckman J-6B centrifuge (Beckman Instruments, Palo Alto, CA) at 4°C, as previously described (7). The buffy coat was diluted 1:1 with sterile 0.9% NaCl (Sigma), layered onto a polysucrose and sodium diatrixoate gradient (Histopaque-1077; Sigma), and centrifuged at 1,500 × g for 40 min at 4°C. The cell suspension was collected, washed with HBSS, and centrifuged at 500 × g for 10 min, and contaminating erythrocytes were removed by hypotonic lysis. Monocytes were resuspended in Iscove's modified Dulbecco's medium (IMDM; Sigma) and used for same-day experiments or cultured for macrophage differentiation. Cells were counted using a hemocytometer, and viability was assessed by 0.1% trypan blue (Flow Laboratories Inc., McLean, VA) exclusion. Differential cell counts were performed on cytospin preparations stained with DiffQuick to assess monocyte purity. Cells were incubated at 37°C, 5% CO2, in either 24-well plates (Costar, Cambridge, MA) for cell death assays or Labtek chamber slides (Nalge Nune International, Naperville, IL) for nonspecific esterase staining. Nonadherent cells were removed by washing them three times (37°C HBSS), and adherent monocytes were incubated at 37°C, 5% CO2, in IMDM containing 10% HI-FBS, 0.9% penicillin-streptomycin, and 0.9% tylosin (all from Sigma) for 7 days for macrophage differentiation. Esterase staining (Sigma) was done on days 2, 4, 6, and 7 to assess macrophage maturation as previously described (7). Viability of cells isolated for macrophage differentiation were >95% pure throughout the study. Macrophages were >95% mature at 7 days.
The bovine kidney epithelial cell line, MDBK (NBL-1, ATCC, Manassas, VA) and the bovine tracheal fibroblast cell line EBTr (NBL-4, ATCC) were grown in minimum essential medium (Eagle's modification) containing 2 mM l-glutamine and Earle's balanced salt solution containing 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, and 10% HI-FBS (all from Sigma). The bovine pulmonary endothelial cell line CPA47 (ATCC) was grown in Kaighn's modified F-12K media (Invitrogen, Carlsbad, CA) containing 10% HI-FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 80 μg/ml tylosin (all from Sigma). The day of experiments, the culture media were replaced with media containing no antibiotics. All cell lines were cultured at 37°C and 5% CO2.
Apoptotic DNA fragmentation.
Apoptotic cell death was first examined using fluorescent in situ terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. Cytospin preparations were permeabilized in 0.1% Triton X-100 in PBS, and the TUNEL reaction was carried out according to the manufacturer's instructions (Roche Diagnostics, Laval, Quebec, Canada). Slides were counterstained with a Hoechst 33258 nuclear stain (Molecular Probes, Eugene, OR) (data not shown). Wavelengths for fluorescence were monitored with excitation at 488 nm and emissions at 515 nm for fluorescein and with excitation at 350 nm and emission at 461 nm for Hoechst. Quantifications of positive cells in TUNEL were performed by taking images of slides containing cells isolated from the BAL fluid collected from the control and sham- and tulathromycin-treated M. haemolytica-infected calves at a magnification of ×400 using a Leica DMR fluorescent microscope. Micrographs were taken using a Retiga 2000x (Q Imaging, Surrey, British Columbia) and analyzed with Velocity (Improvision, Waltham, MA).
To further assess the proapoptotic effects of tulathromycin, a cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche) was used according to the manufacturer's instructions, as previously described (8). Photometric development was measured at 405 nm using a THERMOmax microplate reader (Molecular Devices Corp., Menlo Park, CA). Colorimetric changes were measured using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA). Apoptosis was expressed as the absorbance ratios of the experimental cell lysates versus absorbances from unmanipulated controls arbitrarily set as 1.0 (100%). For time and dose dependence experiments, purified neutrophils (106 cells) were incubated with tulathromycin (50 μg to 2 mg per ml) at 37°C and 5% CO2 for 0.5 to 2 h. Tulathromycin-induced cell apoptosis was assessed in freshly isolated bovine mononuclear leukocytes, monocyte-derived macrophages, CPA47 endothelial cells, MDBK epithelial cells, and EBTr fibroblasts and compared with induction of apoptosis in neutrophils, using 24-well plates as described above. To assess and compare the effects of tulathromycin and other drugs used to treat bovine respiratory disease on neutrophil apoptosis, purified neutrophils were coincubated with 2 mg/ml tulathromycin or equimolar concentrations of penicillin G, ceftiofur sodium, or oxytetracycline (all from Sigma) for 0.5 h at 37°C and 5% CO2. For all experiments, cells treated with HBSS containing 10% HI-FBS or 1.0 μM staurosporine were used as negative and positive controls, respectively.
Translocation of phosphatidylserine.
Neutrophil apoptosis in individual cells was also examined using an annexin V FLUOS staining kit (Roche) according to the manufacturer's instructions. A wet mount of 15 μl of the stained cells was prepared and was visualized under fluorescent microscopy. Apoptotic cells appeared solid green (excitation at 450 nm, emission at 490 nm) and necrotic cells appeared green with red nuclei (excitation at 535 nm, emission at 550 nm). Images were taken using a Leica DMR fluorescent microscope and a Retiga 2000x (Q Imaging) camera and analyzed with Velocity (Improvision).
Caspase activity assays.
Caspase activity in bovine neutrophils was assessed in triplicate using a caspase-3 activity assay (Calbiochem, La Jolla, CA) according to the manufacturer's instructions. Briefly, 1 × 106 cells were treated with the caspase-3 substrate (Asp-Glu-Val-Asp; DEVD) labeled with a fluorescent molecule, 7-amino-4-trifluoromethyl coumarin (AFC). The synthetic peptide substrate DEVD-AFC was added and cleaved by caspase-3 to also generate fluorescent AFC. The reaction was monitored by a blue-to-green shift in fluorescence upon cleavage of the AFC fluoroflore using a SpectraMax M2e microplate reader (Molecular Devices).
Leukotriene B4 assay.
Levels of CXCL8 could not be detected in the BAL fluid in these studies (data not shown). Therefore, the effect of tulathromycin treatment in experimentally infected calves on the bronchoalveolar accumulation of another marker of severe inflammation, LTB4, was measured in BAL fluid from experimentally infected calves. Concentrations of LTB4 in the bronchoalveolar lavage fluid supernatants were determined (THERMOmax microplate reader; Molecular Devices Corp., Menlo Park, CA) at 405 nm using a competitive enzyme immunometric assay kit (Leukotriene B4 EIA kit; Cayman Chemical Co., Ann Arbor MI). The specificity of the assay is 100% for LTB4, 0.03% for 5(S)-HETE, and <0.01% for LTC4, LTE4, LTD4, and LTF4 and has a detection limit of 7 pg/ml. Colorimetric changes were measured using a SpectraMax M2e microplate reader (Molecular Devices).
Western blotting.
Changes in phosphorylation of IκBα, nuclear translocation of the p65 subunit of NF-κB, and cleaved caspase-3 were assessed using Western blotting techniques. Treated cells were washed with HBSS and lysed using a lysis buffer (1% Igepal CA-630, 0.1% SDS, 0.5% sodium deoxycholate diluted in 1× PBS; all from Sigma) containing a protease inhibitor cocktail tablet (Complete Mini; Roche Diagnostics). Total protein concentration was determined using a Bradford protein assay (Bio-Rad Laboratories, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Whole-cell lysates were diluted at a 1:1 ratio in 2× electrophoresis buffer and boiled at 90°C for 5 min. Proteins were resolved on 10% SDS-polyacrylamide gel by electrophoresis and were electro-transferred to nitrocellulose membranes (Sigma). Membranes were blocked in 5% nonfat dry milk in TBS containing 0.5% Tween 20 (TBS-T) for 1 h at room temperature (RT). The blots were probed with monoclonal mouse anti-phospho-IκBα (Ser32/36), rabbit anti-cleaved caspase-3 antibodies (Cell Signaling Technology, Danvers, MA), and mouse anti-NF-κB p65 (F-6; Santa Cruz) at a dilution of 1:500. After exposure to each primary antibody, the blots were incubated with anti-mouse/rabbit secondary antibodies conjugated with horseradish peroxidase (all from Cell Signaling) at a final dilution of 1:1,000 for 1 h at RT and subjected to band visualization with an enhanced chemiluminescence detection system as described by the manufacturer (ECL plus; GE Healthcare, Pittsburgh, PA). All membranes were stripped in 0.5 M acetic acid for 1 h at RT and reprobed for goat anti-actin and anti-goat (Santa Cruz Biotechnologies, Santa Cruz, CA) at a dilution of 1:1,000. Densitometry analysis was performed using a Canon CanoScan 4400F scanner and Image J densitometry software (http://rsbweb.nih.gov/ij/).
RNA isolation and real-time PCR analysis.
Total RNA was isolated from vehicle- or drug-treated neutrophils using the RNeasy minikit (Qiagen, Mississauga, Ontario, Canada), and mRNA was reverse transcribed using the SuperScript first-strand synthesis system (Invitrogen) and a MyCycler thermocycler (Bio-Rad), according to the manufacturer's instructions. The first-strand cDNA was used as a template in the subsequent PCR analyses. Transcript levels were determined by real-time PCR using the Sybr green PCR master mix reagent kit and an iCycler real-time PCR detection system (Bio-Rad Laboratories). The PCR primer sequences used were designed as previously described (33). The following primers were used: CXCL8 forward (CATGTTCTGTGTGGGTCTGG) and reverse (CAGGTGAGGGTTGCAAGATT;); GAPDH (glyceraldehyde-3-phosphate dehydrogenase) forward (CCTGGAGAAACCTGCCAAGT) and reverse (GCCAAATTCATTGTCGTACCA). Cycling conditions for real-time PCR were as follows: 95°C for 3 min, followed by 40 cycles at 95°C for 30 s, and an annealing temperature of 62°C for 30 s and 72°C for 30 s. Relative levels of CXCL8 transcripts were calculated using individual standard curves and normalized to relative GAPDH mRNA levels.
Statistical analysis.
Statistical analyses of the data were made using one-way analysis of variance (ANOVA). Multicomparison post hoc analysis was performed with the Tukey test. Statistical significance was established at a P value of <0.05. Numeric values were expressed as means ± standard errors of the means (SEM).
RESULTS
Tulathromycin induces leukocyte apoptosis in calves challenged intratracheally with live M. haemolytica and inhibits the increase of BAL fluid LTB4 caused by infection.
Calves infected with M. haemolytica, but not control animals, contained elevated concentrations of bacteria and high numbers of neutrophils (>90% of BAL fluid cells) in their bronchoalveolar lavage specimens at 3 h postinfection (Table 1). Tulathromycin-treated animals had significantly less bacteria in the lungs 24 h postinfection, but neutrophil numbers were not different (Table 1). There was no significant difference in rectal temperatures between any of the groups (Table 1).
TABLE 1.
Calf rectal temperatures, numbers of M. haemolytica CFU, and percentages of leukocytes recovered from the bronchoalveolar lavage fluid of calves at 3 h and 24 h postinfection
| Parameter | Control |
M. haemolytica |
M. haemolytica + TUL |
|||
|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |
| Bacteria (CFU/ml) | 0 | 0 | 1.54 × 106a | 2.45 × 106b | 8.85 × 105a | 6.0 × 101c |
| Neutrophils (%) | 31.3 | 76.4 | 90.9d | 83.3 | 91.8d | 71.3 |
| Rectal temp (°C) | 39.5 | 39.3 | 39.5 | 39.8 | 39.5 | 38.9 |
P < 0.05 compared to 3-h control.
P < 0.05 compared to 24-h control.
P < 0.05 compared to 24-h M. haemolytica.
P < 0.05 compared to 3-h control.
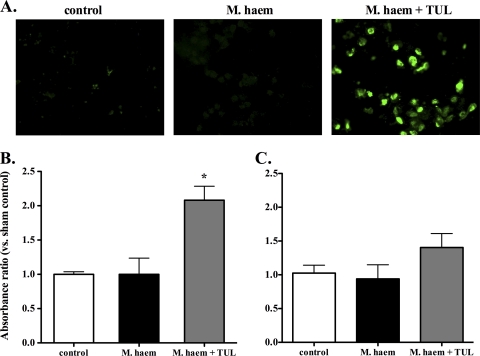
Three hours postchallenge, there were greater numbers of BAL fluid leukocytes exhibiting TUNEL-positive staining in infected animals treated with tulathromycin than in the other groups, in which TUNEL-positive leukocytes were few (Fig. 1A). Hoechst staining also revealed an increase in leukocytes containing broken nuclear structures in the BAL fluid isolated from tulathromycin-treated animals infected 3 h postchallenge (data not shown). A cell death detection ELISA confirmed that tulathromycin-treated calves contained levels of apoptotic leukocytes significantly higher than those of controls or vehicle-treated infected calves at 3 h, but not 24 h, postchallenge (Fig. 1B and C).
FIG. 1.
Tulathromycin induces cell apoptosis in calves challenged intratracheally with live M. haemolytica. (A) Fluorescent staining of bronchoalveolar lavage (BAL) samples fixed with 4% paraformaldehyde. Green represents TUNEL staining of apoptotic cells. Levels of apoptotic mono-/oligonucleosomes in BAL samples isolated from sham-treated (control), M. haemolytica-challenged calves (M. haem) and tulathromycin-treated calves challenged with M. haemolytica (M. haem + TUL) were measured using a cell death ELISA at 3 h (B) and 24 h (C) postinfection. Values were calculated as absorbance ratios versus values measured with cells from uninfected control calves. Values are means ± standard errors of the means. n = 5 to 8/group. *, P < 0.05.
By 24 h postchallenge, BAL fluid LTB4 was significantly higher in infected untreated calves than that in controls; treatment with tulathromycin prevented this increase in LTB4 (Fig. 2B).
FIG. 2.
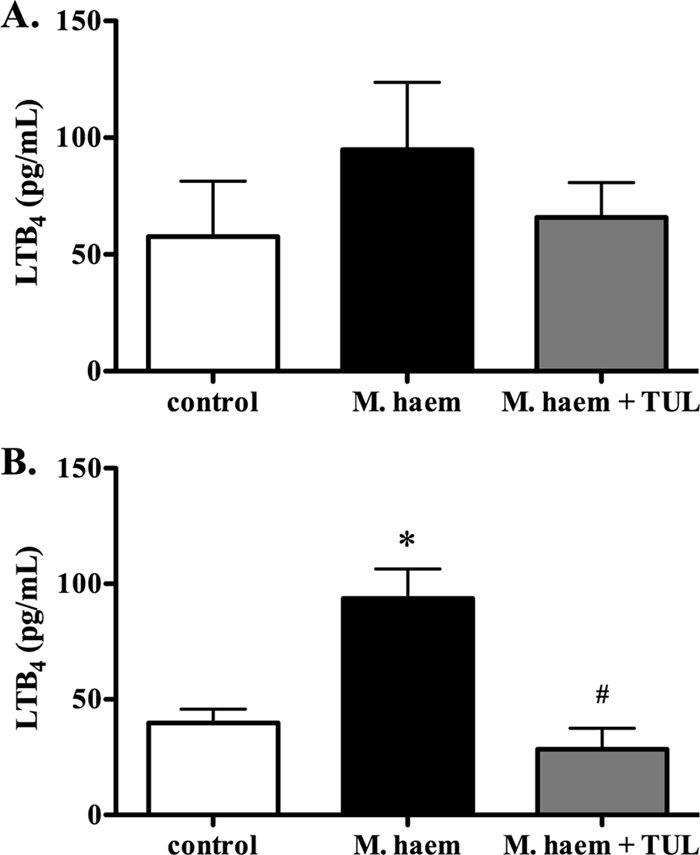
Tulathromycin reduces levels of leukotriene B4 in calves challenged intratracheally with live M. haemolytica. LTB4 synthesis in the bronchoalveolar lavage fluid isolated from sham-treated calves (control), M. haemolytica-challenged calves (M. haem) and tulathromycin-treated calves challenged with M. haemolytica (M. haem + TUL) at 3 h (A) and 24 h (B) postinfection. Values are means ± standard errors of the means. n = 5 to 8/group. *, P < 0.05 versus control; #, P < 0.05 versus M. haemolytica-challenged calves.
Tulathromycin induces apoptosis, but not necrosis, in bovine neutrophils in vitro.
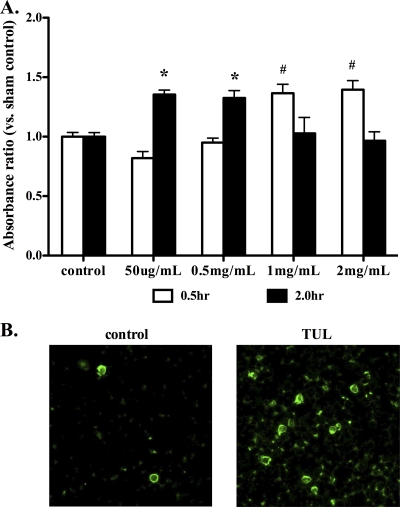
To further investigate the proapoptotic effects of tulathromycin observed in vivo, freshly isolated neutrophils were incubated with various concentrations of tulathromycin. After 30 min of incubation, 1 mg/ml and 2 mg/ml tulathromycin significantly increased neutrophil apoptosis compared to controls (Fig. 3A). After 2 h of incubation, the lower concentrations of 50 μg/ml and 0.5 mg/ml tulathromycin also induced neutrophil apoptosis (Fig. 3A). Consistent with previous studies that showed that tulathromycin accumulates in neutrophils at intracellular concentrations 26-fold greater than extracellular concentrations (14) and in view of our experimental findings, 2 mg/ml of tulathromycin was selected for the majority of our subsequent studies in vitro in an attempt to best reflect physiological conditions in which the drug accumulates at high concentrations within the cell. Annexin V-fluorescein isothiocyanate (FITC) and propidium iodine staining confirmed that tulathromycin (2 mg/ml) induces neutrophil apoptosis, but not necrosis (Fig. 3B). Staurosporine (1 μM), used as a positive control in all experiments, also induced apoptosis in neutrophils (data not shown).
FIG. 3.
Tulathromycin induces apoptosis in bovine neutrophils in a time- and concentration-dependent manner in vitro. (A) Levels of apoptotic mono-/oligonucleosomes in neutrophils incubated with tulathromycin (50 μg/ml to 2 mg/ml) for 0.5 h and 2.0 h were measured using a cell death ELISA. Values were calculated as absorbance ratios versus values measured in neutrophils from controls incubated with HBSS (control), arbitrarily set to 1.0. (B) Fluorescent staining of apoptotic neutrophils after 0.5 h of incubation with HBSS (control) or tulathromycin (TUL; 2 mg/ml), as determined by annexin V-FITC fluorescent staining of externalized phosphatidyl serine. Values are means ± standard errors of the means. n = 6/group. * and #, P < 0.05 versus control.
Antibiotic-induced apoptosis in bovine neutrophils is caspase-3 dependent and occurs in the presence or absence of live M. haemolytica.
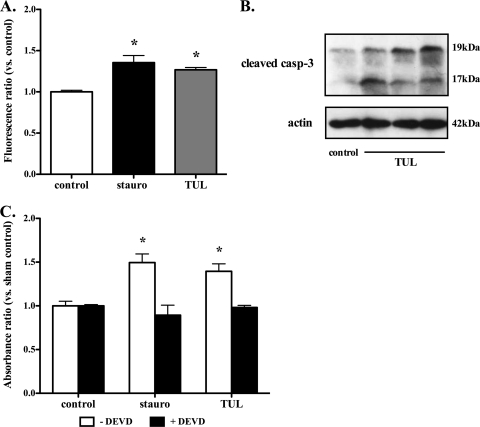
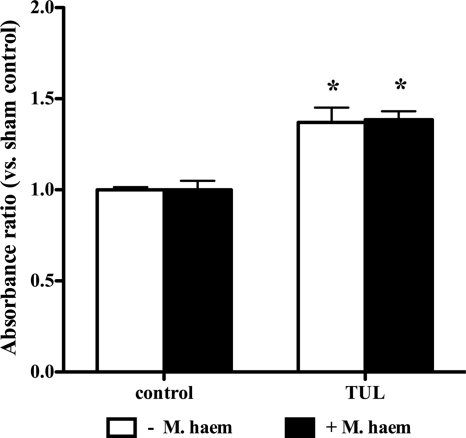
Another set of experiments determined whether the proapoptotic effects of tulathromycin were associated with the activation of caspase-3. Using a caspase-3-specific activity assay and Western blotting, experiments revealed a significant increase in caspase-3 activity (Fig. 4A) and cleaved caspase-3 fragments (Fig. 4B) in bovine neutrophils treated with tulathromycin. This effect was observed in tulathromycin-treated neutrophils at 15 min, as well as at 30 min (data not shown). Pretreatment of bovine neutrophils with the selective caspase-3 inhibitor, DEVD, blocked the proapoptotic effects of tulathromycin, or staurosporine, in these cells (Fig. 4C). Moreover, tulathromycin induced similar levels of neutrophil apoptosis, regardless of the presence or absence of live M. haemolytica (Fig. 5).
FIG. 4.
Induction of apoptosis in bovine neutrophils by tulathromycin is caspase-3 dependent. (A) Activity of caspase-3 in neutrophils treated with HBSS (control) or 2 mg/ml tulathromycin after 0.25 h. (B) Western blot analysis of cleaved caspase-3 in control and tulathromycin-treated (TUL; 2 mg/ml) bovine neutrophils for 0.25 h. Data illustrate 1 control and 3 tulathromycin-treated samples from 3 separate experiments. (C) Cell death ELISA of bovine neutrophils pretreated with a caspase-3 inhibitor (DEVD; 50 μM) for 1 h, followed by treatment with HBSS or tulathromycin (TUL; 2 mg/ml) for 0.5 h. Neutrophils treated with staurosporine (stauro; 1 μM) served as a positive control. Values are means ± standard errors of the means. n = 3 to 6/group. *, P < 0.05 versus control.
FIG. 5.
Tulathromycin induces apoptosis in bovine neutrophils in the presence and absence of live M. haemolytica. Levels of apoptotic mono-/oligonucleosomes in neutrophils incubated with HBSS (control) or tulathromycin (TUL; 2 mg/ml) for 0.5 h in the presence or absence of 2 × 107 CFU M. haemolytica were measured using a cell death ELISA. Values were calculated as absorbance ratios versus values measured with neutrophils from controls incubated with HBSS, arbitrarily set to 1.0. Values are means ± standard errors of the means. n = 4 to 6/group. *, P < 0.05 versus control.
Tulathromycin-induced apoptosis is, at least in part, cell and drug specific.
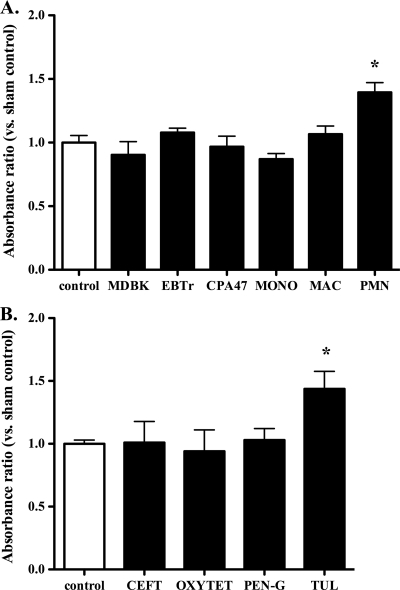
To investigate the cell selectivity of tulathromycin's proapoptotic effects, various bovine cell types were treated with 2 mg/ml tulathromycin for 30 min. Tulathromycin induced apoptosis in neutrophils, but not in cultured bovine pulmonary endothelial (CPA47) cells, kidney epithelial cells (MDBK), tracheal fibroblasts (EBTr), freshly isolated bovine blood monocytes, or monocyte-derived macrophages (Fig. 6A). Staurosporine induced apoptosis in all cell types (data not shown). At equimolar concentrations equal to 2 mg/ml of tulathromycin, tulathromycin induced neutrophil apoptosis, but penicillin, ceftiofur, or oxytetracycline failed to do so (Fig. 6B).
FIG. 6.
Tulathromycin-induced apoptosis is cell (A) and drug (B) selective. (A) Administration of tulathromycin increases levels of apoptotic mono-/oligonucleosomes in bovine neutrophils (PMN) but not in bovine epithelial cells (MDBK), fibroblasts (EBTr), endothelial cells (CPA47), freshly isolated blood monocytes (MONO), or monocyte-derived macrophages (MAC). (B) Tulathromycin (TUL; 2 mg/ml) but not ceftiofur (CEFT), oxytetracycline (OXYTET), or penicillin G (PEN-G) added at equimolar concentrations for 0.5 h increases levels of apoptosis in bovine neutrophils. Values were calculated as absorbance ratios versus values measured in neutrophils from control samples incubated with HBSS, arbitrarily set to 1.0. Values are means ± standard errors of the means. n = 4/group. *, P < 0.05 versus control.
Tulathromycin reduces expression of phosphorylated IκBα and nuclear translocation of the NF-κB p65 subunit.
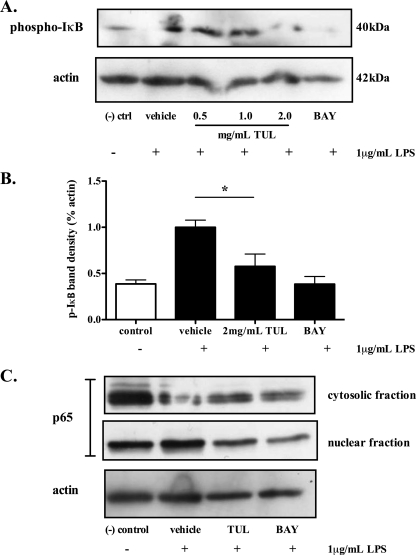
As caspase-3 may cleave a variety of NF-κB proteins (4, 24, 28), further experiments assessed whether the proapoptotic effects of tulathromycin were associated with a modulation of NF-κB signaling. LPS significantly increased the levels of IκBα phosphorylation in bovine neutrophils; treatment with tulathromycin inhibited LPS-induced IκBα phosphorylation in these cells (Fig. 7A and B). Additionally, LPS also promoted the cytosol-to-nucleus translocation of the NF-κB p65 subunit in bovine neutrophils; treatment with tulathromycin prevented the translocation of the NF-κB p65 subunit (Fig. 7C).
FIG. 7.
Tulathromycin reduces phosphorylation of IκBα and prevents nuclear translocation of NF-κB p65. Representative immunoblotting (A) and densitometry analysis (B) of phosphorylated IκBα in LPS (1 μg/ml)-stimulated bovine neutrophils pretreated with MG132 proteosome inhibitor (20 μM), and cytosolic and nuclear p65 in LPS-stimulated bovine neutrophils treated with HBSS (vehicle) or tulathromycin (TUL; 2 mg/ml) for 0.5 h (C). BAY 11-7085 (BAY; 50 μM) inhibitor served as a positive control. Unstimulated neutrophils treated with HBSS served as a negative control. Densitometry data are expressed as percent actin. Values are means ± standard errors of the means. n = 3 to 4/group. *, P < 0.05 versus LPS-stimulated control.
TUL inhibits mRNA transcription of CXCL8.
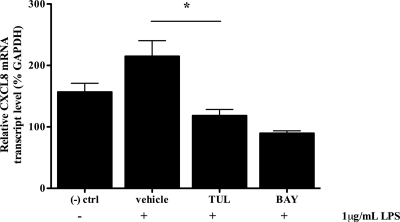
Interleukin-8 (CXCL8) is a proinflammatory gene product of the NF-κB signaling pathway. Our findings demonstrated that stimulation of bovine neutrophils with LPS (1 μg/ml) significantly increased mRNA transcription of CXCL8 (1.4-fold increase) (Fig. 8). Moreover, in addition to significantly reducing phosphorylated levels of IκBα and reducing translocation of p65 into the nucleus, treatment with tulathromycin (2 mg/ml for 1.0 h) in LPS-stimulated bovine neutrophils significantly reduced mRNA transcription of CXCL8 (0.55-fold decrease) (Fig. 8).
FIG. 8.
Tulathromycin inhibits mRNA transcription of CXCL8. Real-time PCR measuring relative CXCL8 mRNA levels of bovine neutrophils coincubated with LPS (1 μg/ml) and HBSS (vehicle) or tulathromycin (TUL; 2 mg/ml) for 1.0 h. The inhibitor BAY 11-7085 (BAY; 50 μM) served as a positive control. Unstimulated neutrophils treated with HBSS served as a negative control. Values are means ± standard errors of the means. n = 4/group. *, P < 0.05 versus LPS-stimulated control.
DISCUSSION
Selective drug-induced activation of neutrophil apoptosis offers a promising avenue for the development of new therapeutics with anti-inflammatory properties. Results from this study illustrate how tulathromycin, in addition to its antimicrobial effects, has immuno-modulatory benefits. Findings indicate that tulathromycin significantly reduces the accumulation of proinflammatory LTB4 in the bronchoalveolar spaces of M. haemolytica-infected calves. Preceding this effect, a striking increase in the numbers of apoptotic leukocytes, as determined by TUNEL staining and quantification of apoptotic mono- and oligo-nucleosomes, was observed with the lungs of the animals treated with the antibiotic. The effect was observed without a reduction in the numbers of total neutrophils infiltrating the bronchoalveolar spaces early in the infection. The observations prompted investigations into the proapoptotic mechanisms induced by tulathromycin, and we sought to assess how in turn this may modulate proinflammatory signaling using freshly isolated bovine neutrophils. The results demonstrate that tulathromycin directly induces neutrophil apoptosis in both a concentration- and time-dependent manner, regardless of the presence or absence of live M. haemolytica. The proapoptotic effects of the antibiotic are caspase-3 dependent. Tulathromycin induced apoptosis in bovine neutrophils, but not in bovine fibroblasts, epithelial cells, endothelial cells, freshly isolated bovine blood monocytes, or monocyte-derived macrophages. Moreover, the proapoptotic effect of the antibiotic could not be induced with equimolar concentrations of penicillin, oxytetracycline, or ceftiofur. Importantly, the data demonstrate that the proapoptotic effect of tulathromycin is associated with a significant decrease in phosphorylation of IκBα, nuclear translocation of the NF-κB p65 subunit, and an inhibition of CXCL8 mRNA transcription. Together, the present findings reveal novel mechanisms through which induction of neutrophil apoptosis and modulation of proinflammatory signaling may confer anti-inflammatory benefits to an antibiotic.
Local necrosis and self-perpetuating recruitment of neutrophils are responsible for severe tissue damage in a variety of inflammatory disorders, including bacterial pneumonia. The model described herein offers a new opportunity to investigate how drug-induced neutrophil apoptosis may deliver anti-inflammatory benefits in a target species. Bovine pneumonia caused by M. haemolytica is responsible for severe pulmonary inflammation, leading to respiratory failure and death within 48 to 72 h, via mechanisms that are shared with human pneumonia (9). In the bovine lung, M. haemolytica leukotoxins are known to induce necrosis in neutrophils and alveolar macrophages, which in turn impairs antibacterial defenses, further amplifying the inflammatory response (12, 46). The toxic compounds and proinflammatory mediators released by necrotic leukocytes exacerbate the inflammatory injury. Findings from this study suggest that tulathromycin may interrupt this proinflammatory cascade by inducing neutrophil apoptosis and by reducing the local accumulation of the potent neutrophil chemoattractant LTB4. Results from studies of bovine cells in vitro lend further support to this hypothesis and uncover a novel mechanism through which drug-induced neutrophil apoptosis may inhibit NF-κB activation and the subsequent transcription of CXCL8.
In addition to their antimicrobial properties, macrolides have a broad range of nonantibiotic properties. Some have anti cancer activity and anti angiogenic effects (58). Macrolides are also known to modulate host immunity and inflammation (5, 22). Recent observations indicate that the macrolides azithromycin and 16-ring tilmicosin, another veterinary antibiotic, may induce neutrophil apoptosis and promote the resolution of inflammation via mechanisms that remain obscure (5, 7, 8, 25, 26). Once terminally differentiated neutrophils reach the site of inflammation, they may alter their proinflammatory signals to an anti-inflammatory response, and induction of neutrophil apoptosis can contribute to this effect (17, 42, 45). Consistent with these observations, the findings reported here demonstrate for the first time that tulathromycin, a 15-ring triamilide macrolide with superior clinical efficacy, promotes neutrophil apoptosis, which in turn leads to the inhibition of the NF-κB pathway and the downstream production of proinflammatory mediators. Caspases cleave NF-κB proteins during apoptosis, preventing the transcription of many proinflammatory and prosurvival genes (4, 28, 37). In chicken spleen cells, active caspase-3 cleaves IκBα, producing a dominant inhibitory form of the protein and ultimately suppressing NF-κB activity (4). Additionally, reports have demonstrated that some macrolides, including erythromycin, inhibit NF-κB signaling and expression of proinflammatory mediators, such as CXCL8, via mechanisms that have yet to be fully elucidated (2, 27, 36, 56). Our results demonstrate that the caspase-3-dependent proapoptotic effects of tulathromycin are associated with the inhibition of IκBα phosphorylation, reduction of the nuclear translocation of the NF-κB p65 subunit, and decrease in mRNA levels of CXCL8.
LTB4 is a potent neutrophil chemoattractant, and during severe inflammation, this arachidonic acid metabolite may be released in a self-perpetuating manner by activated neutrophils; LTB4 also stimulates the release of neutrophil elastase and the generation of superoxide radicals (6). M. haemolytica leukotoxins stimulate the secretion of LTB4 during infection (9, 19). This study demonstrates that tulathromycin treatment is associated with a reduction of LTB4 in the infected lung, subsequent to the induction of apoptosis in bronchoalveolar leukocytes. Similar observations have been reported for tilmicosin, which also promotes neutrophil apoptosis (26). Further research is warranted to assess whether and how macrolides such as tulathromycin may directly inhibit LTB4 synthesis, independently of its antibacterial effects in vivo. To shed new light on such processes may help uncover new mechanisms whereby drug-induced neutrophil apoptosis may directly deliver anti-inflammatory benefits.
Some antibiotics can modulate neutrophil recruitment (8, 23, 31). In contrast, data from our in vivo calf studies reveal that tulathromycin does not significantly alter bronchoalveolar neutrophil numbers in the first 24 h of infection. The findings indicate that the cell's trafficking properties may remain unchanged, at least early during infection, despite the drug's proapoptotic effects, in keeping with other studies investigating similar properties of macrolides (12, 25, 46).
Apoptosis is a key mechanism for the removal of neutrophils from sites of injury and significantly contributes to the resolution of inflammation (17, 45). Neutrophils lose some of their secretory properties and undergo distinct morphological changes during apoptosis, including cell shrinkage, compaction of chromatin, and DNA fragmentation (29, 41, 43). An early biochemical change during apoptosis is the loss of membrane phospholipid asymmetry, as phosphatidylserine translocates to the outer leaflet of the plasma membrane (20, 34). The externalization of this molecule facilitates the selective recognition of apoptotic neutrophils by macrophages (40, 42). Our results demonstrate that tulathromycin promotes phosphatidylserine translocation in bovine neutrophils. However, the exact mechanisms whereby antibiotics like tulathromycin and tilmicosin (1, 10) may activate programmed cell death in neutrophils have remained elusive. Apoptosis can be initiated by one of two main caspase signaling cascades, namely, the extrinsic death receptor Fas pathway and the intrinsic mitochondrial pathway (1, 10). In an attempt to identify new mechanisms of drug-induced neutrophil apoptosis, the present study reveals that the proapoptotic effect of tulathromycin is caspase-3 dependent. However, whether tulathromycin's proapoptotic effects result from extrinsic activation and/or via the mitochondrial pathway requires further investigation. Tilmicosin-induced apoptosis does not result from a change in surface Fas expression (26). The effects of tulathromycin on Fas are unknown. Future research will determine whether the apparent cell selectivity of tulathromycin-induced apoptosis may be linked to its preferential uptake within neutrophils (14) and hence may possibly be initiated via the intrinsic apoptotic pathway.
In summary, using a clinically relevant model of tulathromycin treatment of calves infected with M. haemolytica and complementary experimental systems with bovine neutrophils in vitro, findings from the present study describe immuno-modulatory effects for a macrolide antibiotic. Together, the findings illustrate that tulathromycin, in addition to its antimicrobial properties, promotes neutrophil apoptosis in a caspase-3-dependent fashion and reduces the accumulation of proinflammatory LTB4 in the infected lung. The effects are time and concentration dependent and, at least in part, cell and drug selective. Moreover, tulathromycin directly decreases the phosphorylation of IκBα, reduces nuclear translocation of the NF-κB p65 subunit, and inhibits CXCL8 mRNA transcription. We propose that these anti-inflammatory effects help explain, at least in part, the superior clinical efficacy of this triamilide macrolide in the treatment of bovine respiratory disease. The data are of great significance for the treatment of bacterial pneumonia in general but also shed light on pivotal mechanisms through which neutrophil programmed cell death helps resolve inflammation. Perhaps most importantly, the findings describe a new pathway through which drug-induced neutrophil apoptosis confers anti-inflammatory benefits.
Acknowledgments
We thank Troy Feener, James Cotton, and Heather Sayer for their assistance with all of the calf studies.
This research was funded by the Natural Sciences and Engineering Research Council of Canada, Pfizer Animal Health, and the Margaret Gunn Endowment for Animal Health Research.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Akgul, C., D. A. Moulding, and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-322. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, Y., and P. N. Kao. 1999. Erythromycin inhibits transcriptional activation of NF-kappaB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrob. Agents Chemother. 43:2678-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoshiba, K., A. Nagai, and K. Konno. 1995. Erythromycin shortens neutrophil survival by accelerating apoptosis. Antimicrob. Agents Chemother. 39:872-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkett, M., D. Xue, H. R. Horvitz, and T. D. Gilmore. 1997. Phosphorylation of IkappaB-alpha inhibits its cleavage by caspase CPP32 in vitro. J. Biol. Chem. 272:29419-29422. [DOI] [PubMed] [Google Scholar]
- 5.Buret, A. G. 2010. Immuno-modulation and anti-inflammatory benefits of antibiotics: the example of tilmicosin. Can. J. Vet. Res. 74:1-10. [PMC free article] [PubMed] [Google Scholar]
- 6.Canetti, C., J. S. Silva, S. H. Ferreira, and F. Q. Cunha. 2001. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br. J. Pharmacol. 134:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin, A. C., W. D. Lee, K. A. Murrin, D. W. Morck, J. K. Merrill, P. Dick, and A. G. Buret. 2000. Tilmicosin induces apoptosis in bovine peripheral neutrophils in the presence or in the absence of Pasteurella haemolytica and promotes neutrophil phagocytosis by macrophages. Antimicrob. Agents Chemother. 44:2465-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, A. C., D. W. Morck, J. K. Merrill, H. Ceri, M. E. Olson, R. R. Read, P. Dick, and A. G. Buret. 1998. Anti-inflammatory benefits of tilmicosin in calves with Pasteurella haemolytica-infected lungs. Am. J. Vet. Res. 59:765-771. [PubMed] [Google Scholar]
- 9.Clinkenbeard, K. D., C. R. Clarke, C. M. Hague, P. Clinkenbeard, S. Srikumaran, and R. J. Morton. 1994. Pasteurella haemolytica leukotoxin-induced synthesis of eicosanoids by bovine neutrophils in vitro. J. Leukoc. Biol. 56:644-649. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, J. J., R. C. Duke, V. A. Fadok, and K. S. Sellins. 1992. Apoptosis and programmed cell death in immunity. Annu. Rev. Immunol. 10:267-293. [DOI] [PubMed] [Google Scholar]
- 11.Crooks, S. W., and R. A. Stockley. 1998. Leukotriene B4. Int. J. Biochem. Cell Biol. 30:173-178. [DOI] [PubMed] [Google Scholar]
- 12.Cudd, L. A., C. L. Ownby, C. R. Clarke, Y. Sun, and K. D. Clinkenbeard. 2001. Effects of Mannheimia haemolytica leukotoxin on apoptosis and oncosis of bovine neutrophils. Am. J. Vet. Res. 62:136-141. [DOI] [PubMed] [Google Scholar]
- 13.Daigle, I., and H. U. Simon. 2001. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int. Arch. Allergy Immunol. 126:147-156. [DOI] [PubMed] [Google Scholar]
- 14.Evans, N. A. 2005. Tulathromycin: an overview of a new triamilide antibiotic for livestock respiratory disease. Vet. Ther. 6:83-95. [PubMed] [Google Scholar]
- 15.Fadok, V. A., D. L. Bratton, A. Konowal, P. W. Freed, J. Y. Westcott, and P. M. Henson. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadok, V. A., J. S. Savill, C. Haslett, D. L. Bratton, D. E. Doherty, P. A. Campbell, and P. M. Henson. 1992. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 149:4029-4035. [PubMed] [Google Scholar]
- 17.Gilroy, D. W., T. Lawrence, M. Perretti, and A. G. Rossi. 2004. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 3:401-416. [DOI] [PubMed] [Google Scholar]
- 18.Gompertz, S., and R. A. Stockley. 2000. Inflammation—role of the neutrophil and the eosinophil. Semin. Respir. Infect. 15:14-23. [DOI] [PubMed] [Google Scholar]
- 19.Henricks, P. A., G. J. Binkhorst, A. A. Drijver, and F. P. Nijkamp. 1992. Pasteurella haemolytica leukotoxin enhances production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect. Immun. 60:3238-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homburg, C. H., M. de Haas, A. E. von dem Borne, A. J. Verhoeven, C. P. Reutelingsperger, and D. Roos. 1995. Human neutrophils lose their surface Fc gamma RIII and acquire annexin V binding sites during apoptosis in vitro. Blood 85:532-540. [PubMed] [Google Scholar]
- 21.Hsuan, S. L., M. S. Kannan, S. Jeyaseelan, Y. S. Prakash, C. Malazdrewich, M. S. Abrahamsen, G. C. Sieck, and S. K. Maheswaran. 1999. Pasteurella haemolytica leukotoxin and endotoxin induced cytokine gene expression in bovine alveolar macrophages requires NF-kappaB activation and calcium elevation. Microb. Pathog. 26:263-273. [DOI] [PubMed] [Google Scholar]
- 22.Ianaro, A., A. Ialenti, P. Maffia, L. Sautebin, L. Rombola, R. Carnuccio, T. Iuvone, F. D'Acquisto, and M. Di Rosa. 2000. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 292:156-163. [PubMed] [Google Scholar]
- 23.Ichikawa, Y., H. Ninomiya, H. Koga, M. Tanaka, M. Kinoshita, N. Tokunaga, T. Yano, and K. Oizumi. 1992. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am. Rev. Respir. Dis. 146:196-203. [DOI] [PubMed] [Google Scholar]
- 24.Kang, K. H., K. H. Lee, M. Y. Kim, and K. H. Choi. 2001. Caspase-3-mediated cleavage of the NF-kappa B subunit p65 at the NH2 terminus potentiates naphthoquinone analog-induced apoptosis. J. Biol. Chem. 276:24638-24644. [DOI] [PubMed] [Google Scholar]
- 25.Koch, C. C., D. J. Esteban, A. C. Chin, M. E. Olson, R. R. Read, H. Ceri, D. W. Morck, and A. G. Buret. 2000. Apoptosis, oxidative metabolism and interleukin-8 production in human neutrophils exposed to azithromycin: effects of Streptococcus pneumoniae. J. Antimicrob. Chemother. 46:19-26. [DOI] [PubMed] [Google Scholar]
- 26.Lee, W. D., A. N. Flynn, J. M. LeBlanc, J. K. Merrill, P. Dick, D. W. Morck, and A. G. Buret. 2004. Tilmicosin-induced bovine neutrophil apoptosis is cell-specific and downregulates spontaneous LTB4 synthesis without increasing Fas expression. Vet. Res. 35:213-224. [DOI] [PubMed] [Google Scholar]
- 27.Leiva, M., A. Ruiz-Bravo, E. Moreno, and M. Jimenez-Valera. 2008. Telithromycin inhibits the production of proinflammatory mediators and the activation of NF-kappaB in in vitro-stimulated murine cells. FEMS Immunol. Med. Microbiol. 53:343-350. [DOI] [PubMed] [Google Scholar]
- 28.Levkau, B., M. Scatena, C. M. Giachelli, R. Ross, and E. W. Raines. 1999. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat. Cell Biol. 1:227-233. [DOI] [PubMed] [Google Scholar]
- 29.Maianski, N. A., A. N. Maianski, T. W. Kuijpers, and D. Roos. 2004. Apoptosis of neutrophils. Acta Haematol. 111:56-66. [DOI] [PubMed] [Google Scholar]
- 30.McDonald, P. P., A. Bald, and M. A. Cassatella. 1997. Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood 89:3421-3433. [PubMed] [Google Scholar]
- 31.Mikasa, K., E. Kita, M. Sawaki, M. Kunimatsu, K. Hamada, M. Konishi, S. Kashiba, and N. Narita. 1992. The anti-inflammatory effect of erythromycin in zymosan-induced peritonitis of mice. J. Antimicrob. Chemother. 30:339-348. [DOI] [PubMed] [Google Scholar]
- 32.Morck, D. W., J. K. Merrill, M. S. Gard, M. E. Olson, and P. N. Nation. 1997. Treatment of experimentally induced pneumonic pasteurellosis of young calves with tilmicosin. Can. J. Vet. Res. 61:187-192. [PMC free article] [PubMed] [Google Scholar]
- 33.Mount, J. A., N. A. Karrow, J. L. Caswell, H. J. Boermans, and K. E. Leslie. 2009. Assessment of bovine mammary chemokine gene expression in response to lipopolysaccharide, lipotechoic acid + peptidoglycan, and CpG oligodeoxynucleotide 2135. Can. J. Vet. Res. 73:49-57. [PMC free article] [PubMed] [Google Scholar]
- 34.Naito, M., K. Nagashima, T. Mashima, and T. Tsuruo. 1997. Phosphatidylserine externalization is a downstream event of interleukin-1 beta-converting enzyme family protease activation during apoptosis. Blood 89:2060-2066. [PubMed] [Google Scholar]
- 35.Nutsch, R. G., T. L. Skogerboe, K. A. Rooney, D. J. Weigel, K. Gajewski, and K. F. Lechtenberg. 2005. Comparative efficacy of tulathromycin, tilmicosin, and florfenicol in the treatment of bovine respiratory disease in stocker cattle. Vet. Ther. 6:167-179. [PubMed] [Google Scholar]
- 36.Ou, X. M., Y. L. Feng, F. Q. Wen, K. Wang, J. Yang, Z. P. Deng, D. S. Liu, and Y. P. Li. 2008. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-kappaB. Respirology 13:63-72. [DOI] [PubMed] [Google Scholar]
- 37.Reuther, J. Y., and A. S. Baldwin, Jr. 1999. Apoptosis promotes a caspase-induced amino-terminal truncation of IkappaBalpha that functions as a stable inhibitor of NF-kappaB. J. Biol. Chem. 274:20664-20670. [DOI] [PubMed] [Google Scholar]
- 38.Rooney, K. A., R. G. Nutsch, T. L. Skogerboe, D. J. Weigel, K. Gajewski, and W. R. Kilgore. 2005. Efficacy of tulathromycin compared with tilmicosin and florfenicol for the control of respiratory disease in cattle at high risk of developing bovine respiratory disease. Vet. Ther. 6:154-166. [PubMed] [Google Scholar]
- 39.Rossi, A. G., D. A. Sawatzky, A. Walker, C. Ward, T. A. Sheldrake, N. A. Riley, A. Caldicott, M. Martinez-Losa, T. R. Walker, R. Duffin, M. Gray, E. Crescenzi, M. C. Martin, H. J. Brady, J. S. Savill, I. Dransfield, and C. Haslett. 2006. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12:1056-1064. [DOI] [PubMed] [Google Scholar]
- 40.Savill, J. 1997. Recognition and phagocytosis of cells undergoing apoptosis. Br. Med. Bull. 53:491-508. [DOI] [PubMed] [Google Scholar]
- 41.Savill, J., V. Fadok, P. Henson, and C. Haslett. 1993. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today 14:131-136. [DOI] [PubMed] [Google Scholar]
- 42.Savill, J. S., A. H. Wyllie, J. E. Henson, M. J. Walport, P. M. Henson, and C. Haslett. 1989. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83:865-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheel-Toellner, D., K. Q. Wang, P. R. Webb, S. H. Wong, R. Craddock, L. K. Assi, M. Salmon, and J. M. Lord. 2004. Early events in spontaneous neutrophil apoptosis. Biochem. Soc. Trans. 32:461-464. [DOI] [PubMed] [Google Scholar]
- 44.Serhan, C. N., S. D. Brain, C. D. Buckley, D. W. Gilroy, C. Haslett, L. A. O'Neill, M. Perretti, A. G. Rossi, and J. L. Wallace. 2007. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 21:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serhan, C. N., and J. Savill. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6:1191-1197. [DOI] [PubMed] [Google Scholar]
- 46.Shewen, P. E., and B. N. Wilkie. 1982. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect. Immun. 35:91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 48.Skogerboe, T. L., K. A. Rooney, R. G. Nutsch, D. J. Weigel, K. Gajewski, and W. R. Kilgore. 2005. Comparative efficacy of tulathromycin versus florfenicol and tilmicosin against undifferentiated bovine respiratory disease in feedlot cattle. Vet. Ther. 6:180-196. [PubMed] [Google Scholar]
- 49.Squier, M. K., A. J. Sehnert, and J. J. Cohen. 1995. Apoptosis in leukocytes. J. Leukoc. Biol. 57:2-10. [DOI] [PubMed] [Google Scholar]
- 50.Takeshita, K., I. Yamagishi, M. Harada, S. Otomo, T. Nakagawa, and Y. Mizushima. 1989. Immunological and anti-inflammatory effects of clarithromycin: inhibition of interleukin 1 production of murine peritoneal macrophages. Drugs Exp. Clin. Res. 15:527-533. [PubMed] [Google Scholar]
- 51.Tang, G., J. Yang, Y. Minemoto, and A. Lin. 2001. Blocking caspase-3-mediated proteolysis of IKKbeta suppresses TNF-alpha-induced apoptosis. Mol. Cell 8:1005-1016. [DOI] [PubMed] [Google Scholar]
- 52.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchihashi, Y., K. Oishi, H. Yoshimine, S. Suzuki, A. Kumatori, T. Sunazuka, S. Omura, K. Matsushima, and T. Nagatake. 2002. Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils. Antimicrob. Agents Chemother. 46:1101-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker, R. D., F. M. Hopkins, T. W. Schultz, M. D. McCracken, and R. N. Moore. 1985. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am. J. Vet. Res. 46:2429-2433. [PubMed] [Google Scholar]
- 55.Ward, C., E. R. Chilvers, M. F. Lawson, J. G. Pryde, S. Fujihara, S. N. Farrow, C. Haslett, and A. G. Rossi. 1999. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 274:4309-4318. [DOI] [PubMed] [Google Scholar]
- 56.Wu, L., J. H. Lin, K. Bao, P. F. Li, and W. G. Zhang. 2009. In vitro effects of erythromycin on RANKL and nuclear factor-kappa B by human TNF-alpha stimulated Jurkat cells. Int. Immunopharmacol. 9:1105-1109. [DOI] [PubMed] [Google Scholar]
- 57.Wyllie, A. H. 1997. Apoptosis: an overview. Br. Med. Bull. 53:451-465. [DOI] [PubMed] [Google Scholar]
- 58.Yatsunami, J., Y. Fukuno, M. Nagata, M. Tominaga, S. Aoki, N. Tsuruta, M. Kawashima, S. Taniguchi, and S. Hayashi. 1999. Antiangiogenic and antitumor effects of 14-membered ring macrolides on mouse B16 melanoma cells. Clin. Exp. Metastasis 17:361-367. [DOI] [PubMed] [Google Scholar]