Abstract
Recent reports on the decline of the efficacy of artemisinin-based combination therapies (ACTs) indicate a serious threat to malaria control. The endoplasmic/sarcoplasmic reticulum Ca2+-ATPase ortholog of Plasmodium falciparum (PfSERCA) has been suggested to be the target of artemisinin and its derivatives. It is assumed that continuous artemisinin pressure will affect polymorphism of the PfSERCA gene (serca) if the protein is the target. Here, we investigated the polymorphism of serca in parasite populations unexposed to ACTs to obtain baseline information for the study of potential artemisinin-driven selection of resistant parasites. Analysis of 656 full-length sequences from 13 parasite populations in Africa, Asia, Oceania, and South America revealed 64 single nucleotide polymorphisms (SNPs), of which 43 were newly identified and 38 resulted in amino acid substitutions. No isolates showed L263E and S769N substitutions, which were reportedly associated with artemisinin resistance. Among the four continents, the number of SNPs was highest in Africa. In Africa, Asia, and Oceania, common SNPs, or those with a minor allele frequency of ≥0.05, were less prevalent, with most SNPs noted to be continent specific, whereas in South America, common SNPs were highly prevalent and often shared with those in Africa. Of 50 amino acid haplotypes observed, only one haplotype (3D7 sequence) was seen in all four continents (64%). Forty-eight haplotypes had frequencies of less than 5%, and 40 haplotypes were continent specific. The geographical difference in the diversity and distribution of serca SNPs and haplotypes lays the groundwork for assessing whether some artemisinin resistance-associated mutations and haplotypes are selected by ACTs.
Artemisinin-based combination therapies (ACTs) are currently the first-line treatment for uncomplicated falciparum malaria in most areas of endemicity (23, 34). The deployment of ACT has greatly reduced malaria morbidity and mortality (8). However, recently there has been accumulating evidence which suggests a decline of the efficacy of ACTs and artemisinin monotherapy in western Cambodia (7, 22). Although the molecular mechanism of the antimalarial action of artemisinin and its derivatives (artemisinins) remains to be clarified, the endoplasmic and sarcoplasmic reticulum Ca2+-ATPase ortholog of Plasmodium falciparum (PfSERCA or PfATP6) has been suggested to be the target of artemisinins (9, 15). A replacement of L at codon 263 of the PfSERCA gene (serca) with E (L263E) results in abrogation of inhibition of PfSERCA by artemisinin (32). A recent allelic exchange study also showed reduced (though not significantly) susceptibility to artemisinins in parasites expressing the L263E allele (33). Mutations(s) of serca has also been associated with in vitro artemether resistance, with field isolates from French Guiana having an artemether 50% inhibitory concentration (IC50) of 1.7 nM whereas that for parasites having an S769N substitution was 79.4 nM (13, 15, 16). The association of these mutations with artemisinin resistance, however, has not been confirmed for other geographic areas (2, 3, 5-7, 11, 12, 14, 18, 20, 26, 35).
Limited sequence analyses have previously shown that P. falciparum serca contains a number of single nucleotide polymorphisms (SNPs) (6, 14, 28). Thus, a large number of field isolates would be required to properly assess whether drug pressure imposed by continuous deployment of ACTs causes a potential selection of a mutation(s) in serca that is associated with artemisinin resistance. We consider that baseline information on serca polymorphism occurring before the implementation of ACTs would provide necessary information to infer whether some serca mutations are likely selected by ACTs. We have recently used serca as a genetic marker to study the geographical distribution of genetic diversity of P. falciparum and obtained 514 full-length serca sequences from nine P. falciparum populations in Africa, Asia, Oceania, and South America (30). In this study we newly obtained 139 full-length serca sequences, mostly from Malawi, Madagascar, Iran, and Bangladesh. Together with published sequences, a total of 656 sequences were analyzed for serca polymorphism. Importantly, all parasite isolates examined hereto were unexposed to ACTs. The results show that serca has many spontaneous mutations and haplotypes, which are geographically distinctive. It is predicted that further studies will reveal more SNPs/haplotypes. This information has several implications for inferring whether some serca mutations and haplotypes are selected by the current continuous deployment of ACTs.
MATERIALS AND METHODS
Parasite isolates.
We collected P. falciparum isolates from Malawi, Madagascar, Iran, and Bangladesh. In Malawi, samples were collected from infected individuals in all age groups during cross-sectional surveys in June and July 2000 at two primary schools in the Salima District (4). The study was approved by the local ethics committee of the Malaria Control Programme and the Malawi Ministry of Health. In Madagascar, blood samples containing parasites were collected from consenting symptomatic outpatients in 2005 as part of the national network activities for the surveillance of drug-resistant Plasmodium spp. (24). Administrative authorizations and ethical clearances were provided by the Ministry of Health and the national ethics committee. Samples from rural areas of Ampasimpotsy and Saharevo were sent to the malaria research unit of the Institut Pasteur de Madagascar and kept frozen at −20°C until use. In Iran, blood samples were collected from P. falciparum-infected individuals, 1 to 70 years old, with symptomatic uncomplicated malaria attending the Malaria Health Center in Chabahar and the Public Health Department in Sistan and Baluchistan province, southeastern Iran, during 2001 and 2002. The study was approved by the Ethical Review Committee of Research of the Pasteur Institute of Iran. In Bangladesh, samples were collected from symptomatic malaria patients in all age groups at Bandarban district hospital from October to December 2007 (17). Approval of the study was obtained from the Bangladesh Medical Research Council and the local health regulatory body in Bandarban, Bangladesh. All isolates examined in this study were from parasite populations not exposed to ACTs (including rural areas in Bangladesh and Papua New Guinea [PNG] which have had no previous access to ACT, despite the countries' support for the WHO policy). We also used three cultured strains originally isolated from Sudan (29).
DNA sequences.
Parasite genomic DNA was extracted using the QIAamp DNA blood minikit (Qiagen, Hilden, Germany). Full-length serca was amplified by PCR using Takara LA Taq polymerase (Takara Bio, Japan) in a 20-μl reaction mixture as previously described (28) with slight modifications: Primers U1 and 4099R were used (see Table S1 in the supplemental material). Forty cycles of amplification (20 s at 93°C and 5 min at 62°C) were preceded by denaturation at 93°C for 1 min and followed by a final elongation at 72°C for 10 min. The PCR product was diluted 10-fold, and a 2-μl aliquot was used as the template for a second PCR amplification of 20 cycles in a 50-μl reaction mixture using primers U1 and 4094R. The PCR products were purified using the QIAquick PCR purification kit (Qiagen). DNA sequencing was performed directly from two independent PCR products, using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems) and an ABI 3130 Genetic Analyzer (Applied Biosystems). Sequencing primers were designed to cover target regions in both directions (see Table S1 in the supplemental material). Mixed-genotype infections, judged from superimposed electropherogram peaks, were excluded from further analysis.
Sequence analyses.
We obtained a total of 139 full-length sequences of serca coding regions from Malawi (n = 38), Madagascar (n = 19), Iran (n = 35), Bangladesh (n = 44), and Sudan (n = 3). Multiple infections were detected for 36 isolates (18.0%). Also, 514 full-length sequences that we recently published (30) were included for analysis; these were from Ghana (n = 38), Tanzania (n = 69), Thailand (n = 82), Philippines (n = 53), Papua New Guinea (PNG) (n = 89), Solomon Islands (n = 51), Vanuatu (n = 80), Brazil (n = 42), and Venezuela (n = 10). Table S2 in the supplemental material summarizes all serca sequences used in this study. Three additional sequences from cultured strains (3D7, Dd2, and HB3) (28) were also included. Nucleotide diversity was estimated by θπ, the average pairwise nucleotide distance, and θS, the standardized number of polymorphic sites per site (Watterson's estimator), using DnaSP version 4.10 (25). Sequences were aligned using Clustal W (31) implemented in MEGA version 4.0 (27). Polymorphic sites and synonymous and nonsynonymous substitutions were determined using DnaSP.
The allele frequency of SNPs was calculated using Arlequine version 3.1 (10). We categorized SNPs as either common or uncommon, defined as those with a minor allele frequency of ≥0.05 or with a minor allele frequency of <0.05, respectively (19). (Excluding samples with multiple infection did not affect the frequency of common SNPs in this study, because analysis of 36 multiply infected samples showed a frequency of common SNPs [27/39 = 69%] that was comparable to that for 139 singly infected samples [103/139 = 74%] [P = 0.88, chi-square test].) Nucleotide and amino acid positions were numbered according to the 3D7 sequence (PlasmoDB gene identification no. PFA0310c). Amino acid haplotype diversity (h) was calculated using the formula h = [n/(n − 1)] × (1 − Σpi2), where pi is the frequency of the ith serca amino acid haplotype (21). The variance (V) of h was calculated using a formula modified from Nei's formula for a haploid genome: V = [2/n(n − 1)]{2[n − 2][Σpi3 − (Σpi2)2] + Σpi2 − [Σpi2]2}.
Nucleotide sequence accession numbers.
The sequences reported in this study have been deposited in the DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank database (accession numbers AB576210 to AB576348).
RESULTS
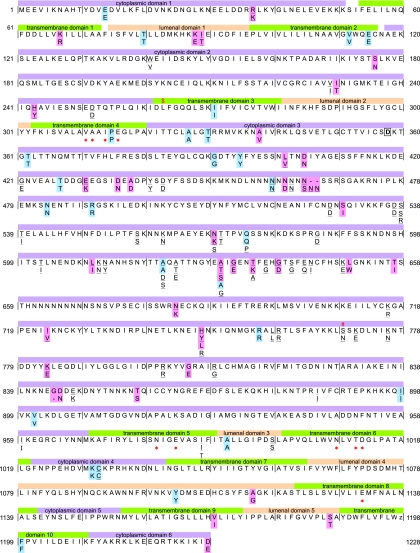
An alignment of 656 sequences (3,687 to 3,693 bp) revealed 62 polymorphic nucleotide sites with 64 SNPs, of which 38 resulted in amino acid substitutions (Fig. 1; see Table S3 in the supplemental material). Among the 64 SNPs, 43 SNPs (20 synonymous SNPs and 23 nonsynonymous SNPs) were newly identified in this study. Together with partial serca sequences obtained by other investigators, there were 110 SNPs (42 synonymous SNPs and 68 nonsynonymous SNPs) in total. In addition to SNPs, a variation in the number of Asn residues (9 to 11) in the Asn-tandem repeat region at codons 457 to 465 (of the 3D7 sequence) and a deletion of the Gly residue at codon 844 were also noted (Fig. 1; see Table S3 in the supplemental material). In our samples, we found no polymorphism at codons 263 and 769; these polymorphisms have been shown to affect the PfSERCA activity or to be associated with increased artemisinin IC50s (13, 32). An E431K SNP, which has been reported to be associated with increased artesunate IC50s in Senegal (13), was detected in Africa, Asia, and South America. I89T and N465K, which have been inferred not to be associated with artemisinin resistance (7), were observed only in Asia and Oceania. Amino acid replacements were largely clustered in cytoplasmic domain 3 (Fig. 1). In contrast, 10 transmembrane domains contained only two amino acid changes (at codons 67 and 1169), which had similar residue properties (i.e., basic residues K67R and hydrophobic residues V1169I).
FIG. 1.
Polymorphism in the P. falciparum SERCA (PfATP6) genes from worldwide parasite populations unexposed to artemisinin-based combination therapies. Nonsynonymous substitutions and synonymous substitutions observed in 656 samples examined in this study are highlighted in pink and blue, respectively, alongside the 3D7 sequence (PlasmoDB, PFA0310c). Substitutions reported by other investigators are underlined. Sequence regions for 10 transmembrane domains, 6 cytoplasmic domains, 5 luminal domains, putative calcium-binding sites (*), and a phosphorylation site (boxed D at 358) were inferred from the rabbit serca gene (Swiss-Prot Protein Data Bank [PDB] code P04191). The L at position 263, where experimental substitution to E results in abrogation of inhibition of PfSERCA by artemisinin (32), is indicated by $. The S769N substitution, which has been shown to be associated with an increased artemether IC50 in French Guiana (13), is indicated by #. Dashes between positions 465 and 466 and at position 844 denote deletions.
The number of SNPs was relatively high in Africa (44 SNPs with 27 nonsynonymous SNPs) compared to other continents (10 to 21 SNPs with 5 to 10 nonsynonymous SNPs) (Table 1 and Fig. 2a). Two nucleotide diversity indices, i.e., θS, the number of polymorphic sites per site, and θπ, the average number of pairwise nucleotide differences, showed substantially different levels in the four continents. Overall, θS was higher than θπ in Africa, Asia, and Oceania, whereas θπ was somewhat higher than θS in South America. This indicates that the majority of the alleles in Africa, Asia, and Oceania are uncommon (allele frequency of <5%) but that this is not so in South America. The rank order of nucleotide diversity (θS and θπ) was Africa > Asia > Oceania ∼ South America, with the exception of θπ in South America (Table 1).
TABLE 1.
SNPs and amino acid haplotypes in 656 full-length serca sequences from 13 P. falciparum populations (656 isolates)
| Geographic area | n | No. of SNPs |
Nucleotide diversity (mean ± SD) |
No. of amino acid haplotypes |
Haplotype diversity, h (mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Nonsynonymous | Commonb | Continent specific | θπ | θS | Total | Commonb | Continent specific | |||
| Worldwidea | 656 | 64 | 38 | 61 | 50 | 0.00045 ± 0.00002 | 0.00238 ± 0.00030 | 50 | 48 | 40 | 0.579 ± 0.023 |
| Africa | 164 | 44 | 27 | 38 | 35 | 0.00061 ± 0.00003 | 0.00206 ± 0.00031 | 34 | 30 | 27 | 0.760 ± 0.033 |
| Ghana | 38 | 13 | 7 | 9 | 0.00041 ± 0.00006 | 0.00084 ± 0.00023 | 9 | 5 | 0.563 ± 0.094 | ||
| Tanzania | 69 | 31 | 18 | 26 | 0.00067 ± 0.00005 | 0.00170 ± 0.00031 | 19 | 15 | 0.771 ± 0.051 | ||
| Malawi | 38 | 14 | 10 | 7 | 0.00056 ± 0.00006 | 0.00090 ± 0.00024 | 13 | 8 | 0.801 ± 0.058 | ||
| Madagascar | 19 | 10 | 6 | NA | 0.00074 ± 0.00010 | 0.00078 ± 0.00025 | 10 | NA | 0.918 ± 0.036 | ||
| Asia + Oceania | 434 | 24 | 11 | 21 | 16 | 0.00028 ± 0.00002 | 0.00098 ± 0.00020 | 19 | 17 | 12 | 0.420 ± 0.030 |
| Asia | 214 | 21 | 10 | 15 | 9 | 0.00037 ± 0.00003 | 0.00096 ± 0.00021 | 17 | 15 | 8 | 0.581 ± 0.036 |
| Iran | 35 | 11 | 5 | 4 | 0.00070 ± 0.00005 | 0.00073 ± 0.00022 | 7 | 2 | 0.805 ± 0.032 | ||
| Bangladesh | 44 | 8 | 5 | 4 | 0.00025 ± 0.00004 | 0.00050 ± 0.00018 | 7 | 4 | 0.432 ± 0.091 | ||
| Thailand | 82 | 11 | 7 | 8 | 0.00027 ± 0.00004 | 0.00060 ± 0.00018 | 9 | 7 | 0.404 ± 0.068 | ||
| Philippines | 53 | 4 | 2 | 1 | 0.00024 ± 0.00003 | 0.00024 ± 0.00012 | 4 | 1 | 0.565 ± 0.040 | ||
| Oceania | 220 | 11 | 5 | 8 | 3 | 0.00018 ± 0.00003 | 0.00050 ± 0.00015 | 6 | 4 | 2 | 0.217 ± 0.036 |
| PNG | 89 | 9 | 5 | 7 | 0.00016 ± 0.00004 | 0.00048 ± 0.00016 | 6 | 5 | 0.211 ± 0.058 | ||
| Solomon Islands | 51 | 4 | 2 | 2 | 0.00010 ± 0.00004 | 0.00024 ± 0.00012 | 2 | 1 | 0.077 ± 0.050 | ||
| Vanuatu | 80 | 3 | 2 | 0 | 0.00024 ± 0.00005 | 0.00016 ± 0.00009 | 2 | 0 | 0.292 ± 0.055 | ||
| South America | 52 | 10 | 7 | 3 | 3 | 0.00083 ± 0.00005 | 0.00060 ± 0.00019 | 7 | 2 | 3 | 0.798 ± 0.024 |
| Brazil | 42 | 9 | 6 | 2 | 0.00072 ± 0.00006 | 0.00057 ± 0.00019 | 6 | 1 | 0.783 ± 0.035 | ||
| Venezuela | 10 | 4 | 2 | NA | 0.00026 ± 0.00013 | 0.00038 ± 0.00019 | 2 | NA | 0.200 ± 0.154 | ||
Three sequences from Sudan and three sequences from cultured parasites are included. See Materials and Methods for details.
Common SNPs are those with a minor allele frequency of ≥5%. Common haplotypes are those with a frequency of ≥5%. Countries for which there were fewer than 20 isolates are excluded (NA).
FIG. 2.
Frequency distributions of SNPs and amino acid haplotypes in serca of P. falciparum isolates from Africa, Asia, Oceania, and South America. (a) Alleles in SNPs are divided into those with a minor allele frequency of <5% (open bars) and those with a minor allele frequency of ≥5% (shaded bars). (b) SNPs are divided into those that are continent specific (open bars) or not (shaded bars). (c) Amino acid haplotypes are divided into those with a minor haplotype frequency of <5% (open bars) and those with a minor haplotype frequency of ≥5% (shaded bars). (d) Amino acid haplotypes are divided into those that are continent specific (open bars) or not (shaded bars). The vertical axes represent the number of isolates.
The geographical distribution of SNPs in serca was remarkably different, particularly between Africa/Asia/Oceania and South America; there were two notable features. First, common SNPs with allele frequencies of ≥0.05 were less prevalent in Africa, Asia, and Oceania, at 6/44 SNPs (14%), 6/21 SNPs (29%), and 3/11 SNPs (27%), respectively (Table 1 and Fig. 2a). However, in South America the prevalence of common SNPs was high (7/10 SNPs [70%]). Second, substantial numbers of SNPs were continent specific in Africa and Asia: 35/44 SNPs (80%) were found only in Africa and 9/21 SNPs (43%) in Asia (Table 1 and Fig. 2b). Most SNPs found in Oceania (8/11 [73%]) were also found in Asia, and thus when SNPs in Asia and Oceania are combined, 16/24 SNPs (67%) are specific to these geographic areas. Limited numbers of SNPs are shared between Africa and Asia/Oceania: of 24 SNPs (33%) found in Asia/Oceania, only 8 are shared with Africa. In contrast, in South America, only 3/10 SNPs (30%) found are specific to the continent, and the remaining 70% are shared with Africa.
In total, there are 50 serca amino acid haplotypes in our sample set (Fig. 3). Haplotype 1 (3D7 strain) is the most prevalent (422/656 [64%]) and is the only haplotype found in all four continents. Next was haplotype 36 (35/656 [5%]), which was found in Asia and Oceania. All other haplotypes are rare (<5%), with numerous singleton haplotypes (n = 28), and 40 haplotypes are continent specific (Fig. 2c and d). These results indicate that there are unexpectedly numerous serca haplotypes that are specific to a geographic area. The frequencies and geographical distribution of haplotypes differ greatly among geographic areas (Fig. 3). The number of haplotypes and haplotype diversity (h) are high in Africa (34 and 0.760, respectively) compared to Asia and Oceania (17 haplotypes and h = 0.581 in Asia; 6 haplotypes and h = 0.217 in Oceania) (Table 1). In South America, the value for h (0.798) was comparable to that in Africa.
FIG. 3.
Amino acid haplotypes of P. falciparum serca observed in 656 worldwide samples. Amino acid positions are numbered according to the 3D7 serca sequence (PFA0310c). Deletions of N next to position 465 are dashed. “Others,” haplotypes of cultured parasites: 1, 3D7 and Dd2; 9, two Sudanese isolates; 10, one Sudanese isolate; 50, HB3.
DISCUSSION
Continuous implementation of ACTs as first-line treatment for uncomplicated malaria is likely imposing artemisinin pressure to P. falciparum populations in wide geographic areas. At present, however, no alternative classes of antimalarial drugs are available to replace the artemisinin derivatives. We therefore believe it important to see whether and how the polymorphism profiles of serca change over time and spatially and in particular whether specific mutations/haplotypes are selected for under artemisinin pressure. The present sequence data provide baseline information on spontaneous mutations in serca from global P. falciparum populations that were unexposed to artemisinin or its derivatives. Natural variations in serca are characterized by abundance of geographic area-specific SNPs, the majority of which are observed at frequencies of less than 5%. The highest diversity of geographic area-specific haplotypes is observed in African parasite populations. The low prevalence of common SNPs in all parasite populations, except in South America, underscores several implications for detecting potential artemisinin-driven selection of resistant parasites and lends valuable insight on whether some serca haplotypes are selected by ACTs in natural parasite populations.
First, the occurrence of numerous SNPs in serca may make the rapid detection of artemisinin-driven selection of resistant parasites difficult. Resistant parasites, when they appear, would be geographically restricted, and their prevalence would initially be low. It is therefore rather difficult to distinguish a mutation associated with artemisinin resistance from abundantly present resistance-unrelated mutations, which are low in frequency and also geographic area specific. This is particularly true for African parasite populations. Identification of a resistance-associated mutation(s) is likely to be missed until such a time that the resistance-associated mutation becomes considerably prevalent. In this context, it should also be mentioned that some SNPs, though limited in number, are already highly prevalent. Those SNPs are probably unrelated to artemisinin resistance; for example, an I89T SNP was common in Asia, but the mutation has been suggested to be unrelated to artemisinin resistance (7). Such highly prevalent SNPs found in populations unexposed to ACTs therefore must be excluded from candidate mutations for consideration.
Second, the detection of potential artemisinin-driven selection would require a better understanding of the parasite population structure. This is particularly true for South America. The characteristics of serca polymorphism in South America are distinct from those in other continents: overall, the number of SNPs (and haplotypes) is limited, and SNPs with a minor allele frequency of ≥0.05 are prevalent. In such a scenario, distinguishing between an increase in allele frequency of a mutation due to artemisinin resistance and an increase of spontaneous mutations unrelated to artemisinin resistance would not be straightforward. South American parasite populations are strongly structured and genetic differentiation among local populations is remarkably high, probably due to epidemic expansion of some parasite genotypes (1). Consistently, serca SNPs/haplotypes were found to be limited in numbers but multiply represented (Table 1 and Fig. 3). Thus, the population structure must be taken into account to properly assess artemisinin-driven selection, preferably using neutral markers such as microsatellites and synonymous SNPs.
Third, the abundance of minor haplotypes may confound potential artemisinin-driven selection in some cases. If an artemisinin resistance-associated mutation(s) was generated in the major wild genotype (3D7 type), the identification of that mutation would not be difficult. A selective sweep of resistance parasites during continuous exposure to artemisinins would greatly reduce within-population diversity of serca haplotypes, with a fixation or predominance of a resistance-associated haplotype. A simple comparison of haplotypes from artemisinin-sensitive and -resistant parasites should reveal the resistance-associated mutation(s). However, if the resistance-associated mutation(s) was selected from one of the less prevalent haplotypes, the identification could not be readily made. Of the 48 minor haplotypes observed in this study (Table 1), 21 haplotypes have two to four amino acid substitutions compared with the major (3D7) haplotype. Since these haplotypes are in most cases continent specific, if resistant haplotypes were generated independently in several geographic areas, minor haplotypes would possess multiple SNPs, most of which might be unrelated to resistance (in some cases continent specific) but possibly selected by genetic hitchhiking linked to a nearby resistance-affording mutation. In these cases, comparison of resistance haplotypes originating from different continents with different evolutionary histories of serca would lead to identification of resistance-conferring mutations with high confidence.
In summary, the present analysis of a total of 656 full-length serca sequences identified numerous SNPs and haplotypes from geographically widespread P. falciparum populations that were unexposed to ACTs. The SNPs and haplotypes observed were in most cases present at a low frequency and were geographic area specific, with the exception of sequences from South America. The geographical difference in the diversity and distribution of serca SNPs and haplotypes observed in this study lays the groundwork for assessing whether some artemisinin resistance-associated mutations and haplotypes are selected by the current continuous and increasing deployment of ACTs.
Supplementary Material
Acknowledgments
We thank all the people who participated in the epidemiological studies for their kind cooperation.
This work was supported by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (18073013), from the Japan Society for Promotion of Sciences (18GS03140013, 20390120, 22406012), and from the Ministry of Health, Labor and Welfare (H20-Shinkou-ippan-013).
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Anderson, T. J., B. Haubold, J. T. Williams, J. G. Estrada-Franco, L. Richardson, R. Mollinedo, M. Bockarie, J. Mokili, S. Mharakurwa, N. French, J. Whitworth, I. D. Velez, A. H. Brockman, F. Nosten, M. U. Ferreira, and K. P. Day. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17:1467-1482. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, D. J., A. M. McCollum, S. M. Griffing, C. Salas, V. Soberon, M. Santolalla, R. Haley, P. Tsukayama, C. Lucas, A. A. Escalante, and V. Udhayakumar. 2009. Dynamics of malaria drug resistance patterns in the Amazon Basin region following changes in Peruvian national treatment policy for uncomplicated malaria. Antimicrob. Agents Chemother. 53:2042-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertaux, L., L. H. Quang, V. Sinou, N. X. Thanh, and D. Parzy. 2009. New PfATP6 mutations found in Plasmodium falciparum isolates from Vietnam. Antimocrob. Agents Chemother. 53:4570-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bwijo, B., A. Kaneko, M. Takechi, I. L. Zungu, Y. Moriyama, J. K. Lum, T. Tsukahara, T. Mita, N. Takahashi, Y. Bergqvist, A. Bjorkman, and T. Kobayakawa. 2003. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first line treatment in Malawi. Acta Trop. 85:363-373. [DOI] [PubMed] [Google Scholar]
- 5.Cojean, S., V. Hubert, J. Le Bras, and R. Durand. 2006. Resistance to dihydroartemisinin. Emerg. Infect. Dis. 12:1798-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlström, S., M. I. Veiga, P. Ferreira, A. Mårtensson, A. Kaneko, B. Andersson, A. Björkman, and J. P. Gil. 2008. Diversity of the sarco/endoplasmic reticulum Ca2+-ATPase orthologue of Plasmodium falciparum (PfATP6). Infect. Genet. Evol. 8:340-345. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp, A. M., F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakpong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. Day, N. Lindegardh, D. Socheat, and N. J. White. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eastman, R. T., and D. A. Fidock. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 7:864-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckstein-Ludwig, U., R. J. Webb, I. D. Van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 10.Excoffier, L., G. Laval, and S. Schneider. 2005. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinform. 1:47-50. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira, I. D., A. Martinelli, L. A. Rodrigues, E. L. do Carmo, V. E. do Rosário, M. M. Póvoa, and P. Cravo. 2008. Plasmodium falciparum from Pará state (Brazil) shows satisfactory in vitro response to artemisinin derivatives and absence of the S769N mutation in the SERCA-type PfATPase6. Trop. Med. Int. Health 13:199-207. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim, M. L., N. Khim, H. H. Adam, F. Ariey, and J.-B. Duchemin. 2009. Polymorphism of PfATPase in Niger: detection of three new point mutations. Malar. J. 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jambou, R., E. Legrand, M. Niang, N. Khim, P. Lim, B. Volney, M. T. Ekala, C. Bouchier, P. Esterre, T. Fandeur, and O. Mercereau-Puijalon. 2005. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 366:1960-1963. [DOI] [PubMed] [Google Scholar]
- 14.Jambou, R., A. Martinelli, J. Pinto, S. Gribaldo, E. Legrand, M. Niang, N. Kim, L. Pharath, B. Volnay, M. T. Ekala, C. Bouchier, T. Fandeur, P. Berzosa, A. Benito, I. D. Ferreira, C. Ferreira, P. P. Vieira, M. G. Alecrim, O. Mercereau-Puijalon, and P. Cravo. 2010. Geographic structuring of the Plasmodium falciparum sarco (endo) plasmic reticulum Ca2+ATPase (PfSERCA) gene diversity. PLoS One 5:e9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishna, S. 2006. Re-evaluation of how artemisinins work in light of emerging evidence of in vitro resistance. Trend Mol. Med. 12:200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrand, E., B. Volney, J. B. Meynard, P. Esterr, and O. Mercereau-Puijalon. 2007. Resistance to dihydroartemisinin. Emerg. Infect. Dis. 13:808-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marma, A. S., T. Mita, H. Eto, T. Tsukahara, S. Sarker, and H. Endo. 2010. High prevalence of sulfadoxine/pyrimethamine resistance alleles in Plasmodium falciparum parasites from Bangladesh. Parasitol. Int. 59:178-182. [DOI] [PubMed] [Google Scholar]
- 18.Menegon, M., A. R. Sannella, G. Majori, and C. Severini. 2008. Detection of novel point mutations in the Plasmodium falciparum ATPase6 candidate gene for resistance to artemisinins. Parasitol. Int. 57:233-235. [DOI] [PubMed] [Google Scholar]
- 19.Mu, J., P. Awadalla, J. Duan, K. M. McGee, J. Keebler, K. Seydel, G. A. T. McVean, and X.-Z. Su. 2007. Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat. Genet. 39:126-130. [DOI] [PubMed] [Google Scholar]
- 20.Mugittu, K., B. Genton, H. Mshindal, and H. P. Beck. 2006. Molecular monitoring of Plasmodium falciparum resistance to artemisinin in Tanzania. Malar. J. 5:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, NY.
- 22.Noedl, H., Y. Se, K. Schaecher, B. L. Smith, D. Socheat, and M. M. Fukuda. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619-2620. [DOI] [PubMed] [Google Scholar]
- 23.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77(Suppl. 6):181-192. [PubMed] [Google Scholar]
- 24.Randrianarivelojosia, M., J. L. Harisoa, L. P. Rabarijaona, L. A. Raharimalala, L. Ranaivo, V. Pietra, J. B. Duchemin, F. Rakotomanana, V. Robert, P. Mauclere, and F. Ariey. 2002. In vitro sensitivity of Plasmodium falciparum to amodiaquine compared with other major antimalarials in Madagascar. Parassitologia 44:141-147. [PubMed] [Google Scholar]
- 25.Rozas, J., J. C. Sánchez-DelBarrio, X. Messeguer, and R. Rozas. 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496-2497. [DOI] [PubMed] [Google Scholar]
- 26.Tahar, R., P. Ringwald, and L. K. Basco. 2009. Molecular epidemiology of malaria in Cameroon. XXVIII. In vitro activity of dihydroartemisinin against clinical isolates of Plasmodium falciparum and sequence analysis of the P. falciparum ATPase 6 gene. Am. J. Trop. Med. Hyg. 81:13-18. [PubMed] [Google Scholar]
- 27.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe, K., N. Sakihama, T. Hattori, L. Ranford-Cartwright, I. Goldman, A. A. Escalante, and A. A. Lal. 2004. Genetic distance in housekeeping genes between Plasmodium falciparum and Plasmodium reichenowi and within P. falciparum. J. Mol. Evol. 59:687-694. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe, K., N. Sakihama, D. Walliker, H. Babiker, A. A. Abdel-Muhsin, B. Bakote'e, H. Ohmae, N. Arisue, T. Horii, I. Rooth, A. Färnert, A. Björkman, and L. Ranford-Cartwright. 2007. Allelic dimorphism-associated restriction of recombination in Plasmodium falciparum msp1. Gene 397:153-160. [DOI] [PubMed] [Google Scholar]
- 30.Tanabe, K., T. Mita, T. Jombart, A. Eriksson, S. Horibe, N. Palacpac, L. Ranford-Cartwright, H. Sawai, N. Sakihama, H. Ohmae, M. Nakamura, M. U. Ferreira, A. A. Escalante, F. Prugnolle, A. Björkman, A. Färnert, A. Kaneko, T. Horii, A. Manica, H. Kishino, and F. Balloux. 2010. Plasmodium falciparum accompanied the human expansion out of Africa. Curr. Biol. 70:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlemann, A. C., A. Cameron, U. Eckstein-Ludwig, J. Fischbarg, P. Iserovich, F. A. Zuniga, M. East, A. Lee, L. Brady, R. K. Haynes, and S. A. Krishna. 2005. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat. Struct. Mol. Biol. 12:628-629. [DOI] [PubMed] [Google Scholar]
- 33.Valderramos, S. G., D. Scanfeld, A. C. Uhlemann, D. A. Fidock, and S. Krishna. 2010. Investigations into the role of the Plasmodium falciparum SERCA (PfATP6) L263E mutation in artemisinin action and resistance. Antimicrob. Agents Chemother. 54:3842-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. 2006. WHO guidelines for the treatment of malaria. World Health Organization, Geneva, Switzerland.
- 35.Zhang, G., Y. Guan, B. Zheng, S. Wu, and L. Tang. 2008. No PfATPase6 S769N mutation found in Plasmodium falciparum isolates from China. Malar. J. 7:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.