Abstract
Inhalational anthrax, a zoonotic disease caused by the inhalation of Bacillus anthracis spores, has a ∼50% fatality rate even when treated with antibiotics. Pathogenesis is dependent on the activity of two toxic noncovalent complexes: edema toxin (EdTx) and lethal toxin (LeTx). Protective antigen (PA), an essential component of both complexes, binds with high affinity to the major receptor mediating the lethality of anthrax toxin in vivo, capillary morphogenesis protein 2 (CMG2). Certain antibodies against PA have been shown to protect against anthrax in vivo. As an alternative to anti-PA antibodies, we produced a fusion of the extracellular domain of human CMG2 and human IgG Fc, using both transient and stable tobacco plant expression systems. Optimized expression led to the CMG2-Fc fusion protein being produced at high levels: 730 mg/kg fresh leaf weight in Nicotiana benthamiana and 65 mg/kg in N. tabacum. CMG2-Fc, purified from tobacco plants, fully protected rabbits against a lethal challenge with B. anthracis spores at a dose of 2 mg/kg body weight administered at the time of challenge. Treatment with CMG2-Fc did not interfere with the development of the animals' own immunity to anthrax, as treated animals that survived an initial challenge also survived a rechallenge 30 days later. The glycosylation of the Fc (or lack thereof) had no significant effect on the protective potency of CMG2-Fc in rabbits or on its serum half-life, which was about 5 days. Significantly, CMG2-Fc effectively neutralized, in vitro, LeTx-containing mutant forms of PA that were not neutralized by anti-PA monoclonal antibodies.
The Gram-positive bacterium Bacillus anthracis is the causative agent of anthrax, an acute zoonotic disease that is highly lethal in its most virulent form. Inhalational anthrax begins with the inhalation of dormant endospores, which are engulfed by alveolar macrophages and dendritic cells in the lungs. Spores germinate and vegetative bacteria multiply within these cells, and the dendritic cells carry them to the mediastinal lymph nodes, where they multiply and gain access to the bloodstream, reaching concentrations of >108/ml (6). B. anthracis owes its pathogenicity to two major determinants of virulence: the formation of a poly-d-glutamyl capsule, which inhibits the phagocytosis of the vegetative cells, leading to a rapid increase in bacterial count in the bloodstream (47), and an ensemble of three proteins, protective antigen (PA), edema factor (EF), and lethal factor (LF), that combine at the surface of host cells to form two toxic noncovalent complexes, edema toxin (EdTx) and lethal toxin (LeTx).
PA binds to specific cell surface receptors on host cells, initially as an 83-kDa protein (PA83). PA83 is then cleaved by a membrane-associated furin-like protease, releasing a smaller PA20 fragment from the N terminus (17). The remaining fragment, PA63, is able to heptamerize into a membrane-bound prepore (18). In addition, PA83 cleavage exposes binding sites for EF and LF, of which up to three molecules can be bound by the prepore (28). The fully assembled toxin complex is internalized by receptor-mediated endocytosis. Acidification induces a conformational change of the prepore conducive to the formation of a channel inserted into the endosomal membrane. EF and LF are translocated into the cytosol, where they can exert their toxic activity (7). EF is a calcium- and calmodulin-dependent adenylate cyclase that stimulates a dramatic elevation of cyclic AMP concentration in eukaryotic cells (21). LF is a zinc metalloprotease that cleaves mitogen-activated protein kinase kinases, and it leads to the disruption of intracellular signal transduction pathways (9, 43).
Letters containing B. anthracis endospores, sent in 2001, alerted the world to the threat of anthrax as a weapon of terror. The spores can be produced and stored, and they remain viable for decades in storage or after release. As a biological threat agent, it is expected that a cloud of anthrax spores could be released at a strategic location to be inhaled by the personnel under attack (1). In the anthrax attack on the U.S. Capitol in 2001, people known or suspected to have been exposed to spores received antibiotic therapy, and none became sick (8). However, 11 people outside the Capitol area were not diagnosed with anthrax until they became symptomatic, about 1 to 6 days after exposure. Five of these patients died despite receiving antibiotic therapy (11). Because of this high rate of mortality, additional treatment strategies for anthrax are needed.
Two human cell surface receptors for PA have been identified: tumor endothelial marker 8 (TEM8) and capillary morphogenesis protein 2 (CMG2) (5, 39). CMG2 has the higher affinity for PA and is the major receptor mediating anthrax toxin lethality in vivo (24). Soluble forms of both of these proteins have been shown to protect cultured cells from intoxication by LeTx, with CMG2 being the more potent of the two (5, 39). However, these materials may have limited value as therapeutics, because they are likely to be rapidly cleared from the blood. To prepare an improved therapeutic, we sought to develop an immunoadhesin form of CMG2. Immunoadhesins are recombinant proteins that combine the target-binding region of a receptor, a cell adhesion molecule, a ligand, or an enzyme with the Fc region of an immunoglobulin (4). Immunoadhesins retain the binding ability of the receptor and gain the advantage of a long circulating half-life and interaction with immune effector cells (30). The CMG2-Fc immunoadhesin described here was produced in high yields in plants. Its protective potency in a rabbit model of inhalational anthrax is comparable to that of monoclonal anti-PA antibodies. In addition, it was able to neutralize, in vitro, mutant forms of PA that are not neutralized by an anti-PA monoclonal antibody. The use of the plant platform for production offers advantages in terms of cost and scalability for stockpiling CMG2-Fc as a therapy in the case of a future anthrax attack (42).
MATERIALS AND METHODS
DNA constructs.
DNA constructs, encoding combinations of various portions of human CMG2 and human IgG1, were assembled using standard methods in binary vectors for plant expression. Construction details are provided in the online supplemental material. Binary expression vectors were transfected into Agrobacterium tumefaciens via electroporation (41).
Transient expression in plants.
The transient expression of recombinant proteins was accomplished by the whole-plant vacuum infiltration of Nicotiana benthamiana (16) using strains of A. tumefaciens EHA105 (13) containing binary expression vectors along with A. tumefaciens C58C1 containing the binary vector pBIN61-35S-P19, encoding the silencing suppressor P19 (45). After infiltration, the plants were maintained in the greenhouse under standard conditions for 7 days prior to protein purification.
Tobacco stable transformation and regeneration.
The transformation of tobacco leaf tissue (Nicotiana tabacum, cultivar Wisconsin 38) by A. tumefaciens strain EHA105, containing binary expression vectors, and the regeneration of transgenic plants were accomplished using standard techniques (14). During the transformation and regeneration of whole plants, the plant growth room was maintained at 26°C under continuous light. Between 7 and 9 weeks after transformation, leaf samples from putative transgenic plants (T0) were screened by enzyme-linked immunosorbent assay (ELISA) (see the next section) for the expression of CMG2-Fc. Plants expressing CMG2-Fc were transferred to potting mix in the greenhouse and subjected to additional screening as they matured. The best-expressing plants were self fertilized to produce T1 seeds. T1 seeds were germinated and allowed to grow to maturity and produce T2 seeds. The expression of CMG2-Fc in plants in the greenhouse was quantified. Leaf tissue punches from the plants were homogenized in a bead beater and then centrifuged for 5 min at maximum speed in a microcentrifuge, and supernatants were assayed by ELISA.
ELISA quantification of CMG2-Fc in plants.
Microtiter plates were coated with unlabeled protein A (0.34 μg/ml; SouthernBiotech, Birmingham, AL) and then blocked with phosphate-buffered saline (PBS) plus 5% nonfat dry milk. Plant extracts (and purified CMG2-Fc standards) were added to wells and incubated at 37°C for 1 h. Plates then were washed with PBS. Bound CMG2-Fc was detected by adding horseradish peroxidase (HRP)-labeled goat anti-human gamma chain (SouthernBiotech). Antibody complexes were quantified by adding HRP substrate (0.4 mg/ml o-phenylenediamine and 0.015% hydrogen peroxide in 0.1 M sodium citrate, pH 5.0) and read at 490 nm. Samples were quantified at least three times during their growth and development in the greenhouse environment.
Protein purification.
Leaves were collected 7 days after vacuum infiltration, washed in ice water, and blotted dry, and then they were homogenized in a blender with extraction buffer (150 mM NaCl, 50 mM sodium phosphate, 10 mM sodium thiosulfate, 1 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.4). The homogenate was filtered through Miracloth (Calbiochem, La Jolla, CA) and the pH adjusted to between 6.5 and 7.0. The homogenate was centrifuged at 15,000 × g for 30 min at 4°C. The supernatant was recovered and further clarified by passage through a 0.45-μm capsule filter. The clarified juice was pumped over a column of protein A-Sepharose 4B (Invitrogen, Carlsbad, CA). The column was washed with PBS and the bound protein eluted with 100 mM glycine, pH 3.0. Protein concentrations were quantified using absorption at 280 nm and extinction coefficients predicted from the expected amino acid sequences.
Protein analysis.
Purified protein samples were analyzed using standard methods. Samples were subjected to SDS-polyacrylamide gel electrophoresis (under reducing and nonreducing conditions) on 4 to 15% Criterion Tris-HCl gradient gels (Bio-Rad, Hercules, CA) (20) and visualized by Coomassie G250 staining or by Western blot analysis. For immunodetection, blots were probed with alkaline phosphatase (AP)-conjugated goat anti-human IgG (SouthernBiotech) and visualized by incubation with AP developing buffer (0.33 mg/ml nitroblue tetrazolium chloride and 0.165 mg/ml 5-bromo-4-chloro-3′-indolylphosphate p-toluidine in 100 mM diethanolamine, 10 mM MgCl2, 100 mM NaCl, pH 9.5). Some blots also were probed with HRP-labeled concanavalin A (ConA; Sigma, St. Louis, MO) and then detected by adding HRP substrate solution (0.27 mg/ml aminoethylcarbazole plus 0.003% H2O2 in 0.1 M Na acetate, pH 5.2) (10).
Toxin neutralization assay (TNA).
The survival of RAW264.7 mouse macrophage-like cells (ATCC TIB-71) after the administration of CMG2-Fc along with toxin was determined essentially as described previously (22), except that the LeTx used was 100 ng/ml (1.2 nM) PA + 100 ng/ml (1.1 nM) LF. PA was from List Biological Laboratories; the recombinant LF used here was prepared by S. H. Leppla and has the native AGG N-terminal sequence (12). EC50s (concentrations of antitoxin at which 50% of toxin activity is neutralized) were calculated using the dose-response software GraphPad Prism (GraphPad Software, La Jolla, CA). The effective molar ratio, or the ratio of antitoxin to toxin at the EC50, also was calculated for each chimeric protein.
Measurement of affinity and binding kinetics.
The affinity of CMG2-Fc for PA was measured by surface plasmon resonance (SPR; Biacore T100, Uppsala, Sweden). Anti-human IgG (Affinipure goat anti-human IgG, Fcγ fragment specific; Jackson ImmunoResearch, West Grove, PA) was immobilized on the sensor surface using an amine coupling kit (Biacore). CMG2-Fc (in PBS plus 1 mM MgCl2) was then captured through the Fc portion so that the CMG2 portion would be exposed. PA at various concentrations (in 10 mM HEPES, 150 mM NaCl, 3 mM EGTA, and 0.005% Tween 20) was flowed past the surface while surface plasmon resonance measurements were taken. The data were analyzed with the Bia-evaluation wizard.
Challenge of rabbits with B. anthracis Ames spores.
Female Dutch belted rabbits (Myrtle's Rabbitry, Thompsons Station, TN) were challenged with 107 CFU of B. anthracis Ames spores (100 50% lethal doses [LD50]) by nasal instillation as described previously (32). After 30 to 60 min, each animal received a single subcutaneous (s.c.) injection of CMG2-Fc (3.6 mg/kg body weight; first challenge study), a single intravenous (i.v.) injection of CMG2-FcA or CMG2-FCG (0.5, 1, 2, or 4 mg/kg; second challenge study), or an equivalent injection of PBS. Rabbits were placed back into their cages and were monitored twice daily for signs of illness and death for 15 days.
Serological analyses.
The adaptive immune responses of animals in the first spore challenge study to B. anthracis antigens were quantified. Briefly, blood samples were collected from surviving rabbits in serum separator microtubes containing silicone (Becton Dickinson, Franklin Lakes, NJ) 15 days postchallenge, and the sera were harvested by centrifugation at 519 × g for 5 min. Sera were sterile filtered and aliquots cultured on rich medium prior to release from the biosafety level 3 facility. Rabbit anti-PA titers were determined by direct ELISA. Microtiter plates were coated with purified PA (List Biological Laboratories, Campbell, CA) in 50 μl PBS at 2.5 μg/ml. Plates were blocked with PBS plus 5% nonfat dry milk. Dilutions of serum (or reference standard) were added to wells and incubated at 37°C for 1 h. Plates were washed three times with PBS. Bound rabbit anti-PA IgG was detected with goat anti-rabbit IgG conjugated with HRP (50 μl at 0.25 μg/ml; SouthernBiotech). After 1 h at 37°C, plates were washed and peroxidase activity was detected by adding HRP substrate. Quantified rabbit anti-PA reference serum from BEI resources (NR-3839) was used as a standard, and the rabbit anti-PA IgG in the serum was reported in μg/ml. Controls included normal rabbit preimmune serum and serum samples spiked with up to 7.5 μg/ml of CMG2-Fc. Spiking the serum of surviving treated animals with CMG2-Fc did not influence the quantification of rabbit anti-PA antibodies.
The toxin-neutralizing ability of the serum samples was measured using the TNA as described above. However, because of the absence of a standard, the data are reported as a titer, which is the reciprocal of the serum dilution at the EC50. The titer that resulted in the 50% inhibition of cell death (EC50) was calculated using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA).
Pharmacokinetic study.
Two groups of Dutch belted rabbits (5 animals each) received CMG2-FcA or CMG2-FcG (5 or 3.6 mg/kg, respectively) by i.v. bolus injection. Serum samples were collected from the central ear artery (32) prior to injection and at 6, 12, 24, 48, 72, 168, 216, and 240 h postinjection.
The serum concentrations of CMG2-FcA or CMG2-FcG were measured by a tiered sandwich ELISA. Microtiter plates were coated with donkey anti-human IgG at 2.5 μg/ml. Plates were blocked with PBS plus 5% nonfat dry milk. Dilutions of serum (or a standard curve of purified CMG2-FcA) were added to wells and incubated at 37°C for 1 h. Plates were washed with PBS and then incubated with goat anti-CMG2 (R&D Systems, Minneapolis, MN) and donkey anti-goat IgG HRP conjugate (Jackson ImmunoResearch). After 1 h at 37°C, plates were washed and peroxidase activity was detected by adding HRP substrate, and the optical density was read at 490 nm. Preliminary control experiments demonstrated that preimmune rabbit serum did not contribute to signal in this assay, and it did not interfere with the detection of purified CMG2-Fc. Serum concentration values of CMG2-FcA and CMG2-FcG versus time for each animal were analyzed using WinNonlin professional version 5.2.1. Residual concentrations of CMG2-Fc in the sera of surviving animals in the first challenge study also were measured using this assay.
RESULTS
Expression of a CMG2-Fc immunoadhesin in plant leaves.
A number of different CMG2-Fc immunoadhesin expression plasmids were constructed and used to produce protein using a transient N. benthamiana expression system before selecting an optimal design (detailed in the supplemental material). The final construct selected for evaluation is comprised of the Arabidopsis thaliana 2S2 signal peptide, amino acids 34 to 220 of human CMG2, followed by two serines and the hinge and Fc region of human IgG1 (C-terminal 232 amino acids of IgG1). This protein therefore will contain 421 amino acids (excluding the signal peptide) having a subunit molecular mass of 46.8 kDa. The resulting CMG2-Fc protein, expressed using the N. benthamiana system and purified by protein A chromatography, was subjected to SDS-PAGE under reducing and nonreducing conditions, Western blotting, and size-exclusion chromatography (SEC). These analyses (detailed in the supplemental material) indicate that the CMG2-Fc protein exists in solution primarily as a single homodimeric species held together by interchain disulfide bonds between the Fc domains. The affinity (KD) of CMG2-Fc for PA, measured by SPR, was 0.4 nM (Ka [association constant] = 5.2 × 104 M−1s−1; Kd [dissociation constant] = 2.2 × 10−5 s−1).
In vitro toxin-neutralizing activity of CMG2-Fc.
The in vitro TNA was used to quantify the toxin-neutralizing activity of CMG2-Fc. Figure 1 is a typical dose-response curve, from which an EC50 can be calculated. In this representative assay, CMG2-Fc had an EC50 of 60 ng/ml. A control, human IgG1 also transiently expressed and purified from tobacco, had no toxin-neutralizing activity in this assay. The average EC50 for CMG2-Fc is 52.8 ng/ml (standard deviation [SD], ±8.6 ng/ml). The molar ratio of CMG2-Fc (dimeric) to PA at the average EC50 is 0.47.
FIG. 1.
Neutralization of lethal toxin activity by CMG2-Fc. Various concentrations of purified CMG2-Fc (circles) or an irrelevant plant-made IgG (squares) were premixed with anthrax lethal toxin before addition to RAW264.7 cells. Viability was determined after 4 h of incubation by a colorimetric assay. Results are expressed as the percentages of cells still viable. Points represent the means of results from triplicate wells of a representative experiment, with error bars representing the standard errors of the means. The best-fit curve and 50% effective concentration (EC50) were calculated using GraphPad Prism.
CMG2-Fc protects rabbits against intranasal challenge with B. anthracis.
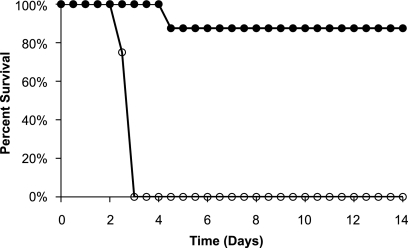
The ability of CMG2-Fc to protect rabbits against a lethal challenge of B. anthracis spores was tested in two studies. In the first challenge study, 16 Dutch belted rabbits were challenged intranasally with a suspension of 100 LD50 of B. anthracis Ames spores, followed shortly by subcutaneous injection with 3.6 mg/kg of CMG2-Fc or PBS. All eight animals in the PBS control group died by day 3, while the only death among the eight animals treated with CMG2-Fc occurred on day 4, giving an overall protection of 87.5% (Fig. 2).
FIG. 2.
Survival of Dutch belted rabbits after intranasal challenge with 107 CFU (100 LD50) of B. anthracis spores, followed by subcutaneous injection with 3.6 mg/kg of CMG2-Fc (solid circles) or PBS (empty circles).
In addition to determining efficacy, we tested whether surviving animals developed protective immunity that would allow them to survive rechallenge. Two serological correlates of immunity to anthrax have been identified in rabbits. The first is the quantitative anti-PA ELISA titer, and the second is the titer in the TNA (23, 33). Day-15 serum samples were first assayed to quantify the concentration of rabbit anti-PA using a direct ELISA. The titer of rabbit anti-PA antibodies varied considerably (range, 1 to 187 μg/ml; median, 32 μg/ml). Serum samples also were subjected to the TNA, which is the same assay used to quantify the in vitro potency of the CMG2-Fc. Titers in the TNA were expressed as the reciprocal of the serum dilution that protected 50% of the cells against LeTx cytotoxic activity (ED50) and also varied considerably (range, 33 to 4,266; median, 345) (Fig. 3).
FIG. 3.
Measurement of anti-PA antibody (black bars) and TNA titer (white bars) in serum of rabbits 15 days after challenge with 100 LD50 of B. anthracis spores.
The residual concentrations of CMG2-Fc in the day-15 sera were also measured (by ELISA) to determine if these contributed to the TNA titer. Animals 2 and 12, with the highest TNA assay titers and the highest levels of anti-PA, had no detectable CMG2-Fc in their serum (the detection limit was 4 ng/ml). In three animals, numbers 10, 14, and 13, residual CMG2-Fc detected in serum may have contributed, at most, 38, 19, and 3%, respectively, to the measured TNA titer. Taken together, these results suggest that treatment with CMG2-Fc at the time of spore challenge allows for the development of a potentially protective immune response against PA in some of the treated animals.
Glycosylation has no effect on the potency of CMG2-Fc.
Our initial CMG2-Fc construct carried the Asn297→Gln mutation in the Fc region, making it nonglycosylated. However, it was of interest to compare glycosylated and nonglycosylated versions of CMG2-Fc because of the potential impact of glycosylation on the in vivo protective efficacy. The elimination of the N-glycan has been shown to have a dramatic impact on the binding of IgG1 to the three major Fc gamma receptor isoforms in vitro, which in turn can affect a number of antibody effector functions, including antibody-dependent cellular cytotoxicity (15). Thus, two additional CMG2-Fc genes were constructed: CMG2-FcG and CMG2-FcA, with the amino acid at position 297 of the Fc being Asn (glycosylated) and Gln (aglycosyl), respectively. In addition, both of these constructs differed from the CMG2-Fc fusion described above by having the endoplasmic reticulum retention signal amino acid sequence SEKDEL appended to the carboxyl-terminal end, the intention of which was to maximize expression and ensure that glycosylation would be of the high-mannose form. Both proteins were produced using the transient expression system and purified by protein A chromatography. More than 26 kg of N. benthamiana leaves expressing CMG2-FcA and 2.4 kg expressing CMG2-FcG were harvested, and the average expression levels were 420 and 730 mg/kg (4.7 and 8.2% total soluble protein), respectively.
The electrophoretic mobilities of both new variants were similar to that of CMG2-Fc (Fig. 4A), with the glycosylated variant displaying a slightly lower mobility on the gel. Both variants were recognized by anti-human IgG antibodies (Fig. 4B). CMG2-FcG could be distinguished from CMG2-FcA by the ability of the lectin concanavalin A to bind to the former but not the latter (Fig. 4C). The in vitro toxin-neutralizing activities of both variants were similar to that of CMG2-Fc, with both having EC50s of 50 ng/ml (Fig. 4D).
FIG. 4.
Comparison of glycosylated and aglycosyl CMG2-Fc. Purified CMG2-FcA (2.5 μg/lane, lanes 1 and 3) or CMG2-FcG (2.5 μg/lane, lanes 2 and 4) were electrophoresed through an SDS-polyacrylamide gel. (A) The gel was stained with Coomassie. Additional gels were blotted onto nitrocellulose and probed with anti-human IgG (B) or concanavalin A (C). Lanes 1 and 2, protein reduced with DTT; lanes 3 and 4, nonreduced protein. M, molecular mass standards, indicated in kDa. (D) Toxin neutralization assay. CMG2-FcA, solid black line with solid circles; CMG2-FcG, dotted white line with squares. Each point is the mean of three replicates, and error bars represent the standard errors of the means.
The in vivo protective efficacies of CMG2-FcG and CMG2-FcA were compared at four different doses (4, 2, 1, and 0.5 mg/kg, administered i.v.) in the second rabbit spore challenge study. CMG2-FcA was 100% protective at either 4 or 2 mg/kg. CMG2-FcG was partially protective at those dosage levels but still was significantly more protective than placebo (Fig. 5A). At 1 mg/kg of either CMG2-FcG or CMG2-FcA, two of six treated rabbits survived spore challenge, but this level of protection was not significantly different from the placebo control: no animals survived spore challenge when treated with 0.5 mg/kg of either variant (Fig. 5B). Thirty days after the initial challenge, the four rabbits that survived spore challenge after treatment with 1 mg/kg of either CMG2-FcG or CMG2-FcA were rechallenged with 100 LD50 of Ames spores and all 4 survived at least 15 days. Four age-matched control animals, challenged but dosed only with PBS, all died (data not shown).
FIG. 5.
Survival of Dutch belted rabbits after intranasal spore challenge. Following spore challenge, groups of six animals were dosed (intravenously) with CMG2-FcA, CMG2-FcG, or PBS at a dose of 4 or 2 mg/kg (A) and a dose of 1 or 0.5 mg/kg (B). The four surviving animals from the two low-dose groups were rechallenged with 107 CFU (100 LD50) of B. anthracis spores on day 30. An asterisk indicates results that are significantly different from the control (P < 0.05 by Pearson's chi-square test for independent binomials).
CMG2-Fc protects against domain 4 variants of PA.
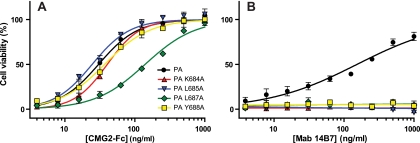
Previous studies identified mutations within domain 4 of PA that do not alter toxicity against cells (when combined with LF) but prevent neutralization by the anti-PA monoclonal antibody 14B7 (37). It was previously shown that soluble CMG2 can neutralize four of these mutated toxins (40). We found that CMG2-FcA also neutralized these four mutated toxins, whereas antibody 14B7 neutralized only the wild-type lethal toxin (Fig. 6). We tested seven additional PA mutants in the TNA: PA K679A, PA K680A, PA S690A, PA N691A, PA P692A, and PA N693A (all having mutations in domain 4). CMG2-FcA neutralized all of them as well as it neutralized wild-type PA. Another anti-PA monoclonal antibody, 4A12 (35), was significantly less effective against two of these mutants: the EC50 of 4A12 against PA K679A and PA K680A was 5-fold higher than that against wild-type PA (data not shown).
FIG. 6.
Neutralization of lethal toxin cytotoxicity by CMG2-FcA and MAb 14B7. RAW264.7 mouse macrophage-like cells were exposed to PA variants + LF in the presence of CMG2-FcA (A) or anti-PA monoclonal antibody 14B7 (B). Cell viability was assessed 4 h later. Each point is the means of three replicates. PA mutants K684A, L685A, L687A, and Y688A were not neutralized by MAb 14B7 at the concentration that neutralized wild-type PA (solid black circles), but all were neutralized by CMG2-FcA.
Pharmacokinetic analysis of CMG2-Fc.
The pharmacokinetics of CMG2-FcA and CMG2-FcG in rabbits were compared following single intravenous injections. Serum-concentration-versus-time data from each animal were modeled using both one-compartment and two-compartment models using WinNonlin professional software. The two-compartment model clearly describes the data better than the one-compartment model. The one-compartment model consistently underestimates the serum concentrations in the terminal phase, while the line predicted by the two-compartment model hits nearly all the observed points (data not shown). Pharmacokinetic parameter estimates are shown in Table 1. The terminal half-lives for both CMG2-FcA and CMG2-FcG appear to be approximately 5 days. However, the AUC (area under the serum concentration versus time curve) was significantly greater for CMG2-FcA, and the clearance (CL) of CMG2-FcG was significantly more rapid.
TABLE 1.
Pharmacokinetic parameters of i.v. CMG2-Fc in rabbitsa
| Parameter | Value |
|
|---|---|---|
| CMG2-FcA | CMG2-FcG | |
| No. of animals | 5 | 5 |
| Dose (mg/kg) | 3.6 | 5 |
| Cmax (μg/ml) | 66 ± 19 | 65 ± 7 |
| AUC0-240h (μg day/ml) | 218 ± 57** | 95 ± 16** |
| CL (ml/h/kg) | 1.02 ± 0.34** | 1.60 ± 0.24** |
| T1/2α (h) | 13.0 ± 9.1 | 7.0 ± 1.3 |
| T1/2β (days) | 5.0 ± 1.1 | 4.4 ± 0.5 |
| Vss (ml/kg) | 217 ± 133 | 187 ± 15 |
| V1 (ml/kg) | 81 ± 23* | 56 ± 5* |
Cmax, maximum calculated concentration; AUC0-240h, area under the serum concentration versus time curve from time zero through 240 h; CL, serum clearance determined from dose/AUC0-∞; T1/2α, initial half-life for 2 compartment model; T1/2β, terminal half-life for 2 compartment model; Vss, volume of distribution at steady state; V1, initial volume of distribution for two-compartment model. Differences between parameters for CMG2-FcA and CMG2-FcG were compared using a two-sided t test. *, P ≤ 0.05; **, P ≤ 0.001.
Stable transgenic tobacco producing high levels of CMG2-Fc.
While the transient expression system has the advantage of being able to produce testable quantities of CMG2-Fc relatively quickly (up to 1 g from 150 N. benthamiana plants), expression from stably transformed plants will be more economical for eventual commercial production. From a single transformation we identified four stably transformed tobacco plants producing CMG2-FcA. The best expressing of these T0 plants produced 35 mg/kg fresh weight at the time of flowering. The expression trait segregated in a ratio of 31/40 in the T1 progeny of this plant, indicating that the transgene existed at a single genetic locus. Two of the T1 plants were identified as homozygous for the transgene by backcrossing to wild-type N. tabacum. Expression among 30 T2 progeny from one of these T1 plants averaged 32 ± 10 mg/kg (maximum of 65 mg/kg) fresh weight. CMG2-FcA purified from plants of this line was indistinguishable in appearance (by SDS-PAGE) and in vitro potency (by TNA) from the same protein produced in the transient expression system (data not shown).
DISCUSSION
Several monoclonal antibodies against PA are under development as anthrax therapeutics (27, 29, 32, 44, 48). While efficacious, these agents are expensive to produce. In the work presented here, we describe an alternative protein therapeutic that promises to be as potent as monoclonal antibodies while being inexpensive to produce and having the further advantage of neutralizing any altered anthrax toxins that retain the essential ability to target receptors. This immunoadhesin is comprised of the anthrax toxin receptor CMG2 extracellular domain and human Fc, and it is expressed in plants. Its design, production, characterization, and initial tests in animal models are described here.
Several different designs of CMG2 fusions to IgG and Fc sequences were explored in preliminary work that is detailed in the supplemental material. A number of constructs were evaluated as to suitability for expression in plants, protein folding and stability, etc. This work led to the selection of an optimal CMG2-Fc fusion protein that was carried into further development.
The Fc portion of the fusion protein contains the C-terminal 232 amino acids of human IgG1, sequences known to be sufficient for dimerization and for binding to Fc receptors. This region contains two cysteines that form an interchain disulfide bond that serves to stabilize the dimer. In fact, the protein produced by transient transfection was shown by several methods to behave in solution as a dimer (see the supplemental material). The Fc region also contains an Asn297 residue that normally is glycosylated. The replacement of this residue with Gln produced a variant (CMG2-FCA) that was not glycosylated, allowing the determination of the impact of glycan modifications on activity, as will be detailed below.
The CMG2-Fc protein had potent activity in the standard in vitro TNA, with an EC50 of 53 ng/ml. This corresponds well to the Kd of 0.4 nM (19 ng/ml) for the affinity of CMG2 to PA as measured by SPR. Additionally, this value closely matches the apparent affinity of PA for the CMG2 receptor on cultured cells, measured as 0.9 nM in competitive cytotoxicity assays (24). These values also are similar to EC50s previously reported for the anti-PA antibodies raxibacumab (EC50 = 50 ng/ml) (49), ETI-204 (EC50 = 80 ng/ml) (29), and AVP-21D9 (EC50 = 30 to 70 ng/ml) (38). In addition, CMG2-Fc was able to neutralize PA mutants that either were not neutralized or were poorly neutralized by two anti-PA antibodies, extending similar observations made with a recombinant soluble form of CMG2 (40). These results suggest that it would be difficult to deliberately engineer strains of B. anthracis that evade the neutralizing activity of CMG2-Fc. This advantageous feature of a CMG2-based drug follows from the obvious fact that engineered variants of PA must retain binding to the cell surface CMG2 or TEM8 to initiate intoxication, so that such variants also must remain subject to inhibition by CMG2-Fc.
An immunoadhesin like CMG2-Fc has several other advantages not possessed by soluble CMG2: (i) the ability to be purified by well-established affinity chromatography with protein A, and (ii) a half-life of many days, comparable to that of human monoclonal antibodies. We confirmed the ease of purification from tobacco leaf tissue by protein A chromatography and achieved a high level of purity. We also demonstrated that the half-life of CMG2-Fc in rabbits (∼5 days) is similar to that reported for three human monoclonal anti-PA antibodies (29, 32, 48). The in vivo half-life of soluble monomeric CMG2 is not known but is expected to be much shorter than that of CMG2-Fc. The longer half-life of CMG2-Fc can be attributed in part to the larger size of the homodimer, which limits clearance by the kidneys (34, 36). In addition, the interaction of the Fc region with the neonatal Fc receptor (FcRn) salvages the protein from degradation in lysosomes. The long half-life of CMG2-Fc may explain the excellent in vivo protective potency of CMG2-Fc that we observed.
We also considered whether the inclusion of the Fc region has a functional effect beyond its role in increasing serum half-life. It has been reported that anthrax toxin neutralization by the anti-PA antibody Valortim is dependent on Fc receptor engagement (44), although the mechanism of this is not understood. Glycosylation is known to affect Fc receptor binding, so the two forms of CMG2-Fc that we generated having either Gln or Asn at the normal site of N glycosylation in Fc allowed a test of whether glycosylation would have an impact on potency. ConA blotting confirmed that CMG2-FcG was glycosylated and CMG2-FcA was not. The two forms of CMG2-Fc had identical potency in the TNA, and both protected rabbits against anthrax. The aglycosyl form, CMG2-FcA, was 100% protective in rabbits at 2 mg/kg, which compares favorably to published results for monoclonal antibodies to PA (27, 29, 44). Our results indicate that glycosylation is certainly not required for the in vivo efficacy of CMG2-Fc against inhalational anthrax. On the contrary, the more-rapid serum clearance of the glycosylated form could be a disadvantage if the protein were to be used prophylactically.
While a CMG2-Fc construct has been expressed in CHO cells and its potency demonstrated against the Sterne strain of B. anthracis in mice (46), our work is the first report of the use of CMG2-Fc as a postexposure treatment for inhalational anthrax in a rabbit model using the more-virulent Ames strain.
The analysis of sera from animals that survived following postexposure treatment with CMG2-Fc in the first challenge study indicated that at least some of the animals developed titers of anti-PA antibodies (titers of >1,000) that were comparable to those found in rabbits immunized with anthrax vaccine (AVA) (33). The fact that animals that survived B. anthracis spore challenge following treatment with CMG2-Fc in the second study were able to survive rechallenge 30 days later suggests that CMG2-Fc allows the development of the animals' own protective immune response. This may seem surprising given that it normally requires two doses of AVA given 4 weeks apart to protect rabbits from a lethal dose of B. anthracis spores (33). However, this protection upon rechallenge also was observed in rabbits and cynomolgus monkeys treated with the anti-PA monoclonal antibodies AVP-21D9 and Raxibacumab (27, 31). It may be that these therapeutic agents, while blocking toxin activity, allow bacterial replication and antigen production to occur at a low level that is sufficient to induce protective immunity, somewhat like a live attenuated strain (2, 26).
Immunoadhesins like the one described here have a number of potential and demonstrated advantages over alternative therapeutic agents. The inclusion of the Fc domain allows efficient purification with protein A, causes dimerization (which increases size), and induces recycling and retention by interaction with FcRn. The latter two properties confer plasma residence times that compare well to those of therapeutic monoclonal antibodies. It also is notable that the immunoadhesin described here contains only human sequences and is unlikely to be immunogenic, potentially allowing repeated administration.
The CMG2-Fc protein described here was produced in plants, which offer a highly attractive system for the large-scale, economical production of antibodies and therapeutic proteins (25). Advantages include reduced production costs compared to those of the bioreactors and specialized media required for CHO cells, as well as safety, since plants, unlike mammalian cell culture systems and transgenic animals, are not known to harbor animal viruses, prions, or mycoplasmas. The accumulation of CMG2-Fc in leaves of the T2-generation plants that we report here (32 mg/kg) is 10 times higher than was reported in a recent publication on the expression of a CMG2-Fc protein in tobacco (3 mg/kg) (3). The in vitro potency of the CMG2-Fc reported in that paper was also about 10 times lower than that reported here. The reasons for these differences are not entirely clear but may be due to the use in our work of a slightly larger portion of CMG2 that includes the cysteines (C39 and C218) that have been shown to form a disulfide bond (19).
In summary, we have used transgenic tobacco plants to produce large amounts of CMG2-Fc and demonstrated that it has high in vitro toxin-neutralizing potency and the ability to protect rabbits against lethal pulmonary infection by spores of the virulent Ames strain of B. anthracis. Additional animal efficacy studies, including treatment with CMG2-Fc after symptom development, are ongoing.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of Angela Sho and Fang Li.
This work was supported by Public Health Service grants R43AI053005 and U01AI082161 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 18 October 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alibek, K., and S. Handelman. 1999. Biohazard: the chilling true story of the largest covert biological weapons program in the world, told from the inside by the man who ran it, 1st ed. Random House, New York, NY.
- 2.Aloni-Grinstein, R., O. Gat, Z. Altboum, B. Velan, S. Cohen, and A. Shafferman. 2005. Oral spore vaccine based on live attenuated nontoxinogenic Bacillus anthracis expressing recombinant mutant protective antigen. Infect. Immun. 73:4043-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrianov, V., R. Brodzik, S. Spitsin, K. Bandurska, H. McManus, H. Koprowski, and M. Golovkin. 2010. Production of recombinant anthrax toxin receptor (ATR/CMG2) fused with human Fc in planta. Protein Expr. Purif 70:158-162. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi, A., and S. M. Chamow. 1997. Immunoadhesins as research tools and therapeutic agents. Curr. Opin. Immunol. 9:195-200. [DOI] [PubMed] [Google Scholar]
- 5.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 6.Cleret, A., A. Quesnel-Hellmann, A. Vallon-Eberhard, B. Verrier, S. Jung, D. Vidal, J. Mathieu, and J. N. Tournier. 2007. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J. Immunol. 178:7994-8001. [DOI] [PubMed] [Google Scholar]
- 7.Collier, R. J. 2009. Membrane translocation by anthrax toxin. Mol. Aspects Med. 30:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doolan, D. L., D. A. Freilich, G. T. Brice, T. H. Burgess, M. P. Berzins, R. L. Bull, N. L. Graber, J. L. Dabbs, L. L. Shatney, D. L. Blazes, L. M. Bebris, M. F. Malone, J. F. Eisold, A. J. Mateczun, and G. J. Martin. 2007. The US capitol bioterrorism anthrax exposures: clinical epidemiological and immunological characteristics. J. Infect. Dis. 195:174-184. [DOI] [PubMed] [Google Scholar]
- 9.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 10.Faye, L., and M. J. Chrispeels. 1985. Characterization of N-linked oligosaccharides by affinoblotting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal. Biochem. 149:218-224. [DOI] [PubMed] [Google Scholar]
- 11.Guarner, J., J. A. Jernigan, W. J. Shieh, K. Tatti, L. M. Flannagan, D. S. Stephens, T. Popovic, D. A. Ashford, B. A. Perkins, and S. R. Zaki. 2003. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am. J. Pathol. 163:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta, P. K., M. Moayeri, D. Crown, R. J. Fattah, and S. H. Leppla. 2008. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS One 3:e3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood, E. E., G. L. Helmer, R. T. Fraley, and M.-D. Chilton. 1986. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168:1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horsch, R. B., J. E. Fry, N. L. Hoffmann, D. Eichholtz, S. G. Rogers, and R. T. Fraley. 1985. A simple and general method for transferring genes into plants. Science 227:1229-1231. [DOI] [PubMed] [Google Scholar]
- 15.Jefferis, R., and J. Lund. 2002. Interaction sites on human IgG-Fc for FcγR: current models. Immunol. Lett. 82:57-65. [DOI] [PubMed] [Google Scholar]
- 16.Joh, L. D., and J. S. VanderGheynst. 2006. Agro-infiltration of plant tissues for production of high-value recombinant proteins: an alternative to production in transgenic crops. J. Sci. Food Agric. 86:2002-2004. [Google Scholar]
- 17.Klimpel, K. R., S. S. Molloy, G. Thomas, and S. H. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. U. S. A. 89:10277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacy, D. B., D. J. Wigelsworth, R. A. Melnyk, S. C. Harrison, and R. J. Collier. 2004. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc. Natl. Acad. Sci. U. S. A. 101:13147-13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacy, D. B., D. J. Wigelsworth, H. M. Scobie, J. A. Young, and R. J. Collier. 2004. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc. Natl. Acad. Sci. U. S. A. 101:6367-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. U. S. A. 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, H., S. D. Soroka, T. H. Taylor, Jr., K. L. Stamey, K. W. Stinson, A. E. Freeman, D. R. Abramson, R. Desai, L. X. Cronin, J. W. Oxford, J. Caba, C. Pleatman, S. Pathak, D. S. Schmidt, V. A. Semenova, S. K. Martin, P. P. Wilkins, and C. P. Quinn. 2008. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods 333:89-106. [DOI] [PubMed] [Google Scholar]
- 23.Little, S. F., B. E. Ivins, P. F. Fellows, M. L. Pitt, S. L. Norris, and G. P. Andrews. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine 22:422-430. [DOI] [PubMed] [Google Scholar]
- 24.Liu, S., D. Crown, S. Miller-Randolph, M. Moayeri, H. Wang, H. Hu, T. Morley, and S. H. Leppla. 2009. Capillary morphogenesis protein-2 is the major receptor mediating lethality of anthrax toxin in vivo. Proc. Natl. Acad. Sci. U. S. A. 106:12424-12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma, J. K., E. Barros, R. Bock, P. Christou, P. J. Dale, P. J. Dix, R. Fischer, J. Irwin, R. Mahoney, M. Pezzotti, S. Schillberg, P. Sparrow, E. Stoger, and R. M. Twyman. 2005. Molecular farming for new drugs and vaccines. Current perspectives on the production of pharmaceuticals in transgenic plants. EMBO Rep. 6:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelson, I., O. Gat, R. Aloni-Grinstein, Z. Altboum, I. Inbar, C. Kronman, E. Bar-Haim, S. Cohen, B. Velan, and A. Shafferman. 2005. Efficacious, nontoxigenic Bacillus anthracis spore vaccines based on strains expressing mutant variants of lethal toxin components. Vaccine 23:5688-5697. [DOI] [PubMed] [Google Scholar]
- 27.Migone, T.-S., G. M. Subramanian, J. Zhong, L. M. Healey, A. Corey, M. Devalaraja, L. Lo, S. Ullrich, J. Zimmerman, A. Chen, M. Lewis, G. Meister, K. Gillum, D. Sanford, J. Mott, and S. D. Bolmer. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135-144. [DOI] [PubMed] [Google Scholar]
- 28.Mogridge, J., K. Cunningham, D. B. Lacy, M. Mourez, and R. J. Collier. 2002. The lethal and edema factors of anthrax toxin bind only to oligomeric forms of the protective antigen. Proc. Natl. Acad. Sci. U. S. A. 99:7045-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed, N., M. Clagett, J. Li, S. Jones, S. Pincus, G. D'Alia, L. Nardone, M. Babin, G. Spitalny, and L. Casey. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ober, R. J., C. G. Radu, V. Ghetie, and E. S. Ward. 2001. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13:1551-1559. [DOI] [PubMed] [Google Scholar]
- 31.Peterson, J. W., J. E. Comer, W. B. Baze, D. M. Noffsinger, A. Wenglikowski, K. G. Walberg, J. Hardcastle, J. Pawlik, K. Bush, J. Taormina, S. Moen, J. Thomas, B. M. Chatuev, L. Sower, A. K. Chopra, L. R. Stanberry, R. Sawada, W. W. Scholz, and J. Sircar. 2007. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect. Immun. 75:3414-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson, J. W., J. E. Comer, D. M. Noffsinger, A. Wenglikowski, K. G. Walberg, B. M. Chatuev, A. K. Chopra, L. R. Stanberry, A. S. Kang, W. W. Scholz, and J. Sircar. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 34.Pitti, R. M., S. A. Marsters, M. Haak-Frendscho, G. C. Osaka, J. Mordenti, S. M. Chamow, and A. Ashkenazi. 1994. Molecular and biological properties of an interleukin-1 receptor immunoadhesin. Mol. Immunol. 31:1345-1351. [DOI] [PubMed] [Google Scholar]
- 35.Reason, D. C., A. Ullal, J. Liberato, J. Sun, W. Keitel, and J. Zhou. 2008. Domain specificity of the human antibody response to Bacillus anthracis protective antigen. Vaccine 26:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roopenian, D. C., G. J. Christianson, T. J. Sproule, A. C. Brown, S. Akilesh, N. Jung, S. Petkova, L. Avanessian, E. Y. Choi, D. J. Shaffer, P. A. Eden, and C. L. Anderson. 2003. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 170:3528-3533. [DOI] [PubMed] [Google Scholar]
- 37.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278:30936-30944. [DOI] [PubMed] [Google Scholar]
- 38.Sawada-Hirai, R., I. Jiang, F. Wang, S. Sun, R. Nedellec, P. Ruther, A. Alvarez, D. Millis, P. R. Morrow, and A. S. Kang. 2004. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. U. S. A. 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma, S., D. Thomas, J. Marlett, M. Manchester, and J. A. Young. 2009. Efficient neutralization of antibody-resistant forms of anthrax toxin by a soluble receptor decoy inhibitor. Antimicrob. Agents Chemother. 53:1210-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen, W. J., and B. G. Forde. 1989. Efficient transformation of Agrobacterium spp. by high voltage electroporation. Nucleic Acids Res. 17:8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoger, E., J. K. Ma, R. Fischer, and P. Christou. 2005. Sowing the seeds of success: pharmaceutical proteins from plants. Curr. Opin. Biotechnol. 16:167-173. [DOI] [PubMed] [Google Scholar]
- 43.Vitale, G., R. Pellizzari, C. Recchi, G. Napolitani, M. Mock, and C. Montecucco. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706-711. [DOI] [PubMed] [Google Scholar]
- 44.Vitale, L., D. Blanset, I. Lowy, T. O'Neill, J. Goldstein, S. F. Little, G. P. Andrews, G. Dorough, R. K. Taylor, and T. Keler. 2006. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 74:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voinnet, O., S. Rivas, P. Mestre, and D. Baulcombe. 2003. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33:949-956. [DOI] [PubMed] [Google Scholar]
- 46.Vuyisich, M., S. Gnanakaran, J. A. Lovchik, C. Rick Lyons, and G. Gupta. 2008. A dual-purpose protein ligand for effective therapy and sensitive diagnosis of anthrax. Protein J. 27:292-302. [DOI] [PubMed] [Google Scholar]
- 47.Welkos, S. L., and R. Marrero. 1996. Pathogenesis and host resistance to infection: a model system and an analysis of capsule synthesis and regulation by Bacillus anthracis, p. 209-256. In K. W. Adolph (ed.), Microbial genome methods. CRC Press, Boca Raton, FL.
- 48.Wild, M. A., K. Kumor, M. J. Nolan, H. Lockman, and K. S. Bowdish. 2007. A human antibody against anthrax protective antigen protects rabbits from lethal infection with aerosolized spores. Hum. Antibodies 16:99-105. [PubMed] [Google Scholar]
- 49.Zhang, X., J. Askins, R. Fleming, B. Sturm, C. Poortman, P. Viriassov, B. Peterson, M. Flynn, Y. Miao, D. Zukauskas, R. Smith, M. Laird, and G. Choi. 2003. Selection of potent neutralizing human monoclonal antibodies to protective antigen of Bacillus anthracis, abstr. 3976. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.