Abstract
The approved treatment dose of intravenous voriconazole is a weight-based dose of 4 mg/kg of body weight twice daily; the approved oral dosing is fixed at 200 mg twice daily. In our institution, patients frequently receive oral high-dose voriconazole at 4 mg/kg twice daily. It is unknown if higher doses are associated with increased hepatotoxicity. A retrospective cohort study of patients treated with oral voriconazole for presumed invasive fungal infection for ≥7 days was completed. Patients receiving a fixed dose (i.e., labeled dose) were frequency matched and compared to those receiving a weight-based dose (i.e., high dose). The primary endpoint of hepatotoxicity was evaluated by using NCI Common Terminology Criteria (CTC) and components of liver enzymes measuring >3× the upper limit of normal (ULN) and >5× baseline measurements. Secondary endpoints included an incidence of other adverse drug events. Twenty-five labeled-dose and 84 high-dose voriconazole patients were studied. Liver enzyme abnormalities were similar between groups, with the exception of labeled-dose patients experiencing more alkaline phosphatase (ALP) CTC >2× the baseline (P = 0.02) and ALP levels >3× the ULN (P = 0.02). Treatment with high dose was associated with the discontinuation of voriconazole for practitioner attribution of adverse drug events (P = 0.03), although reasons varied and no commonality of biomarker abnormality was identified. Multivariate analysis revealed that the duration of therapy and higher mg/kg total daily doses as interval variables were predictive of some hepatotoxicity outcomes. No difference existed in liver abnormalities for high-dose voriconazole; however, higher mg/kg doses and a longer duration of therapy may be associated with hepatotoxicity.
Voriconazole is a broad-spectrum azole antifungal agent with activity against many invasive yeast and mold species. Favorable efficacy and safety profiles have resulted in its inclusion into guidelines for the treatment of common nosocomial infections such as invasive aspergillosis and candidiasis (14, 23). The drug is fungicidal against Aspergillus species and fungistatic against Candida species (9). While generally regarded as safe, adverse events associated with the use of voriconazole include hepatic toxicity and visual disturbances (2). Therapeutic trials show that significant transaminase abnormalities occurred in 12.4% of patients receiving voriconazole (16).
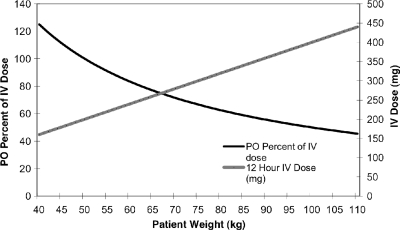
FDA-recommended dosing regimens differ based on the route of drug administration (i.e., intravenously [i.v.] versus orally [p.o.]), and patients at most weights do not receive the same mg/kg dose of voriconazole (Fig. 1). The approved labeled maintenance treatment dose of i.v. voriconazole is a weight-based 4 mg/kg of body weight twice daily; conversely, the approved oral dosing is a fixed 200-mg dose twice daily. Clinicians may increase the dose to 300 mg twice daily for patients weighing greater than 40 kg who do not have an adequate response to the 200-mg twice-daily dose (16, 17). The oral formulation of the drug has a lower recommended dose than the i.v. formulation in large part due to a phase II dose escalation trial in which 1 out of 9 patients who received 300 mg twice daily of oral voriconazole experienced an elevation of aspartate aminotransferase (AST) levels of greater than 3 times the upper limit of normal (ULN) (10). Additionally, a previous report suggested an increased risk of hepatotoxicity with incrementally rising drug concentrations, but there is no known threshold above or below which this occurs (19).
FIG. 1.
Comparison of p.o. versus i.v. dosages according to patient weight with FDA-labeled dosing.
As concerns for increased toxicity with maintenance doses of voriconazole greater than 200 mg twice daily are based on limited data, many clinicians choose to dose oral voriconazole according to a weight-based dosing scheme similar to that of the i.v. formulation. Such a practice is also being evaluated by pending clinical investigations where oral voriconazole doses can be increased up to a fixed 300-mg twice-daily dose (ClinicalTrials.gov identifiers NCT00531479 and NCT00556998; www.clinicaltrials.gov [16 October 2009, accession date]). At our institution, the oral formulation is commonly administered to patients based on the i.v. labeling of 6 mg/kg twice daily on day 1, followed by 4 mg/kg twice daily thereafter. The purpose of this study was to assess whether patients receiving higher-than-FDA-labeled oral dosing of voriconazole have an increased incidence of hepatotoxicity or other adverse events compared to those receiving the labeled oral dose.
MATERIALS AND METHODS
A retrospective cohort study was conducted, evaluating patients treated with oral voriconazole for presumed or proven invasive fungal infections at Northwestern Memorial Hospital between 1 January 2006 and 31 December 2008. Two groups of patients were compared: those receiving the FDA-approved dose of 200 mg twice daily of oral voriconazole and those patients receiving mg/kg-based doses of oral therapy that were higher than 200 mg twice daily. The primary study endpoint was hepatotoxicity, as measured with multiple objective and standardized formats. The National Cancer Institute Common Terminology Criteria (CTC) scores (Table 1) (http://ctep.cancer.gov) were assessed nominally as component liver function scores greater than or equal to 2 times the baseline (5, 12). Similar to other studies, we also assessed for liver enzymes that were >3× the ULN (5, 7, 12, 17) and >5× the baseline (5, 12). Institution normals for the various biomarkers are as follows: bilirubin, 0 to 1.3 mg/dl; alkaline phosphatase (ALP), 30 to 115 U/liter; AST, 0 to 40 U/liter; alanine aminotransferase (ALT), 0 to 48 U/liter. Secondary endpoints included an incidence of other documented drug-related adverse events and discontinuation of therapy due to adverse reactions. This study received approval by the Northwestern University and Midwestern University Institutional Review Boards.
TABLE 1.
NCI CTC for hepatotoxicity
| CTC score | Bilirubin criterion (value [mg/dl]) | ALT criterion (value [U/liter]) | AST criterion (value [U/liter]) | ALP criterion (value [U/liter]) |
|---|---|---|---|---|
| 0 | None (<1.3) | None (<48) | None (<40) | None (<115) |
| 1 | >ULN-1.5× ULN (1.3-1.95) | >ULN-2.5× ULN (48-120) | >ULN-2.5× ULN (40-100) | >ULN-2.5× ULN (115-387.5) |
| 2 | >1.5-3.0× ULN (1.95-3.9) | >2.5-5.0× ULN (120-240) | >2.5-5.0× ULN (100-200) | >2.5-5.0× ULN (387.5-775) |
| 3 | >3.0-10.0× ULN (3.9-13) | >5.0-20.0× ULN (240-960) | >5.0-20.0× ULN (200-800) | >5.0-20.0× ULN (775-2,300) |
| 4 | >10.0× ULN (>13) | > 20× ULN (>960) | > 20× ULN (>800) | > 20× ULN (>2,300) |
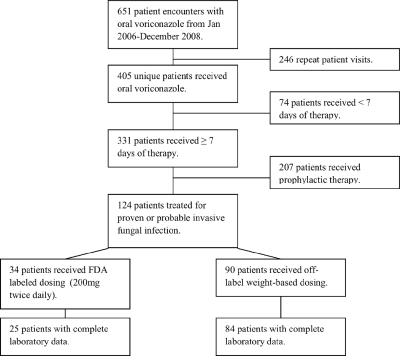
Patients were included if they received ≥7 days of therapy with treatment doses of oral voriconazole for possible, probable, or proven invasive fungal infection (IFI) and had available baseline and follow-up liver enzyme laboratory values. Patients who were determined to have received voriconazole for antifungal prophylaxis or who did not have baseline or follow-up laboratory values were excluded (Fig. 2). For patients receiving more than one course of voriconazole during this time period, only the first exposure was evaluated. Possible, probable, or proven IFI was defined according to physician diagnosis at the time of therapy and standard criteria (1).
FIG. 2.
Patient selection.
The following data were extracted from patients' medical records: age, sex, height, weight, indication for voriconazole therapy, underlying disease state, and other comorbidities. The length of therapy (LOT) based on the start of therapy and end of therapy or the date of last recorded liver function tests (LFTs), baseline and peak LFTs, the number of concomitant potentially hepatotoxic medications, other documented adverse drug reactions, and the reason for the discontinuation of therapy were documented. Medications were defined as potentially hepatotoxic if reports of hepatotoxicity associated with their use existed in published literature or if LFT monitoring was suggested in their product labeling (7, 11, 13). Additionally, patient comorbidities were assessed compositely with the Deyo modification of the Charlson comorbidity score calculated with ICD-9 (International and Statistical Classification of Diseases and Health Related Problems) codes (4, 6). An age-adjusted Charlson score was calculated, assigning 1 point for every decade over 40 years of age (4).
Statistical analysis.
Data analysis was performed by using Intercooled Stata, version 10.1 (Stata, College Station, TX). Descriptive statistics were performed for all study variables. Inferential statistics were performed as follows: Fisher's exact and chi-square analyses were performed where appropriate for nominal data, and Student's t tests were conducted for interval data. Odds ratios (ORs) and adjusted odds ratios with 95% confidence intervals (CIs) were calculated based on bivariate and multivariate logistic regression, respectively. If hepatotoxicity (biomarker) outcomes were significantly associated with treatment group in bivariate comparisons at a P value of <0.2, a multivariate model was created for each potential outcome. Multivariate models were created by adding variables with a plausible relationship to the dependent outcomes and significant at a P value of <0.2 in bivariate analyses (baseline differences between treatment groups or differences between those experiencing an outcome) to the logistic regression equation. To select the most parsimonious and explanatory model describing differences between the treatment groups, a forward selection technique was employed by assessing each variable iteratively added to the multivariate model according to significance. Model selection was completed by assessing twice the difference in the log likelihood ratios between the models and comparing the product against a chi-square distribution with the appropriate degrees of freedom. Final selected variables were assessed for interaction (P < 0.05), and interactive variables were included only with the interaction term present in the model. Probabilities of outcomes adjusting for confounders were calculated from adjusted odds ratios. When potentially confounding interval variables were significant at a P value of <0.05 in the multivariate models, the effect of the independent variable on the dependent variable was explored to determine the incremental effect of the independent variable (other than dosing regimen) on the probability of liver abnormality outcomes. A binary recursive partitioning methodology, Classification and Regression Tree modeling, was utilized to assess the appropriate division between interval variables and outcomes utilizing SPSS v.17 and Decision Trees Add-on v.17 (SPSS Inc., Chicago, IL). All tests were two tailed, and the alpha was fixed at 0.05 for final interpretations.
RESULTS
One hundred nine patients met study inclusion criteria with baseline and follow-up LFTs reported and were included for analysis. Patient demographic data are reported in Table 2 . Twenty-five patients received the labeled 200-mg twice-daily dose, and 84 received higher weight-based doses. The indication for voriconazole and patients' underlying conditions were similar between the two groups, with the majority of patients having probable or proven invasive aspergillosis and diagnosed with malignancy and/or having received a bone marrow transplant. Patients were generally well matched, with a few exceptions. Those who received labeled dosing weighed significantly less than those receiving off-labeled dosing (mean weight, 67.2 versus 81.0 kg, respectively; P = 0.001). Similarly, there was a statistically significant difference in the mean mg/kg dose between groups. Patients receiving labeled dosing received a mean dose of 3.2 mg/kg, compared to 3.8 mg/kg for patients receiving off-label, weight-based dosing (P < 0.001).
TABLE 2.
Baseline characteristics
| Patient demographica | Value for group |
P valueb | |
|---|---|---|---|
| FDA dosing (n = 25) | Non-FDA dosing (n = 84) | ||
| Mean age (yr) (SD) | 57.0 (12.4) | 52.7 (13.7) | 0.160 |
| Mean wt (kg) (SD) | 67.2 (16.3) | 81.0 (18.4) | 0.001 |
| Mean ht (in) (SD) | 67.2 (5.1) | 67.2 (4.7) | 1.000 |
| No. (%) of female patients | 12 (48) | 32 (38) | 0.380 |
| No. (%) of patients of race | |||
| Caucasian | 16 (64) | 57 (67.8) | 0.719 |
| African American | 4 (16) | 9 (10.7) | 0.490 |
| Hispanic | 1 (4) | 8 (9.5) | 0.681 |
| Asian American | 0 (0) | 1 (1.2) | 1.000 |
| Other | 4 (16) | 9 (10.7) | 0.490 |
| No. (%) of patients with reason for voriconazole treatment | |||
| Possible IA | 3 (12) | 17 (20.2) | 0.560 |
| Probable/proven IA | 22 (88) | 64 (76.2) | 0.270 |
| Other | 0 (0) | 3 (3.6) | —c |
| No. (%) of patients with underlying condition | |||
| Hematologic malignancy | 12 (48) | 36 (42.9) | 0.650 |
| BMT | 6 (24) | 34 (40.5) | 0.160 |
| Solid-organ transplant | 3 (12) | 9 (10.7) | 1.000 |
| Alcoholic cirrhosis | 1 (4) | 2 (2.4) | 0.550 |
| Other | 4 (16) | 3 (3.6) | 0.130 |
| Mean duration of therapy (days) (SD) | |||
| i.v. load | 1.0 (2.5) | 1.6 (4.7) | 0.530 |
| Oral | 72.0 (102.0) | 76.1(104.2) | 0.861 |
| Mean voriconazole twice-daily dose (mg/kg) (SD) | 3.2 (0.74) | 3.8 (0.75) | <0.001 |
| Mean voriconazole twice-daily dose (mg) (SD) | 200 (0) | 297.3 (59.2) | <0.001 |
| Mean no. of concomitant hepatotoxins (range) | 3.3 (1.6) | 3.4 (1.7) | 0.800 |
| Mean baseline LFT (SD) | |||
| Direct bilirubin (mg/dl) | 1.6 (1.8) | 1.3 (3.3) | 0.730 |
| AST (U/liter) | 73.4 (127.3) | 50.1 (142.6) | 0.460 |
| ALT (U/liter) | 27.9 (15.8) | 44.6 (56.9) | 0.150 |
| ALP (U/liter) | 175.8 (325) | 100.8 (75.4) | 0.052 |
| Mean Charlson score (SD) | 2 (1) | 2 (1.1) | 0.970 |
| Mean age-adjusted Charlson score (SD) | 4.3 (1.8) | 4.0 (1.7) | 0.350 |
IA, invasive aspergillosis; BMT, bone marrow transplant.
Boldface type indicates significant values.
Analysis not possible to due the low number of events.
The numbers of concomitant hepatotoxins were similar between two groups. Common medications known to be associated with hepatotoxicity in the studied groups included acetaminophen, allopurinol, esomeprazole, ondansetron, HMG-coenzyme A (CoA) reductase inhibitors, zolpidem, antipsychotics, and chemotherapeutic agents. Of note, no patients received medications known to significantly inhibit the metabolism of voriconazole through liver cytochrome (CYP) interactions (16). Patients' baseline LFTs and comorbidities were similar between the two groups.
Liver function outcomes as assessed by biochemical surrogates are reported in Table 3. The incidences of LFT abnormalities were similar in the two groups, with the exception of a significant CTC increase of >2 for ALP (16.0% versus 2.4%; P = 0.024) and an ALP >3× the ULN (32.0% versus 11.9%; P = 0.018) seen for patients receiving 200-mg twice-daily dosing. The incidences of visual disturbances were similar between the two groups. Despite these findings, patients who received higher off-label doses were significantly more likely to have their therapy discontinued for clinician-attributed adverse events (0.0% versus 16.9%; P = 0.028). Reasons for the discontinuation of therapy included visual disturbances (n = 2), possible cause of drug fever (n = 1), and clinician diagnosis of LFT abnormalities (n = 11). No consistency in biomarker abnormalities existed among patients who had their therapy discontinued for adverse events.
TABLE 3.
Adverse outcomes stratified by dosing strategy
| Outcome | No. (%) of patients with adverse outcome |
P valuea | |
|---|---|---|---|
| FDA dosing (n = 25) | Non-FDA dosing (n = 84) | ||
| Bilirubin (mg/dl) | |||
| CTC increase >2 | 6 (24) | 12 (14.3) | 0.356 |
| >3× ULN | 4 (16) | 5 (6) | 0.206 |
| >5× baseline | 3 (12) | 3 (3.6) | 0.132 |
| ALP (U/liter) | |||
| CTC increase >2 | 4 (16) | 2 (2.4) | 0.024 |
| >3× ULN | 8 (32) | 10 (11.9) | 0.018 |
| >5× baseline | 1 (4) | 3 (3.6) | 1.000 |
| AST (U/liter) | |||
| CTC increase >2 | 4 (16) | 18 (21.4) | 0.777 |
| >3× ULN | 4 (16) | 17 (20.2) | 0.777 |
| >5× baseline | 3 (12) | 13 (15.5) | 1.000 |
| ALT (U/liter) | |||
| CTC increase > 2 | 4 (16) | 12 (14.3) | 0.759 |
| >3× ULN | 3 (12) | 13 (15.5) | 1.000 |
| >5× baseline | 6 (24) | 11 (13.1) | 0.214 |
| Visual disturbances | 1 (4) | 3 (3.6) | 1.000 |
| Discontinued due to adverse event | 0 (0) | 14 (16.9) | 0.028 |
Boldface type indicates significant values.
Odds ratios and adjusted odds ratios for specific hepatic outcomes are listed in Table 4. When controlling for potential confounders as detailed above in “Statistical analysis,” those receiving higher non-FDA dosing had decreased odds of experiencing CTC score elevations of >2 for ALP (OR, 0.09; 95% CI, 0.01 to 0.66) and having the ALP level reach 3× the ULN (OR, 0.13; 95% CI, 0.03 to 0.66). Other LFT outcomes that were not statistically different between the groups in bivariate analyses (0.05 ≤ P value < 0.2) remained similar when controlling for potential confounders (Table 4).
TABLE 4.
Bivariate and multivariate odds ratiosg
| Liver function marker | Non-FDA vs FDA OR (95% CI) | Non-FDA vs FDA adjusted OR (95% CI)a |
|---|---|---|
| Bilirubin (mg/dl) | ||
| CTC increase >2 | 0.53 (0.18-1.59) | |
| >3× ULN | 0.33 (0.08-1.35) | 0.50 (0.09-2.70)b |
| >5× baseline | 0.27 (0.05-1.4) | 0.24 (0.04-1.52)c |
| ALP (U/liter) | ||
| CTC increase >2 | 0.13 (0.02-0.75) | 0.09 (0.01-0.66)d |
| >3× ULN | 0.29 (0.10-0.84) | 0.13 (0.03-0.66)e |
| >5× baseline | 0.89 (0.09-8.94) | |
| AST (U/liter) | ||
| CTC increase >2 | 1.43 (0.44-4.70) | |
| >3× ULN | 1.33 (0.40-4.39) | |
| >5× baseline | 1.34 (0.35-5.14) | |
| ALT (U/liter) | ||
| CTC increase >2 | 0.88 (0.26-3.00) | |
| >3× ULN | 1.34 (0.35-5.14) | |
| >5× baseline | 0.48 (0.16-1.46) | 0.56 (0.18-1.75)f |
Only reported for those with a P value of <0.2.
Adjusted OR controlled for initial bilirubin concentration.
Adjusted OR controlled for initial bilirubin concentration and length of voriconazole therapy.
Adjusted OR controlled for length of treatment.
Adjusted OR controlled for initial ALP level, voriconazole dose in mg/kg, and length of voriconazole therapy.
Adjusted OR controlled for initial ALT concentration.
Boldface type indicates significant values.
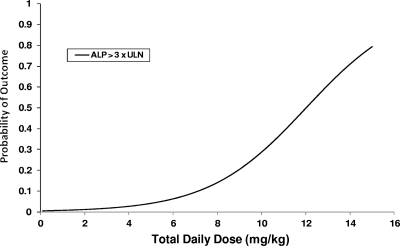
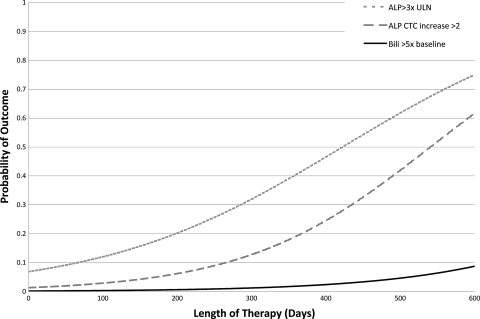
Results of the logistic regression analysis controlling for the variables noted in Table 4 demonstrated that the mg/kg dose that patients received was significantly associated with an ALP level >3× the ULN (Fig. 3). Length of therapy was also predictive of certain liver abnormalities. The probability of experiencing an ALP >3× the ULN, an ALP CTC increase of >2, and a bilirubin level >5× the baseline were found to increase with the patient's length of therapy (Fig. 4). Lengths of therapy of >203 days and >186.5 days were predictive of a bilirubin level >5× the baseline and an ALP level >3× the ULN (P < 0.01 for both), respectively. Length of therapy did not partition well for an ALP CTC increase of >2, as the optimal cut point included only 3 patients in one grouping. Finally, a dose of >6.49 mg/kg was predictive of a bilirubin level >5× the baseline (P < 0.01).
FIG. 3.
Hepatic outcomes by magnitude of dose, controlled for initial ALP concentration, FDA dosing group, and length of therapy.
FIG. 4.
Hepatic outcomes by length of therapy. An ALP CTC increase of >2 controlled for the FDA dosing group, a bilirubin level >5× the baseline controlled for FDA dosing group and initial bilirubin concentration, and an ALP level >3× the ULN controlled for FDA dosing group, initial ALP concentration, and mg/kg dose.
DISCUSSION
We did not identify differences in liver abnormalities between those receiving FDA-labeled oral voriconazole dosing and those receiving higher-mg/kg-based oral dosing. Elevations in ALP levels were seen more frequently in the group receiving lower fixed doses, although clinical explanations for these results are difficult; thus, we would not interpret the results to mean that receiving higher-weight-based dosing of voriconazole is protective against elevations in transaminase levels. As this was a retrospective study, differences may have been related to the baseline characteristics of the study population that were not captured in our models. It is noted that the baseline ALP values for those receiving fixed oral doses of the drug were higher than values for those receiving weight-based therapy by a degree that approached statistical significance (P = 0.052), which might explain the finding of a difference in the ALP of >3× the ULN between the two dosing groups but would not affect the outcome of the ALP CTC increase of >2. Additionally, adding the initial ALP value to the regression did not change our outcomes (data not shown). Perhaps those at the lower extreme of body weight, regardless of the dosing scheme, were the most predisposed to LFT abnormalities, although this was not clear when assessing weight as an interval variable against an ALP CTC increase of >2 (P = 0.20). Still, the fact that adverse outcomes were similar between the two dosing strategies may have important implications for dosing strategies in voriconazole therapy.
Proper dosing for voriconazole remains paramount, as the drug exhibits nonlinear pharmacokinetics and is extensively metabolized via the hepatic CYP450 enzyme system, primarily by CYP2C19 (8). Well-documented genetic polymorphisms of this isoenzyme contribute to the wide interindividual variability in plasma concentrations seen for patients even at fixed doses (19-21). Two retrospective studies have shown a correlation between voriconazole plasma concentrations of ≥2 mg/liter and successful treatment outcomes for patients with invasive aspergillosis (18) or for the prevention of Candida glabrata isolation (22). Recently, a prospective study of 52 patients with invasive fungal infections which incorporated therapeutic drug monitoring to adjust voriconazole dosing to achieve plasma concentrations between 1 and 5.5 mg/liter found significantly more favorable outcomes among patients whose plasma concentrations were maintained within the target range. All 6 patients with subtherapeutic voriconazole plasma concentrations and inadequate clinical responses had successful outcomes when the voriconazole dose was increased to attain plasma concentrations of >1 mg/liter. Those authors also found an increased risk of neurological adverse events, including confusion, agitation, and visual hallucinations, with plasma trough concentrations of >5.5 mg/liter (15). When voriconazole drug concentrations were obtained in our patient population, concentrations were noted, but a lack of universal concentration monitoring precluded useful interpretation. Ten of the concentrations (n = 10/36 for 24 patients) that were recorded in our study were above 5.5 mg/liter. Therapeutic drug monitoring of voriconazole is not standardized or governed by a formal protocol at our institution, and hence, the retrospective nature of our study allowed us only to review events that occurred. Also, the lack of monitoring at our institution may be secondary to the fact that the assay was not completed locally, and thus, a poor correlation of drug concentrations to patient events may often be exacerbated by the temporal delay of reporting.
Regarding hepatotoxicity outcomes, a large retrospective study by Tan et al. did show a weak association with a risk of AST, ALP, and bilirubin concentration increases as a bivariate outcome with an incremental rise in the voriconazole concentration as an interval measure (19). Despite the fact that small increases of the probability of LFT abnormalities were predicted by a log-linear regression line, categorical interpretation of the voriconazole concentrations did not elucidate a cut point (i.e., a single concentration that predicted outcome) by receiver-operating characteristic analysis. These findings suggest that mean voriconazole serum concentrations may not be a useful marker for a prediction of hepatotoxicity.
Although there was not a statistically significant difference in the incidence of adverse events between the two groups, therapy was stopped more frequently for patients receiving off-label dosing. This suggests that perhaps clinicians may be more apt to discontinue higher, nonlabeled doses of voriconazole when liver abnormalities or other events occur. Multivariate analyses did reveal that higher mg/kg doses may be associated with increases in LFTs. This is exclusive of the dosing strategy, possibly suggesting that even some patients who receive the flat 200-mg dose may actually be receiving a dose that is too high for their weight on a mg/kg basis. It has also been postulated that an increased oral dosing of voriconazole can result in hepatotoxicity and that the first-pass metabolism of oral voriconazole results in higher portal vein plasma concentrations, which could result in a higher incidence of liver enzyme abnormalities than with the i.v. formulation (5, 12). In the setting of limited data to support this hypothesis, such concerns remain largely theoretical and are not supported by our data.
Additionally, our data demonstrate that the duration of voriconazole therapy and dose standardized to weight should be considered relative to hepatotoxicity. It is not unusual for patients to receive many months of therapy with voriconazole, and these patients may require a closer monitoring of hepatic enzymes. We acknowledge that due to the sample size and data distribution, our regression models are more predictive at the lower mg/kg and LOT values (data not shown).
There were several limitations to this retrospective analysis. First, a relatively small sample size was reviewed, and it is possible that a larger sample size may have captured more statistically and clinically significant differences in outcomes. However, this represents the largest data set to our knowledge to analyze hepatotoxicity outcomes for non-FDA-approved dosing schemes. Second, the retrospective nature of the study limited the analysis to outcomes that were reported in the medical records and the frequency by which hepatic enzymes were measured. Third, several patients underwent multiple admissions throughout the 3-year time period, and there were many outpatient days during this time frame in which compliance or concomitant medications were not measured. However, the lack of a completely closed health care system precludes the optimal sampling of these data in a retrospective fashion. Fourth, we acknowledge that it is difficult to diagnose drug-induced hepatotoxicity, and there is no true consensus as to how to best capture this endpoint (7, 11, 15). To address this concern, we analyzed several biochemical markers of liver injury and employed three traditional research composite scores for assessments of elevations in enzyme concentrations (i.e., score relative to baseline, score relative to the upper limit of normal, and CTC score). Still, one may question whether the small amount of increased hepatotoxicity predicted by rising mean voriconazole concentrations is a causal relationship or if this represents a correlation in which the precursor variable (voriconazole concentration) is in the causal pathway of the event (i.e., hepatotoxicity). Since voriconazole is hepatically metabolized, a malfunctioning liver will likely result in higher voriconazole concentrations. Finally, we were unable to assess the likelihood of causality between voriconazole administration and hepatic outcomes, and one must consider that an increase in surrogate liver function tests does not necessarily reflect a hepatotoxicity of clinical significance. In a study by Chamilos and colleagues that looked at biopsy specimen evidence of potential drug-induced hepatotoxicity from different amphotericin B formulations, those researchers were unable to confirm that LFT abnormalities seen in leukemia patients corresponded to histopathological evidence of drug toxicity (3). We acknowledge that concomitant disease states such as graft-versus-host disease (GVHD) may contribute to the elevated levels hepatic enzymes seen for our patients, but due to the retrospective nature of this study, it is impossible to differentiate drug-induced hepatotoxicity from liver damage due to other comorbid complications, including GVHD, infection, and chemotherapy, etc. However, as we did not see major differences between the two dosing groups in our primary outcomes (i.e., hepatic enzymes), this limitation is only of potential relevance to our secondary outcomes. Of note, a large percentage of our patients had malignancies and received a bone marrow transplant. However, none of these patients had underlying hepatic cirrhosis or hepatitis C (per protocol, patients with serious concomitant liver disease are excluded). Patients with evidence of prior hepatitis B exposure are placed on antiviral agents, and to our knowledge, no patients have had reactivation posttransplantation. Additionally, we are not aware that any of our oncology patients received a diagnosis of hepatocellular carcinoma or cirrhosis.
Conclusion.
Higher-than-FDA-labeled oral dosing of voriconazole was not associated with hepatotoxicity in our analysis. The fact that this study found a similar incidence of hepatotoxicity between standard 200-mg twice-daily dosing and higher-weight-based dosing of oral voriconazole suggests that future prospective studies should be conducted to assess the efficacy and safety of the weight-based dosing strategy for oral voriconazole. Treatment providers should simultaneously consider possible benefits and harms associated with each dosing strategy. Utilizing mg/kg doses of oral voriconazole may be reasonable for those with a high probability of poor outcomes due to IFIs.
Acknowledgments
No authors have any affiliations or conflicts of interest to disclose.
Footnotes
Published ahead of print on 25 October 2010.
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, H. W., A. H. Groll, C. C. Chiou, and T. J. Walsh. 2004. Newer systemic antifungal agents: pharmacokinetics, safety and efficacy. Drugs 64:1997-2020. [DOI] [PubMed] [Google Scholar]
- 3.Chamilos, G., M. Luna, R. E. Lewis, R. Chemaly, I. I. Raad, and D. P. Kontoyiannis. 2007. Effects of liposomal amphotericin B versus an amphotericin B lipid complex on liver histopathology in patients with hematologic malignancies and invasive fungal infections: a retrospective, nonrandomized autopsy study. Clin. Ther. 29:1980-1986. [DOI] [PubMed] [Google Scholar]
- 4.Charlson, M. E., P. Pompei, K. L. Ales, and C. R. MacKenzie. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373-383. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander, J. G., C. van Arkel, B. J. Rijnders, P. J. Lugtenburg, S. de Marie, and M. D. Levin. 2006. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J. Antimicrob. Chemother. 57:1248-1250. [DOI] [PubMed] [Google Scholar]
- 6.Deyo, R. A., D. C. Cherkin, and M. A. Ciol. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45:613-619. [DOI] [PubMed] [Google Scholar]
- 7.Guo, J. J., P. R. Wigle, K. Lammers, and O. Vu. 2005. Comparison of potentially hepatotoxic drugs among major US drug compendia. Res. Social Adm. Pharm. 1:460-479. [DOI] [PubMed] [Google Scholar]
- 8.Hyland, R., B. C. Jones, and D. A. Smith. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. Dispos. 31:540-547. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, L. B., and C. A. Kauffman. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 10.Lazarus, H. M., J. L. Blumer, S. Yanovich, H. Schlamm, and A. Romero. 2002. Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J. Clin. Pharmacol. 42:395-402. [PubMed] [Google Scholar]
- 11.Lee, W. M. 2003. Drug-induced hepatotoxicity. N. Engl. J. Med. 349:474-485. [DOI] [PubMed] [Google Scholar]
- 12.Levin, M. D., J. G. den Hollander, B. van der Holt, B. J. Rijnders, M. van Vliet, P. Sonneveld, and R. H. van Schaik. 2007. Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms. J. Antimicrob. Chemother. 60:1104-1107. [DOI] [PubMed] [Google Scholar]
- 13.Lucena, M. I., M. Garcia-Cortes, R. Cueto, J. Lopez-Duran, and R. J. Andrade. 2008. Assessment of drug-induced liver injury in clinical practice. Fundam. Clin. Pharmacol. 22:141-158. [DOI] [PubMed] [Google Scholar]
- 14.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, and J. D. Sobel. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascual, A., T. Calandra, S. Bolay, T. Buclin, J. Bille, and O. Marchetti. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201-211. [DOI] [PubMed] [Google Scholar]
- 16.Pfizer, Inc. 2008. VFend (voriconazole) package insert. Pfizer, Inc., New York, NY.
- 17.Scott, L. J., and D. Simpson. 2007. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 67:269-298. [DOI] [PubMed] [Google Scholar]
- 18.Smith, J., N. Safdar, V. Knasinski, W. Simmons, S. M. Bhavnani, P. G. Ambrose, and D. Andes. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan, K., N. Brayshaw, K. Tomaszewski, P. Troke, and N. Wood. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 20.Theuretzbacher, U., F. Ihle, and H. Derendorf. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649-663. [DOI] [PubMed] [Google Scholar]
- 21.Trifilio, S., G. Pennick, J. Pi, J. Zook, M. Golf, K. Kaniecki, S. Singhal, S. Williams, J. Winter, M. Tallman, L. Gordon, O. Frankfurt, A. Evens, and J. Mehta. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532-1535. [DOI] [PubMed] [Google Scholar]
- 22.Trifilio, S., S. Singhal, S. Williams, O. Frankfurt, L. Gordon, A. Evens, J. Winter, M. Tallman, J. Pi, and J. Mehta. 2007. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 40:451-456. [DOI] [PubMed] [Google Scholar]
- 23.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]