Abstract
Neurocysticercosis resulting from Taenia solium infections is a major cause of adult-acquired seizures worldwide. Disease is caused by larval cysts, and treatment consists of the anthelmintic drugs albendazole or praziquantel. There are no standard methods to assess drug activity to T. solium cysts in vitro. Morphological, functional, and biochemical changes that might reflect damaging (inhibiting, cytotoxic) drug effects were analyzed after exposure of cysts to albendazole sulfoxide (ABZ-SO), the major active metabolite of the drug in vivo, praziquantel (PZQ), or combinations of both. PZQ exposure led to a decrease in cyst size and inhibition of evagination, whereas ABZ-SO exposure resulted in minimal changes. Alkaline phosphatase (AP) is normally secreted by cysts, and both drugs inhibited AP secretion at concentrations of 5 and 50 ng/ml for PZQ and ABZ-SO, respectively. Some combinations of both drugs resulted in additive and/or synergistic activities. Parasite-specific antigen, detected in the cerebrospinal fluid and blood of infected patients, is also normally secreted by T. solium cysts. Antigen secretion was similarly inhibited by ABZ-SO and PZQ and a combination of both drugs, suggesting that inhibition of secretion is a common downstream consequence of the activities of both drugs. These studies establish quantitative methods to measure in vitro anthelmintic activity and suggest combination therapy with ABZ-SO and PZQ may have clinical benefit.
Neurocysticercosis (NCC), the most common cause of adult acquired seizures worldwide, results from infection of the brain by the cystic larval stage of Taenia solium. The adult tapeworm resides in the human intestines. It consists of a head with a scolex, which is an attachment organ, a neck, and increasingly maturing segments containing infectious ova. These are excreted as segments in the feces and, when ingested by free-roaming pigs, hatch and are carried to the brain, muscles, and other organs, where they develop into viable cysts in about 2 to 3 months. After ingestion and exposure to bile and other factors in the gastrointestinal tract milieu, the cysts evaginate and develop into tapeworms. Humans develop NCC after accidental ingestion of infectious ova sometimes originating from their own tapeworm or one residing in a family member or coinhabitant.
The clinical manifestations of NCC are due to growth, degeneration, and inflammation associated with the host response to the cyst. Treatment consists of the anthelmintic agents albendazole or praziquantel (PZQ) (4, 5, 15), as well as corticosteroids or other anti-inflammatory agents (4, 14), that are commonly used to control treatment-induced inflammation. However, cure rates are relatively low with currently available regimens, and more effective agents are needed.
T. solium larval cysts (metacestodes) can only be obtained from infected pigs. Studies using T. solium cysts are difficult because many infected pigs are required, harvesting cysts individually is laborious and time-consuming, and culturing and maintaining cysts in the laboratory is difficult. For these reasons, cysts of a related parasite of the same genus as T. solium, Taenia crassiceps, have been commonly used instead of T. solium cysts for in vitro studies of drug effects and parasite biology (16, 17, 32). However, it is not known whether any or all of the conclusions from these studies can be applied to T. solium.
Despite numerous studies of direct drug effects on T. crassiceps cysts (16-18, 31, 32), the best parameters for evaluating the effects of anthelmintics on T. solium have not been established. Traditionally, the effects of drugs on taeniid cysts have been assessed by visual examination of cysts for morphological and histopathological changes on light or electron microscopy suggesting damage (16, 17). Although these approaches have demonstrated effects of the commonly used anthelmintics for treatment of T. solium (17) and other cestodes (7, 11), the techniques require experienced personnel and equipment and are time-consuming for routine use and analysis. We were interested in investigating alternative, potentially more sensitive methods for detection of damage to parasites that had the potential for testing new drugs rapidly and inexpensively. The rationale for the use of enzymatic tests of parasite damage was that damaged parasites would likely release cellular contents through their tegument once the integrity of the barrier was breached. Such approaches have been demonstrated to be sensitive and specific to drug effects on other cestodes, e.g., Echinococcus species, in which alkaline phosphatase (AP) activity has, indeed, been shown to increase in the supernatants of drug-treated metacestodes (20, 22, 23). Other enzymes that are released into the supernatant of metacestodes, such as phosphoglucose isomerase, have been shown to be equally or more sensitive to the effects of drugs on Echinococcus multilocularis metacestodes (21).
In the present study, we characterized the macroscopic, microscopic, and biochemical parameters of drug-induced damage to T. solium cysts by the currently used anthelmintics PZQ and albendazole sulfoxide (ABZ-SO; the active compound of albendazole). We identified inhibition of the release of parasite-derived alkaline phosphatase as a useful and more sensitive measure of drug activity than morphological changes or death for both drugs. Using this assay, we found additive or synergistic effects of both drugs together. In addition, we show that ABZ-SO, as well as a combination of both drugs, inhibits the release of a specific parasite-derived antigen previously reported to be found in the sera of infected patients. These studies present quantitative method(s) to measure in vitro anthelmintic activity and suggest that combination therapy with ABZ-SO and PZQ may enhance cure rates.
MATERIALS AND METHODS
Parasites.
Metacestodes of T. solium were obtained from naturally infected pigs purchased from villages in an endemic region of Peru. The pigs were euthanized according to an approved protocol established by the Faculty of Veterinary Sciences at the Universidad Nacional Mayor de San Marcos. Parasites were individually harvested from muscle tissue and transported in phosphate-buffered saline (PBS; pH 7.4; Gibco-Invitrogen, Gaithersburg, MD).
Drugs.
ABZ-SO was purchased from a commercial supplier (Sigma, St. Louis, MO), and PZQ tablets were purchased from Merck (Merck KgAa, Darmstadt, Germany). Stock solutions of ABZ-SO and powdered PZQ tablets were prepared in dimethyl sulfoxide (DMSO; Sigma) and absolute ethanol, respectively, and diluted in culture medium to the desired concentration.
In vitro culture of cysts.
For in vitro culture, T. solium metacestodes were washed two times with sterile PBS and once with cyst medium composed of RPMI 1640 medium supplemented with HEPES buffer (10 mM; Gibco), penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml; all purchased from Gibco-Invitrogen) and allowed to stabilize in cyst medium for 12 h at 37°C in 5% CO2. For the drug studies, 10 cysts were cultured per well in 12-well plates in culture medium with a final volume of 2 ml. Drugs were added to achieve different final concentrations (range, 1 to 500 ng/ml). Control wells contained medium with drug solvent (DMSO or ethanol) at concentrations equal to the highest concentration of the corresponding drug used in the assay. Plates were incubated for 4 to 6 days at 37°C in 5%CO2. After each 24-h period, the supernatants of each well was harvested and replaced, and a photograph was taken of each culture plate. Each condition was evaluated in triplicate.
Measurement of cyst size.
Cyst size was represented by the average diameter of each cyst derived from the average of maximal length and maximal width of all cysts in a well. These measurements were performed on photographs of the culture plates using a standardized layout of photographic equipment at each time point. The size of each cyst (in centimeters), designated the corrected size, was estimated as follows: corrected cyst size = (cyst size in photograph/well diameter in photograph) × 2 cm, where 2 cm is the diameter of each well of the culture plate. A change in size was expressed as the percent reduction in the average corrected cyst size at the designated time point compared to average of the corrected cyst size at baseline in the well using the following formula: % reduction = (the average corrected cyst size in a given well/the average corrected cyst size in the same well at the start of culture) × 100.
Evagination.
The ability of cysts to evaginate was assessed by addition of porcine bile at selected time points. Culture medium was replaced with 1 ml of culture medium containing 50% porcine bile and incubated for 12 to 18 h at 37°C in 5% CO2, and then the evaginated cysts were counted and the percentage of evaginated cysts was calculated.
Measurement of AP secretion.
The concentration of AP in cyst culture supernatants of T. solium cysts was determined by using a commercially available colorimetric AP detection system (Roche Diagnostics, Branford, CT), adapted to a microassay format. Briefly, the reaction mixture in each well consisted of 50 μl of supernatant mixed with 25 μl of kit reagent 1 and 125 μl of reagent 2. The mixture was incubated at 37°C in the dark for 1 h. The optical densities (ODs) at 492 nm were then determined using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). Culture medium incubated without parasites or freshly prepared medium were included as controls. The results were expressed as corrected OD using the following formula: ODcorrected = ODtest sample − ODcorresponding control.
Parasite Ag detection in supernatants.
T. solium antigen (Ag) secretion into culture supernatants of T. solium metacestodes was detected with an antigen capture enzyme-linked immunosorbent assay (ELISA) as described previously (1, 19). This assay relies on two monoclonal antibodies (MAbs; designated B158 and biotinylated B60), both of which recognize an undefined Taenia saginata antigen but cross-react with T. solium.
RESULTS
Cyst viability in culture.
The size and ability of cysts to evaginate were determined in the absence of drug and after addition of PZQ or ABZ-SO. Viable cysts evaginate spontaneously in culture or after exposure to bile (16, 17). A trend toward decreased evagination after exposure to bile was apparent even in the absence of added drug after 3 days and achieved statistical significance at 6 days (P = 0.01, as determined by analysis of variance [ANOVA]; Fig. 1). Histological examination of cysts revealed minimal changes at day 3 (see Fig. S1 in the supplemental material) compared to day 0, but the tegument and other areas of the cysts were clearly damaged from day 4 onward (data not shown). Therefore, only data up to day 3 of culture were included in the present study, before significant histopathological damage, defined as the loss of morphological integrity of the tegumentary or subtegumentary layers of the cyst on hematoxylin-eosin staining, became apparent (see Fig. S1 in the supplemental material).
FIG. 1.
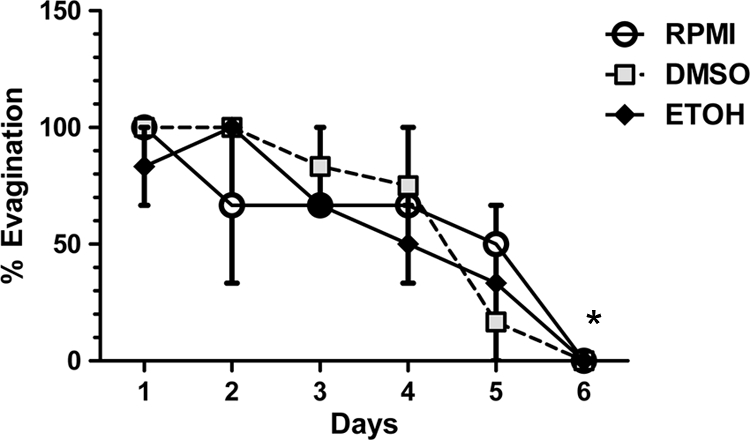
In vitro bile-stimulated evagination of cysts. Cysts of T. solium were cultured in triplicate wells for 6 days (see Materials and Methods), and on each of the indicated days (x axis), the percentage of cysts that evaginated after exposure to bile for 18 h was determined. The graph depicts the total percentage of cysts that evaginated on the indicated day of culture, a sum of spontaneously evaginated and bile-induced evagination, in control medium (RPMI medium alone), RPMI with DMSO (0.00005%), or ethanol (0.00025%), corresponding to the concentration of solvents used for dissolution of ABZ-SO (DMSO) or PZQ (ethanol). Each data point represents the mean of triplicate wells, and the bars indicate the standard errors of the mean (SEM). The asterisk indicates significantly different values compared to day 1.
Changes in cyst size and evagination after drug treatment.
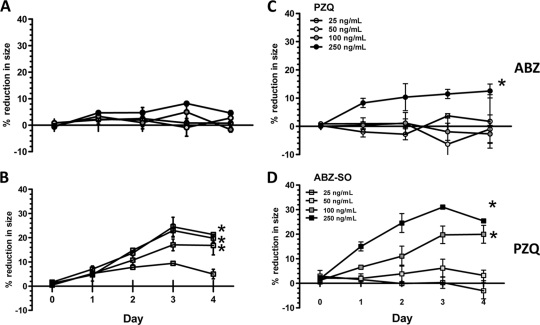
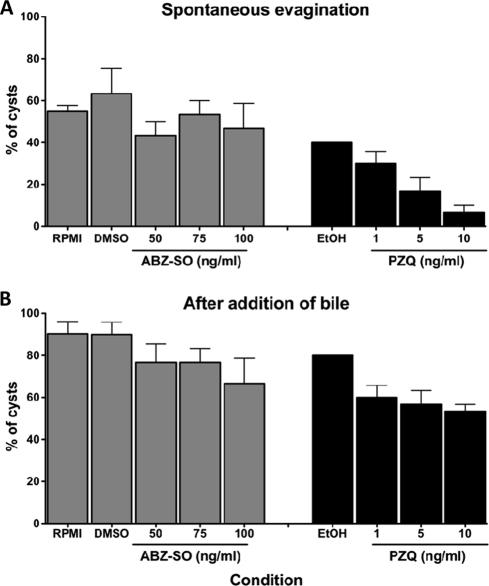
PZQ treatment resulted in up to a 30% decrease in size that was both dose and time dependent (P < 0.05, Fig. 2). The decrease in average size of cysts was evident at PZQ concentrations between 25 and 250 ng/ml on day 2 and maximal by day 3 at 250 ng/ml. In comparison, ABZ-SO demonstrated a small decrease in size, but only at a concentration of 250 ng/ml. When ABZ-SO (25 ng/ml) was added to increasing concentrations (10 to 250 ng/ml) of PZQ, size reduction was enhanced, However, when 25 ng of PZQ/ml was tested in combination with increasing concentrations of ABZ-SO, a decrease in size occurred only at 250 ng of ABZ-SO/ml, the highest concentration of ABZ-SO tested, as was observed with ABZ-SO alone. Thus, the combination of PZQ and ABZ-SO did not significantly enhance the effect of ABZ-SO alone. PZQ, in addition to its effect on parasite size, also inhibited both total (spontaneous and bile-stimulated) evagination at low concentrations (Fig. 3). In contrast, ABZ-SO had no significant effect on evagination, despite the modest size reduction apparent at concentrations of 250 ng/ml after 3 days in culture.
FIG. 2.
Changes in the sizes of parasites during treatment with anthelmintics. Ten cysts were cultured in triplicate wells (see Materials and Methods) with ABZ-SO at a final concentration of 25 to 250 ng/ml (A), PZQ at a final concentration of 25 to 250 ng/ml (B), a fixed concentration of PZQ (25 ng/ml) with the indicated concentrations (25 to 250 ng/ml) of ABZ-SO (C), and a fixed concentration of ABZ-SO (25 ng/ml) with the indicated concentrations (25 to 250 ng/ml) of PZQ (D). The symbols represent the mean, and the error bars indicate the SEM. Asterisks signify conditions that are significantly different from the untreated.
FIG. 3.
Effect of ABZ-SO and PZQ on cyst evagination. Ten cysts of T. solium were cultured in triplicate wells for 4 days in medium alone, ABZ-SO (50, 75, or 100 ng/ml), or PZQ (1, 5, or 10 ng/ml), and the total percentages of cysts that evaginated spontaneously (A) and total evaginated cysts (spontaneous plus after bile) after stimulation with bile (B) for 18 h were determined. Each data point represents the mean of triplicate wells, and the bars indicate the SEM.
Inhibition of AP secretion.
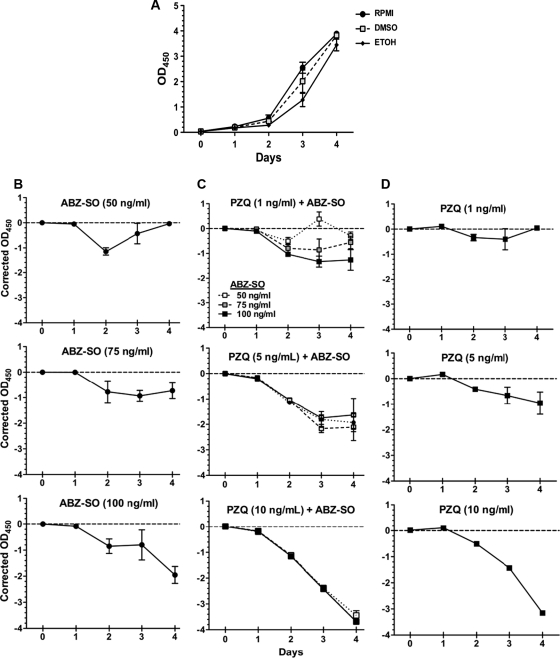
AP activity is normally detected in the culture supernatants of other cestodes (23) and has been used to quantify drug effects in vitro. However, AP secretion has not been investigated in relation to T. solium cysts. In our experiments, AP was found in the supernatants of T. solium cultured cysts. The rate of release of AP in supernatants increased progressively from days 1 to 4 in controls (Fig. 4). No consistent difference in AP secretion rates was seen between ethanol and DMSO control cultures. Both PZQ and ABZ-SO inhibited AP secretion but at different drug concentrations (Fig. 5). PZQ showed inhibition of AP secretion starting between at 1 and 5 ng/ml, whereas inhibition by ABZ-SO was first apparent at between 50 and 75 ng/ml. The ability of both drugs to inhibit AP secretion allowed assessment of the effects of both drugs together.
FIG. 4.
Inhibition of alkaline phosphatase (AP) release in supernatants of cysts by ABZ-SO and PZQ. AP levels were measured colorimetrically (see Materials and Methods). (A) Release of AP in medium containing the solvents used to solubilize the drugs, ABZ-SO (DMSO) and PZQ (ethanol). (B to D) Release of AP (shown as the corrected OD at 450 nm [OD450]; see Materials and Methods) in the supernatants of cysts treated with ABZ-SO alone (B), both ABZ-SO and PZQ (C), or PZQ alone (D). The data shown are means and SEM of triplicate wells from one of three representative experiments.
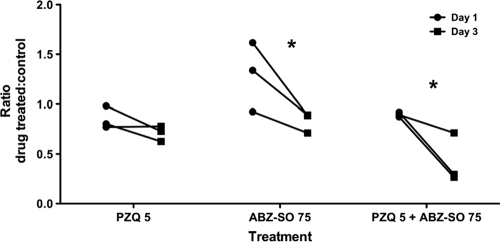
FIG. 5.
Inhibition of parasite antigen secretion by anthelmintic treatment. The levels of parasite antigens in the supernatant of T. solium cysts in culture were assayed by ELISA using MAb B158C11A10 (see Materials and Methods). The graph depicts the mean antigen levels in supernatants from days 1 and 3 of culture, expressed as a ratio of the OD492 values for each test condition to the corresponding control. The differences between days 1 and 3 are statistically significant for ABZ-SO and ABZ-SO plus PZQ (P < 0.05 [Kruskal-Wallis test]).
Submaximal concentrations of both drugs showed various degrees of activity (Fig. 4). Significant synergy (P < 0.05 [nonparametric ANOVA]) was noted at ABZ-SO concentrations of 50 and 75 ng/ml combined with PZQ at 5 ng/ml after 3 days. With 10 ng of PZQ/ml, although there was an increased activity with the addition of ABZ-SO, the effects were additive but less than the sum of the individual activities, indicating an absence of synergy in the action of the two drugs. This degree of enhanced activity is potentially of clinical importance because even a partial (nonsynergistic) increase in activity may enhance the cysticidal effects of the drug on the parasite. Taken together, these results suggest some degree of additive and/or synergistic activity between the two drugs.
Inhibition of antigen secretion by ABZ-SO and PZQ.
We used a double MAb capture ELISA to measure level of released parasite Ag. The MAb B158C11A10 detects genus-specific antigen(s), which are present in the sera and cerebrospinal fluid (CSF) of some patients with T. solium cysticercosis (19). The kinetics and release of the Ag were determined in vitro from cysts treated with PZQ, ABZ-SO, or a combination of the two drugs. Ag was spontaneously released into the supernatant at 1 and 3 days of culture. Ag secretion between days 1 and 3 was inhibited by both drugs alone, or in combination, but the decrease in secretion was statistically significant only for ABZ-SO and the combination of ABZ-SO and PZQ (Fig. 5). The levels of parasite antigens, expressed as a ratio of treated to control conditions, were decreased between day 1 and day 3 by 0.47-fold for ABZ-SO (95% confidence interval [CI] = 0.04 to 0.89), and by 0.47-fold for ABZ-SO plus PZQ (CI = 0.05 to 0.90; Fig. 5). Enhanced inhibition of release of Ag and of AP by the combination of both drugs supports the additive and/or synergistic effects of both drugs on T. solium cysts.
DISCUSSION
Although ABZ-SO and PZQ are clinically useful in the treatment of human cysticercosis due to T. solium, cure rates in parenchymal disease are suboptimal, and the treatment of subarachnoid and ventricular cysts is both uncertain and usually difficult. The discovery of new drugs and modalities of treatment is urgently needed. However, testing drug efficacy in vivo in T. solium-infected pigs is extremely difficult and impractical; testing is further thwarted because there is no accepted in vitro methodology for identifying and testing new agents.
The major findings of the present study are (i) the characterization of the macroscopic, microscopic, and biochemical changes of T. solium cysts over time by anthelmintic drug treatments; (ii) the use of parasite AP secretion to measure anthelmintic activity of ABZ-SO and PZQ; (iii) the detection of the epitope in T. solium recognized by MAb B158C11A10 in the supernatant of cultured cysts and inhibition of release after drug treatment; and (iv) demonstration of synergy/additive effects of combined ABZ-SO and PZQ treatment on T. solium cysts.
We found inhibition of AP secretion to be a quantitative and useful measure of drug activity on T. solium cysts. The changes in AP secretion observed were primarily due to the effects of the anthelmintics, since the effect of stress from the culture conditions appeared to be significant only after day 3 in the culture conditions used. Measurement of AP was previously used to quantitate drug effects in E. multilocularis (24), and E. granulosus (29) but had not been used with T. crassiceps or T. solium cysts. However, in these cestodes, the rate of AP secretion did not increase over time and exposure to both drugs resulted in increased release of AP. For E. multilocularis, effective inhibitor concentrations are relatively high, ranging from 0.1 μg (300 nM) to 100 μg/ml (321 mM). In the present study, inhibition of AP secretion was a sensitive measure of drug activity to both PZQ and ABZ-SO. Inhibition of release was between 1 and 5 ng/ml (3 to 16 nM) and 50 to 75 ng/ml (177 to 268 nM), respectively, for PZQ and ABZ-SO. Of note, ABZ-SO alone did not inhibit AP secretion at doses <50 ng/ml (177 nM; data not shown). The drug concentrations tested in these experiments are well within the range reported in human sera and CSF in individuals treated with PZQ and ABZ-SO (2, 13). Jung et al. (9) reported mean levels of ABZ-SO of 918 ng/ml in plasma and 392 ng/ml in CSF, and PZQ levels of 1,640 ng/ml in serum and 398 ng/ml in CSF. Surprisingly, despite distinct mechanisms of action of PZQ and ABZ-SO, both inhibited secretion.
Secretion of a specific antigen was also inhibited at similar drug concentrations. This antigen, recognized by MAb B158C11A10 that was originally reported to recognize T. saginata cyst excretory-secretory products (1) but later also found to cross-react with T. solium and T. crassiceps, is found in the CSF and sera of patients with cysticercosis (19). Because inhibition occurred with two unrelated drugs and an antigen recognized by MAb B158C11A10, inhibition of excreted-secreted materials may be a direct or more likely a secondary or downstream consequence of these drugs. However, differences in drug activity between ABZ-SO and PZQ were evident because PZQ inhibited evagination at low concentrations, a property that likely allows its use to treat taeniasis, and had a direct effect on cyst size. In contrast, ABZ-SO effects were small or not present.
Combined PZQ and ABZ-SO treatment has been used in vitro and in vivo against a number of cestode infections in humans and animals. Our studies suggest that combined therapy may be useful in vivo in the treatment of NCC. Because both drugs inhibited AP release, we were able to use this assay to determine activities of combination therapy. Various degrees of enhanced activity ranging from synergy/addition to partial enhancement were found. Although this is the first in vitro demonstration of enhanced activity of combination therapy using T. solium larval cysts in vitro, enhanced activity has been described in the cestodes T. crassiceps (16) and E. granulosus and E. multilocularis in vitro (12, 25-30), as well as in imperfect human case series against E. granulosus in vivo (8, 33). Kaur et al. found enhanced but nonsignificant decrease in estimated time to clearance of single enhancing lesions using combined therapy in NCC compared to single therapy in India (10), and Guo et al. reported usefulness of combined treatment of NCC in China (6).
T. crassiceps has been used in vitro and in vivo to evaluate effectiveness of cysticidal drugs. Assessments of drug effects are based upon macroscopic and microscopic changes that occur in T. crassiceps cysts over 11 days (16). Although the results have been useful, there are potential and actual problems in using T. crassiceps. First, T. crassiceps is used as a surrogate for T. solium, but reported differences between the two parasites (3) make it unclear whether results obtained with T. crassiceps cysts apply to T. solium. Second, these assays are laborious and slow, with endpoints at 11 days, and are therefore not easily applied to the screening of new drugs for cysticercosis. There are many biological differences between T. crassiceps and T. solium, but the most obvious difference—the ability of T. crassiceps cysts to multiply by budding—is also the most useful characteristic and advantage for in vitro studies because this growth characteristic allows the regular availability of T. crassiceps cysts through sequential peritoneal passage in mice.
In vitro studies with either T. solium or T. crassiceps show drug activity of ABZ-SO and PZQ at roughly the same concentrations. They also show additive activity/synergy when these drugs are used in combination. Both assays are tedious, time-consuming, and difficult in their own way. Although it would be advantageous to use AP secretion to study drug effects on T. crassiceps, inherent biological differences of AP secretion between the two cestodes (T. crassiceps and T. solium) may limit the generalizability of our findings to other taeniid cestodes. Taken together, our results suggest that functional assays, such as AP activity, may serve as useful parameters for studying drug effects on T. solium; however, easier and better in vitro methods for screening drugs are clearly needed to identify and characterize new anticestode drugs.
Supplementary Material
Acknowledgments
This research was supported (in part) by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Published ahead of print on 1 November 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Brandt, J. R., S. Geerts, R. De Deken, V. Kumar, F. Ceulemans, L. Brijs, and N. Falla. 1992. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int. J. Parasitol. 22:471-477. [DOI] [PubMed] [Google Scholar]
- 2.Cobo, F., C. Yarnoz, B. Sesma, P. Fraile, M. Aizcorbe, R. Trujillo, A. Diaz-de-Liano, and M. A. Ciga. 1998. Albendazole plus praziquantel versus albendazole alone as a pre-operative treatment in intra-abdominal hydatisosis caused by Echinococcus granulosus. Trop. Med. Int. Health 3:462-466. [DOI] [PubMed] [Google Scholar]
- 3.Escobedo, G., M. C. Romano, and J. Morales-Montor. 2009. Differential in vitro effects of insulin on Taenia crassiceps and Taenia solium cysticerci. J. Helminthol. 83:403-412. [DOI] [PubMed] [Google Scholar]
- 4.Garcia, H. H., and O. H. Del Brutto. 2005. Neurocysticercosis: updated concepts about an old disease. Lancet Neurol. 4:653-661. [DOI] [PubMed] [Google Scholar]
- 5.García, H. H., R. H. Gilman, A. E. Gonzalez, M. Verastegui, S. Rodriguez, C. Gavidia, V. C. W. Tsang, N. Falcon, A. G. Lescano, L. H. Moulton, T. Bernal, M. Tovar, and The Cysticercosis Working Group in Peru. 2003. Hyperendemic human and porcine Taenia solium infection in Peru. Am. J. Trop. Med. Hyg. 68:268-275. [PubMed] [Google Scholar]
- 6.Guo, D. M., S. P. Xie, and J. P. Jia. 2003. Therapeutic efficacy of praziquantel, albendazole and a combination of the two drugs in cysticercosis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 21:187-188. (In Chinese.) [PubMed] [Google Scholar]
- 7.Hemphill, A., B. Stadelmann, S. Scholl, J. Muller, M. Spiliotis, N. Muller, B. Gottstein, and M. Siles-Lucas. 2010. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 137:569-587. [DOI] [PubMed] [Google Scholar]
- 8.Jamshidi, M., M. Mohraz, M. Zangeneh, and A. Jamshidi. 2008. The effect of combination therapy with albendazole and praziquantel on hydatid cyst treatment. Parasitol. Res. 103:195-199. [DOI] [PubMed] [Google Scholar]
- 9.Jung, H., M. Hurtado, M. Sanchez, M. T. Medina, and J. Sotelo. 1990. Plasma and CSF levels of albendazole and praziquantel in patients with neurocysticercosis. Clin. Neuropharmacol. 13:559-564. [DOI] [PubMed] [Google Scholar]
- 10.Kaur, S., P. Singhi, S. Singhi, and N. Khandelwal. 2009. Combination therapy with albendazole and praziquantel versus albendazole alone in children with seizures and single lesion neurocysticercosis: a randomized, placebo-controlled double blind trial. Pediatr. Infect. Dis. J. 28:403-406. [DOI] [PubMed] [Google Scholar]
- 11.Markoski, M. M., E. S. Trindade, G. Cabrera, A. Laschuk, N. Galanti, A. Zaha, H. B. Nader, and H. B. Ferreira. 2006. Praziquantel and albendazole damaging action on in vitro developing Mesocestoides corti (Platyhelminthes: Cestoda). Parasitol. Int. 55:51-61. [DOI] [PubMed] [Google Scholar]
- 12.Morris, D. L., D. Taylor, D. Daniels, and K. S. Richards. 1987. Determination of minimum effective concentration of praziquantel in in vitro cultures of protoscoleces of Echinococcus granulosus. Trans. R. Soc. Trop. Med. Hyg. 81:494-497. [DOI] [PubMed] [Google Scholar]
- 13.Na-Bangchang, K., S. Vanijanonta, and J. Karbwang. 1995. Plasma concentrations of praziquantel during the therapy of neurocysticerosis with praziquantel, in the presence of antiepileptics and dexamethasone. Southeast Asian J. Trop. Med. Public Health 26:120-123. [PubMed] [Google Scholar]
- 14.Nash, T. E. 2003. Human case management and treatment of cysticercosis. Acta Trop. 87:61-69. [DOI] [PubMed] [Google Scholar]
- 15.Nash, T. E., G. Singh, A. C. White, V. Rajshekhar, J. A. Loeb, J. V. Proano, O. M. Takayanagui, A. E. Gonzalez, J. A. Butman, C. DeGiorgio, O. H. Del Brutto, A. Delgado-Escueta, C. A. Evans, R. H. Gilman, S. M. Martinez, M. T. Medina, E. J. Pretell, J. Teale, and H. H. Garcia. 2006. Treatment of neurocysticercosis: current status and future research needs. Neurology 67:1120-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palomares, F., G. Palencia, J. R. Ambrosio, A. Ortiz, and H. Jung-Cook. 2006. Evaluation of the efficacy of albendazole sulphoxide and praziquantel in combination on Taenia crassiceps cysts: in vitro studies. J. Antimicrob. Chemother. 57:482-488. [DOI] [PubMed] [Google Scholar]
- 17.Palomares, F., G. Palencia, R. Perez, D. Gonzalez-Esquivel, N. Castro, and H. J. Cook. 2004. In vitro effects of albendazole sulfoxide and praziquantel against Taenia solium and Taenia crassiceps cysts. Antimicrob. Agents Chemother. 48:2302-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palomares-Alonso, F., H. Jung-Cook, J. Perez-Villanueva, J. C. Piliado, S. Rodriguez-Morales, G. Palencia-Hernandez, N. Lopez-Balbiaux, A. Hernandez-Campos, R. Castillo, and F. Hernandez-Luis. 2009. Synthesis and in vitro cysticidal activity of new benzimidazole derivatives. Eur. J. Med. Chem. 44:1794-1800. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez, S., P. Dorny, V. C. W. Tsang, E. J. Pretell, J. Brandt, A. G. Lescano, A. E. Gonzalez, R. H. Gilman, H. H. Garcia, and the Cysticercosis Working Group in Peru. 2009. Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J. Infect. Dis. 199:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spicher, M., C. Roethlisberger, C. Lany, B. Stadelmann, J. Keiser, L. M. Ortega-Mora, B. Gottstein, and A. Hemphill. 2008. In vitro and in vivo treatments of echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrob. Agents Chemother. 52:3447-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadelmann, B., S. Scholl, J. Muller, and A. Hemphill. 2010. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 65:512-519. [DOI] [PubMed] [Google Scholar]
- 22.Stettler, M., R. Fink, M. Walker, B. Gottstein, T. G. Geary, J. F. Rossignol, and A. Hemphill. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stettler, M., M. Siles-Lucas, E. Sarciron, P. Lawton, B. Gottstein, and A. Hemphill. 2001. Echinococcus multilocularis alkaline phosphatase as a marker for metacestode damage induced by in vitro drug treatment with albendazole sulfoxide and albendazole sulfone. Antimicrob. Agents Chemother. 45:2256-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor, D. H., and D. L. Morris. 1988. In vitro culture of Echinococcus multilocularis: protoscolicidal action of praziquantel and albendazole sulphoxide. Trans. R. Soc. Trop. Med. Hyg. 82:265-267. [DOI] [PubMed] [Google Scholar]
- 25.Taylor, D. H., D. L. Morris, and K. S. Richards. 1988. Combination chemotherapy of Echinococcus granulosus: in vitro studies. Trans. R. Soc. Trop. Med. Hyg. 82:263-264. [DOI] [PubMed] [Google Scholar]
- 26.Taylor, D. H., D. L. Morris, and K. S. Richards. 1989. Echinococcus granulosus: in vitro maintenance of whole cysts and the assessment of the effects of albendazole sulphoxide and praziquantel on the germinal layer. Trans. R. Soc. Trop. Med. Hyg. 83:535-538. [DOI] [PubMed] [Google Scholar]
- 27.Taylor, D. H., D. L. Morris, K. S. Richards, and D. Reffin. 1988. Echinococcus multilocularis: in vivo results of therapy with albendazole and praziquantel. Trans. R. Soc. Trop. Med. Hyg. 82:611-615. [DOI] [PubMed] [Google Scholar]
- 28.Urrea-Paris, M. A., M. J. Moreno, N. Casado, and F. Rodriguez-Caabeiro. 1999. Echinococcus granulosus: praziquantel treatment against the metacestode stage. Parasitol. Res. 85:999-1006. [DOI] [PubMed] [Google Scholar]
- 29.Urrea-Paris, M. A., M. J. Moreno, N. Casado, and F. Rodriguez-Caabeiro. 2000. In vitro effect of praziquantel and albendazole combination therapy on the larval stage of Echinococcus granulosus. Parasitol. Res. 86:957-964. [DOI] [PubMed] [Google Scholar]
- 30.Urrea-Paris, M. A., M. J. Moreno, N. Casado, and F. Rodriguez-Caabeiro. 2002. Relationship between the efficacy of praziquantel treatment and the cystic differentiation in vivo of Echinococcus granulosus metacestode. Parasitol. Res. 88:26-31. [DOI] [PubMed] [Google Scholar]
- 31.Vargas-Villavicencio, J. A., C. Larralde, M. A. De Leon-Nava, G. Escobedo, and J. Morales-Montor. 2007. Tamoxifen treatment induces protection in murine cysticercosis. J. Parasitol. 93:1512-1517. [DOI] [PubMed] [Google Scholar]
- 32.Willms, K., and R. Zurabian. 2010. Taenia crassiceps: in vivo and in vitro models. Parasitology 137:335-346. [DOI] [PubMed] [Google Scholar]
- 33.Yasawy, M. I., A. R. Mohamed, and M. A. Al-Karawi. 1992. Albendazole in hydatid disease: results in 22 patients. Ann. Saudi Med. 12:152-156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.