Abstract
Paradoxical growth (PG) has been described for echinocandins and is characterized by cell growth at drug concentrations above the MIC. In this study, two isolates each of Candida albicans, C. tropicalis, C. orthopsilosis, and C. parapsilosis, all of which displaying PG in response to caspofungin, were subjected to MIC, minimal fungicidal concentration (MFC), and time-kill curve assays to evaluate the levels of PG. Cell wall components and ultrastructural modifications of the PG cells were also investigated. The results showed that when cell growth and survival were evaluated by MFC or time-kill curve assays, high concentrations of caspofungin did not show fungicidal activity against PG cells. Furthermore, for C. parapsilosis and C. orthopsilosis, time-kill curves were more discriminatory than MFCs in detecting the PG effect. The four different Candida species studied demonstrated similar alterations in cell wall components and ultrastructure associated with PG. In PG cells, β-1,3-glucan content decreased from 2.7- to 7.8-fold, whereas chitin content increased from 4.0- to 6.6-fold. An electron microscopy study of the PG cells revealed morphological alterations, clumping of cells, enlarged cells, the absence of filamentation, abnormal septa, and accumulation of chitin in the cell wall. Also, PG cells basically exhibited a single dark high-density layer in the cell wall, indicating the loss of the β-1,3-glucan layer. Our results present novel details about the ultrastructural alterations that occur in C. albicans, C. parapsilosis, C. orthopsilosis, and C. tropicalis during PG and show that chitin is the major component of the cell walls of PG cells. Stimulation of chitin synthesis may represent a rescue mechanism against caspofungin activity.
Caspofungin (CAS), the first echinocandin to be commercialized worldwide, exerts its antifungal effect by blocking the Fks1 subunit of glucan synthase, the enzyme responsible for β-1,3-glucan biosynthesis (12, 13, 20). Candida isolates are generally susceptible, in vitro and in vivo, to low concentrations of CAS, typically exhibiting MICs of ≤0.5 μg/ml (29). An exception is Candida parapsilosis, which generally shows MIC90 values ranging from 0.5 to 1.0 μg/ml (18, 30) when tested by broth microdilution assay, according to Clinical and Laboratory Standards Institute (CLSI) guidelines (10). CAS is therefore considered to be fungicidal to most Candida species, with some exceptions, including C. parapsilosis and C. guilliermondii strains, as both of which generate higher MIC and minimal fungicidal concentration (MFC) values (3).
Although CAS shows excellent growth-inhibitory activity at low concentrations, paradoxical growth (PG) in the presence of this compound has been observed in Candida spp. PG has been described as cell growth in the presence of CAS concentrations that are at least two concentrations above the MIC in broth microdilution susceptibility tests, performed according to CLSI guidelines (7, 22, 34). Unlike trailing growth, in which reduced but persistent cell growth at all drug concentrations above the MIC is observed, PG is defined as a resurgence of growth in the presence of supra-MICs of a drug; such growth is often close to the levels achieved in the absence of drug (22).
Studies describing PG in Candida spp. have demonstrated that there are differences in the frequency of PG in vitro that are echinocandin specific and Candida species related (7, 15, 37). Despite the fact that PG occurs in the presence of high concentrations of echinocandins, it has been shown that this phenomenon is not due to the selection of a resistant subpopulation but, in part, to compensatory mechanisms of synthesis of cell wall components (35, 38).
The clinical relevance of PG has not yet been fully determined. Data obtained using animal models are still rare and controversial (9). Interestingly, a randomized comparative study of the use of two micafungin dosages to treat patients with candidemia reported that patients with persistently positive blood cultures were more frequently observed in a group treated with 150 mg/day micafungin (23 patients; 11.6%) than those observed in a group treated with 100 mg/day micafungin (11 patients; 5.8%). The average time to register the first negative blood culture for Candida was 2 days for the group treated with 100 mg/day micafungin and 3 days for the group treated with 150 mg/day of the compound (28).
The occurrence of PG after exposure to echinocandins has been characterized mostly with C. albicans strains (35); thus, it is not clear whether the physiological and morphological adaptations described may be applied to other Candida species. The aim of this study is to characterize the morphological and biochemical features related to PG in the following four different Candida species: C. albicans, C. parapsilosis, C. orthopsilosis, and C. tropicalis.
MATERIALS AND METHODS
Isolates.
PG was screened using the broth microdilution method by testing 77 Candida bloodstream isolates randomly selected from yeast stock cultures from the Laboratório Especial de Micologia, Universidade Federal de São Paulo, São Paulo, Brazil. After being screened, eight Candida strains exhibiting PG at approximately 16 μg/ml of caspofungin were selected. These included two strains of C. albicans, two strains of C. orthopsilosis, two strains of C. parapsilosis, and two strains of C. tropicalis.
Antifungal susceptibility.
Caspofungin (Merck Sharp & Dohme) MICs were determined by microdilution, as described in the CLSI document M27-A3 (10), using RPMI 1640 medium at pH 7.0 buffered with morpholinepropanesulfonic acid (MOPS). MIC endpoints were determined after 24 h based on a prominent decrease in growth compared to that of the drug-free growth control. Following the recommendations of the CLSI guidelines, C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as control strains and were included in each day of testing. The final CAS concentrations ranged from 0.125 to 64.0 μg/ml. In this assay, PG was defined as growth resurgence in the presence of drug at least two dilutions above the MIC.
MFC.
After MIC readings, 100-μl samples above the MIC from each well were withdrawn and plated in triplicate onto 90-mm Sabouraud dextrose agar (SDA) plates. The inoculated plates were incubated at 35°C, and MFC were recorded after 48 h. The MFC was defined as the lowest concentration of drug that reduced the size of the initial inoculum by at least 99%, as adapted from Barchiesi et al. (2).
Time-kill curve (TKC).
This methodology was adapted from Barchiesi et al. (3). The isolates were subcultured twice and grown for 24 h at 35°C on SDA plates. Three to five colonies of each strain were suspended in 10 ml of sterile distilled water, and the turbidity was adjusted spectrophotometrically to that of a 0.5 McFarland standard (approximately 1 × 106 to 5 × 106 CFU/ml). One milliliter of adjusted fungal suspension was added to 9 ml of RPMI 1640 medium buffered with MOPS plus an appropriate amount of CAS. For C. albicans and C. tropicalis, CAS was used at concentrations of 0.25, 0.5, 2.0, 16.0, and 32.0 μg/ml. For C. parapsilosis and C. orthopsilosis, CAS was used at 0.5, 1.0, 4.0, 16.0, and 32.0 μg/ml. Test solutions were incubated at 35°C, and at predetermined time points (0, 3, 6, 9, 12, 24, and 48 h), 100-μl aliquots were removed from each test solution. After 10-fold serial dilutions, a 100-μl aliquot from each dilution was streaked, in triplicate, onto Sabouraud dextrose agar plates for colony count determination after incubation at 35°C for 48 h. Fungicidal activity was considered to be achieved when the number of CFU/ml was reduced by >99% compared to that of the initial inoculum.
Cell wall isolation and carbohydrate composition analysis.
Candida cells obtained from 24-h growth in 50 ml of RPMI 1640 medium buffered with MOPS or in the same medium plus 16.0 μg/ml of CAS were used for cell wall component analysis, as described previously (26, 32, 35). The cells were washed three times in phosphate-buffered saline (PBS) and disrupted by >100 cycles of freezing and thawing, followed by agitation with glass beads at 425 to 600 μm in diameter (Sigma-Aldrich, Inc., St. Louis, MI) until no viable cells could be observed by subculture; disruption was also observed by light microscopy. The cell wall was isolated, dried, lyophilized, and frozen at −80°C. Isolated cell walls were alkali extracted three times for 60 min with 500 μl of 3% NaOH at 75°C. The pellet was washed once with 100 mM Tris-HCl (pH 7.5) and once more with 10 mM Tris-HCl (pH 7.5), then digested with 4 mg/ml Zymolyase 100T (Seikagaku Corporation, Tokyo, Japan) in 10 mM Tris-HCl (pH 7.5) overnight at 37°C, and centrifuged for 10 min at 20,000 × g at 4°C. The supernatant was split into two portions. One portion was used for hexose (β-1,3- plus β-1,6-glucan) quantification by the phenol-sulfuric method, as previously described (14, 26, 35), and the other portion was dialyzed against distilled water using a Spectra/Por membrane, with a molecular weight exclusion of 6,000 to 8,000 (Spectrum Medical Industries, Inc., Rancho Dominguez, CA), and its hexose content was measured (β-1,6-glucans, resistant to zymolyase treatment). The β-1,3-glucan value was determined by subtraction of the β-1,6-glucan value from the value representing the combination of β-1,3- plus β-1,6-glucan. Zymolyase-insoluble pellets were washed once with 10 mM Tris-HCl, pH 7.0, and water. The pellets were used for measurement of glucosamine content to determine chitin concentrations (24, 35). The pellets were hydrolyzed with 6 N HCl at 100°C for 6 h, and the hydrolyzates were freed from excess acid by repeated evaporation at reduced pressure. After removal of the acid, the pellets were resuspended in water, and the glucosamine content was measured and compared to a standard curve ranging from 0 to 200 mg of d-glucosamine, as previously described (24).
Microscopic analysis.
Candida cells obtained from a 24-h culture in RPMI 1640 with 16 μg/ml of CAS or without the drug were used for fluorescent microscopy, transmission electron microscopy (TEM), and scanning electron microscopy (SEM).
(i) Fluorescence microscopy.
The methodology used was adapted from that used by Walker et al. (36). Cells were collected by centrifugation, washed with PBS buffer at pH 7.2, and fixed in 10% (vol/vol) neutral buffered formalin (Sigma-Aldrich). Cells were stained with 5 μM calcofluor white (CFW) (Invitrogen, Oregon) at 37°C for 10 min. Aliquots of 10 μl were placed on glass slides and examined using a Olympus IX81 motorized microscope (Olympus, Tokyo, Japan) equipped with fluorescence. Because CFW bind specifically to chitin, this methodology was used to visualize the chitin in the cell wall. Images were digitally recorded using Cell^P imaging software and a DP-71 microscope digital camera (Olympus, Tokyo, Japan). Mean fluorescence intensities were calculated for at least 20 individual cells for each condition. The exposure time of fluorescence images was fixed to observe intensity differences between PG and control cells. The mean fluorescence intensities of the control cell and PG cells were compared using the Student t test (Microsoft Office Excel 2003). The significance level for the P value was considered to be <0.05.
(ii) SEM.
This methodology was adapted from that used by Bizerra et al. (4). The cells were fixed overnight at 4°C in 2.0% formaldehyde plus 2.5% glutaraldehyde buffered at pH 7.2 with 0.1 M sodium cacodylate. After fixation, small drops of the sample were placed on a specimen support coated with poly-l-lysine. Postfixation was carried out with 1% osmium tetroxide in cacodylate buffer for 1 h. Samples were then treated with 1% tannic acid for 45 min, washed three times with distilled water for 15 min, and treated again with 1% osmium tetroxide in cacodylate buffer for 1 h. Dehydration was carried out in a graded series of ethanol. The dehydrated samples were critical-point dried in CO2, coated with gold, and examined in a Jeol JSM-5300 scanning electron microscope. The perimeter of the cell was measured using Cell^P imaging software (Olympus, Tokyo, Japan). The mean of the cell perimeter was then calculated for at least 20 individual cells from each experimental condition. The perimeter values of control and PG cells were compared using the Student t test (Microsoft Office Excel 2003). The significance level for the P value was considered to be <0.05.
(iii) TEM.
Initially, cells were washed in PBS and fixed overnight at 4°C with 2.0% formaldehyde plus 2.5% glutaraldehyde buffered at pH 7.2 with 0.1 M sodium cacodylate. The fixed cells were postfixed in 1% osmium tetroxide for 1 h at room temperature. After dehydration in a graded series of ethanol and treatment with propylene oxide, the cells were embedded in Epon resin. Ultrathin sections (60 nm) were contrasted with uranyl acetate and lead citrate and examined using a transmission electron microscope (Jeol JEM-1200EX II).
RESULTS
CAS susceptibility and PG effect.
Of the 77 Candida bloodstream isolates initially screened, 42 isolates (54.5%) displayed the phenomenon of PG in the susceptibility test using caspofungin. Of the 42 isolates, two from each species (C. albicans, C. tropicalis, C. orthopsilosis, and C. parapsilosis) were selected for additional tests. CAS MICs generated by the broth microdilution assay were <2.0 μg/ml for the eight selected isolates (Table 1). It should be noted that the C. albicans and C. tropicalis strains showed MIC values between 0.25 and 0.5 μg/ml of CAS, whereas C. parapsilosis and C. orthopsilosis isolates showed MIC values of 1.0 μg/ml of CAS. The appearance of PG was confirmed in all eight selected isolates at CAS concentrations ranging from 8.0 to 32.0 μg/ml. Because all isolates showed PG at 16 μg/ml CAS (Table 1), this concentration was chosen for all subsequent experiments, including electron microscopy, fluorescence microscopy, and cell wall composition analysis.
TABLE 1.
Caspofungin MICs and PG patterns of Candida strains tested
| Species | Strain | CAS MIC (μg/ml) | Drug concn (μg/ml) producing PG |
|---|---|---|---|
| C. albicans | 1399 | 0.5 | 8.0 and 16.0 |
| 1954 | 0.25 | 16.0 | |
| C. tropicalis | 1507 | 0.25 | 8.0 and 16.0 |
| 1490A | 0.5 | 16.0 | |
| C. orthopsilosis | 1825 | 1.0 | 8.0, 16.0, and 32.0 |
| 1238A | 1.0 | 16.0 and 32.0 | |
| C. parapsilosis | 1856 | 1.0 | 16.0 and 32.0 |
| 1529A | 1.0 | 16.0 and 32.0 |
MFC.
The MFC was used to test the fungicidal activity of CAS and to confirm PG in the selected isolates. CAS showed fungicidal activity against the C. albicans and C. tropicalis strains at lower concentrations than those needed to show fungicidal activity against the other two strains. Table 2 shows that, in C. albicans and C. tropicalis, PG was detected at CAS concentrations between 8 and 32 μg/ml; the highest of these concentrations is at least three drug dilutions above the MFC. Against C. parapsilosis and C. orthopsilosis isolates (with the exception of the C. orthopsilosis 1238A isolate) CAS was only fungistatic; MFC was unable to detect PG in these strains (Table 2).
TABLE 2.
Growth distribution of Candida isolates in MFC tests at different CAS concentrations after 24 h of incubation in RPMI 1640
| Isolate | Growth at indicated CAS concn (μg/ml)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.12 | 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 | 16 | 32 | 64 | |
| C. albicans 1399 | + | + | − | − | − | − | − | + | + | − |
| C. albicans 1954 | + | + | − | − | − | − | − | + | + | − |
| C. tropicalis 1490A | + | + | − | − | − | − | + | + | − | − |
| C. tropicalis 1507 | + | + | − | − | − | − | + | + | − | − |
| C. orthopsilosis 1238A | + | + | + | + | − | − | + | + | + | − |
| C. orthopsilosis 1825 | + | + | + | + | + | + | + | + | + | + |
| C. parapsilosis 1529A | + | + | + | + | + | + | + | + | + | + |
| C. parapsilosis 1856 | + | + | + | + | + | + | + | + | + | + |
+, cell growth; −, growth inhibition (fungicidal activity).
TKCs.
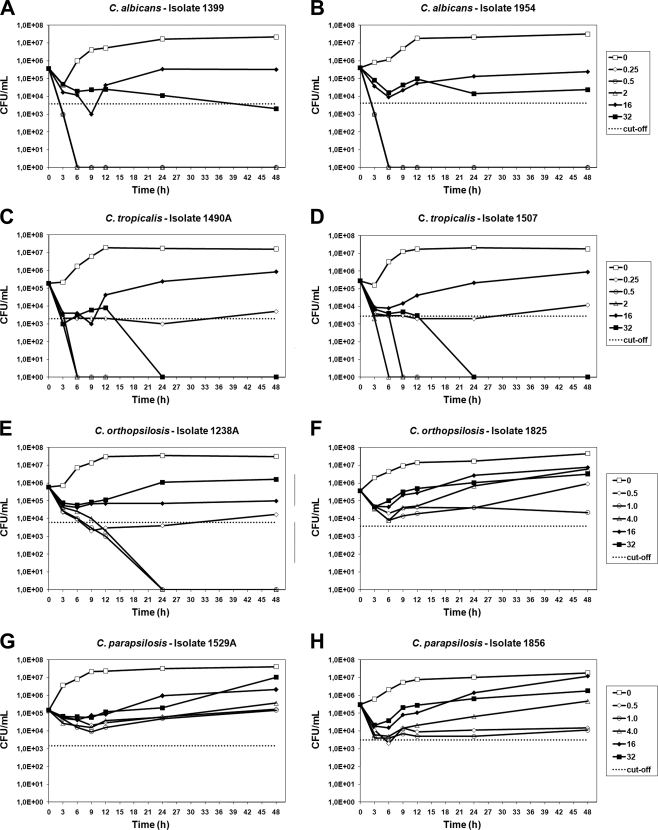
TKCs confirmed the occurrence of PG in all eight isolates tested (Fig. 1); however, some differences were observed among the strains tested. C. albicans and C. tropicalis strains showed similar patterns of TKCs in which the PG effect was characterized by fungicidal activity at low concentrations of CAS (0.25, 0.5, and 2.0 μg/ml) and fungistatic activity at the highest concentration (16.0 μg/ml). On the other hand, CAS at concentrations ranging from 0.25 to 32 μg/ml had only fungistatic activity on C. parapsilosis and C. orthopsilosis isolates, although an exception worth noting is the C. orthopsilosis 1238A isolate, which was killed at lower concentrations of CAS (2.0 and 4.0 μg/ml). In agreement with the MFC assay results, the TKC results showed that the PG effect occurred at concentrations ranging from 16 to 32 μg/ml. At drug concentrations that induced PG, a decrease in the CFU count was observed at early incubation times (3 to 6 h), followed by an increase in the CFU count at later incubation times (9 to 48 h). A concentration of 16 μg/ml CAS proved to be the most effective for producing the PG effect.
FIG. 1.
Time-kill curves of all tested Candida isolates: C. albicans (A and B), C. tropicalis (C and D), C. orthopsilosis (E and F), and C. parapsilosis (G and H). CAS concentrations used for each species are shown in the respective graphs. The dotted lines represent a >99% growth reduction compared with the initial inoculum sizes (fungicidal activities).
Alterations of cell wall components.
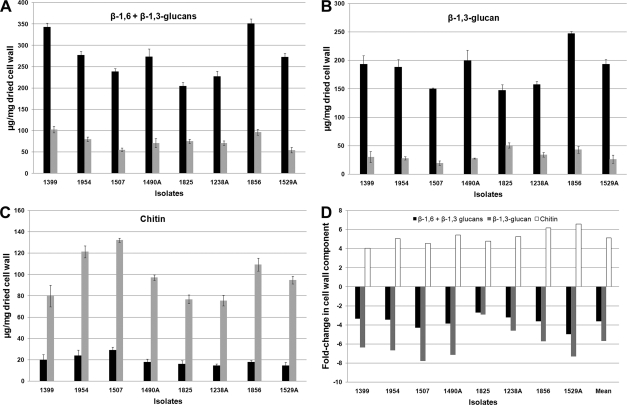
When cultivated in the presence of 16 μg/ml CAS, all Candida species analyzed showed similar cell wall chitin and glucan composition. Cells cultivated under these conditions showed a significant decrease in the total amount of cell wall glucan content (Fig. 2A) and a sharp decrease in the content of β-1,3-glucans (Fig. 2B). An increase in total chitin content was detected in all Candida species (Fig. 2C). Figure 2D shows that treatment of Candida species with CAS led to a decrease of 2.9- to 5.0-fold in β-1,3- plus β-1,6-glucan, a decrease of 2.7- to 7.8-fold in β-1,3-glucan alone, and an increase of 4.0- to 6.6-fold in chitin.
FIG. 2.
Cell wall components of control and PG cells. (A to C) The absolute values of β-1,6- plus β-1,3-glucan (A), β-1,3-glucan (B), and chitin (C) of the control and PG cells (black and gray bars, respectively) were expressed as the number of micrograms of carbohydrate per milligram of dried cell wall. (D) The graph represents the fold change in β-1,6- plus β-1,3-glucan (black), β-1,3-glucan (gray), and chitin (white) contents of PG cells compared to those of control cells.
Microscopic analysis. (i) Fluorescence microscopy.
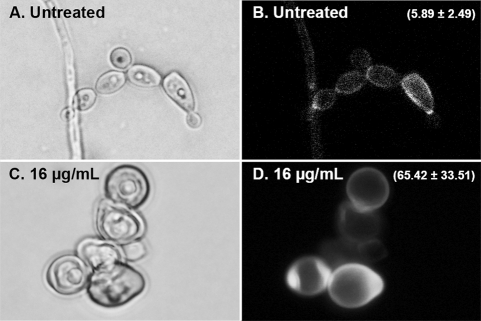
Figure 3 shows fluorescent micrographs of untreated and PG cells of C. albicans strain 1399 after 24 h of incubation. Because CFW binds specifically to chitin, the intensity of CFW fluorescence of cells was comparable to the results of chitin content determined by our assays of cell wall biochemistry. Control cells exhibited particularly intense fluorescence layered between mother cells and buds, with a more homogeneous and less intense fluorescent signal along the remaining surface of the cell. Compared to untreated cells, PG cells showed a more heterogeneous and intense fluorescence around the entire cell surface, with some regions exhibiting excess chitin, as shown by accumulation of fluorescence intensity (Fig. 3).
FIG. 3.
Fluorescence microscopy of C. albicans 1399 control (A and B) and PG (C and D) cells stained with calcofluor white (CFW). The value (mean ± standard deviation) of the CFW fluorescence intensity is related to the chitin content of the cell wall (B and D). PG cells showed regions exhibiting the accumulation of chitin and higher fluorescence intensity values than those of control cells (P < 0.05).
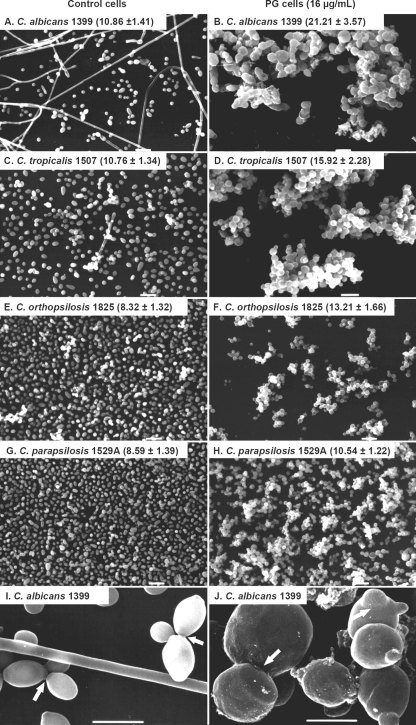
(ii) SEM.
As shown in Fig. 4, PG cells exhibited morphological abnormalities that included (i) budding cells without clear rings around the constrictions between mother and daughter cells, (ii) enlarged yeasts forming clumps, and (iii) the absence of filamentation. These changes were similarly found in the four Candida species studied. Control cells showed a normal elliptical shape, budding cells with clear constriction rings, and the presence of filamentation. Determination of the size of the cell perimeter confirmed the differences between control and PG cells of C. albicans (10.86 ± 1.41 and 21.21 ± 3.57 μm, respectively), C. tropicalis (10.76 ± 1.34 and 15.92 ± 2.28 μm, respectively), C. orthopsilosis (8.32 ± 1.32 and 13.21 ± 1.66 μm, respectively), and C. parapsilosis (8.59 ± 1.39 and 10.54 ± 1.22 μm, respectively). In all cases, the differences were statistically significant (P < 0.001). The average perimeter size of control cells of all Candida species was 9.56 ± 1.83 μm, whereas PG cells showed an average perimeter size of 15.22 ± 4.56 μm (P < 0.001).
FIG. 4.
Scanning electron micrographs of Candida strains, comparing control cells and PG cells grown with 16 μg/ml of CAS. The values represent the perimeters of cells (in μm; mean ± standard deviation). PG cells were larger in size than control cells (P < 0.01). Panels I and J show higher magnifications of panels A and B, respectively; the ring around the constriction between mother cell and bud was noted only in control cells (arrows). Scale bars, 10 μm (A to H) and 5 μm (I and J).
(iii) TEM.
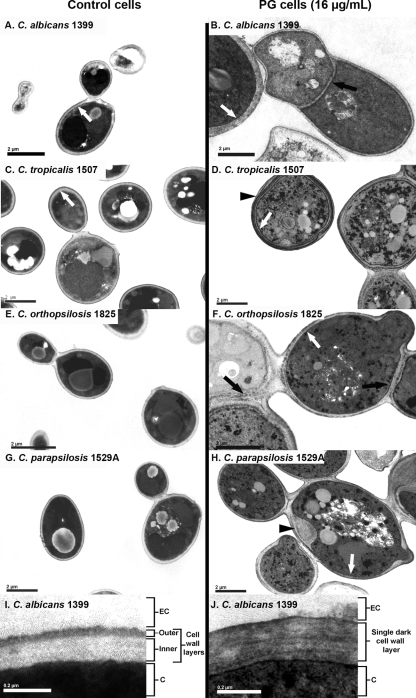
The morphological changes observed in PG cells by TEM are related mainly to the cell wall structure and formation of the budding septa (Fig. 5). An abnormal thickness of the septum was observed between mother and daughter cells, with no clear separation between the cells (Fig. 5B and F, black arrows). The cell walls of control and PG cells presented remarkable differences in morphology (Fig. 5). By means of conventional TEM, we observed that control cells exhibited a cell wall with two layers: (i) an electron-dense outer layer and (ii) an inner layer of low electron density, continuous with the plasma membrane (Fig. 5I). In PG cells, a decrease in the thickness of the inner layer of the cell wall was observed, with a predominance of the more-electron-dense layer (Fig. 5B, D, F, H, and J, white arrows). Some PG cells showed what seemed to be multilayered cell walls or a focal enlargement of the cell walls (Fig. 5D and H, black arrowheads, respectively).
FIG. 5.
Transmission electron micrographs of Candida strains, comparing control cells and PG cells grown with 16 μg/ml of CAS. Black arrows indicate the abnormal septa in PG cells. White arrows show the cell walls of control cells, which exhibited two layers, with a predominance of the more-electron-dense layer in PG cells. Black arrowheads show a multilayered cell wall (D) and a focal enlargement of the cell wall (H) in PG cells. Panels I and J show higher-magnification electron micrographs of control and PG cells, respectively. (I) As described above, control cells exhibited cell walls with two layers, an electron-dense outer layer and an inner layer of low electron density. (J) In PG cells, a decrease of inner cell wall layers and a predominance of the more-electron-dense layers were observed. EC, extracellular environment; C, cytosol. Scale bars, 2 μm (A to H) and 0.2 μm (I and J).
DISCUSSION
Echinocandins have remarkable in vitro activity against Candida spp., including azole-resistant isolates (11, 23, 31). However, the emergence of echinocandin resistance during treatment of Candida infections has been reported, and such resistance is mostly due to mutations in the FKS1 gene (1, 8, 16, 17, 19).
In addition to the possibility that Candida may acquire resistance to echinocandins both in vitro and in vivo by mutation, it has been shown that PG is not an uncommon phenomenon in Candida isolates (5, 7, 15, 33). Melo et al. (22) studied the PG of a number of Candida species in the presence of CAS, reporting PG frequencies of 83%, 43%, 37.5%, and 25% for C. tropicalis, C. parapsilosis, C. orthopsilosis, and C. albicans, respectively, in the presence of the drug. Chamilos et al. (7) observed that, when cells were grown in the presence of CAS, the prevalence of PG was 90%, 60%, and 40% for C. parapsilosis, C. albicans, and C. tropicalis, respectively.
PG has been demonstrated by other authors using a variety of assays, particularly broth microdilution tests for detecting MICs and MFCs of echinocandins against Candida spp. (7, 15, 22). Soczo et al. (33) reported that for C. tropicalis isolates, PG in CAS was more frequently observed using MFC assays (93%) than MIC tests (13%). In our study, MFC assays were helpful in identifying PG for only the C. albicans and C. tropicalis isolates. Considering that CAS is usually fungistatic against C. parapsilosis and C. orthopsilosis strains (3), time-kill curves were more appropriate than MFC assays for identifying PG of Candida strains in the presence of high concentrations of CAS, especially for C. parapsilosis and C. orthopsilosis isolates.
Previous reports on the PG effect in the presence of echinocandins have focused on differences among C. albicans strains. In this study, we have described the ultrastructural and cell wall biochemical changes that occur in isolates representative of four clinically relevant Candida species, C. albicans, C. tropicalis, C. orthopsilosis, and C. parapsilosis, during PG induced by high concentrations of CAS. Our results allow us to conclude that PG of non-albicans Candida strains induces the same ultrastructural and biochemical pattern of cell wall changes as that in C. albicans isolates. PG cells exhibited a significant decrease in β-1,3-glucan content (from 2.7- to 7.8-fold) and a substantial increase in chitin content (from 4.0- to 6.6-fold) of the cell wall, regardless of the type of Candida species considered.
With respect to the ultrastructure of PG cells, non-albicans Candida species and C. albicans strains displayed similar morphology, including enlarged blastospores, clumping of cells, the absence of filamentation, and abnormalities in constriction rings between mother and daughter cells. In the cell wall, untreated cells clearly exhibited two different layers, an outer cell wall layer with high electron density and an inner layer that is less electron dense. PG cells showed a decrease in the inner cell wall layer, which according to Chaffin et al. (6), is composed of β-1,3-glucan. Similar results were described by Nishiyama et al. (27) when testing micafungin against C. albicans strains. It is therefore reasonable to suppose that the observed reduction in the inner cell wall layer is related to the inhibition of β-1,3-glucan synthesis caused by exposition to echinocandins. Our conclusions are supported by fluorescence microscopy analysis of cell walls stained with CFW, in which control cells presented less intense fluorescence and PG cells showed a strong fluorescence with CFW. These data clearly indicate that β-1,3-glucan is replaced by chitin in PG cells.
Munro et al. (25) demonstrated that chitin synthesis is upregulated under conditions in which the integrity of the cell wall is compromised by antifungal treatments. Walker et al. (36) showed that low levels of echinocandins stimulated chitin synthase (CHS) gene expression. Thus, the production of high levels of chitin seems to reduce the efficacy of echinocandins. It is therefore suggested that an increase in chitin synthesis during CAS treatment may play a key role in mediating resistance to echinocandins.
It is not yet fully understood whether the morphological and biochemical modifications exhibited by PG cells confer a fitness disadvantage or contribute to antifungal therapy failure during treatment with high doses of echinocandin. It is well known that β-1,3-glucan plays a critical role in the maintenance of cell shape, mechanical rigidity, and resistance to osmotic pressure (6). The reduction in β-1,3-glucan observed in PG cells suggests that PG cells are osmotically fragile, resulting in morphological alteration; this could possibly lead to a fitness disadvantage for these cells. Moreover, it is known that in yeast, virulence factors are expressed mainly during filamentous growth stages. Because PG cells do not present a filamentous form, this could justify a decrease in virulence factor expression and a consequent loss of infectivity in these cells.
The clinical significance of the occurrence of PG during echinocandin therapy is not well understood. Although animal models have failed to demonstrate PG in murine models of systemic Candida infection (9, 21), these data require further detailed analysis. It is worth mentioning that a randomized comparative study of two different micafungin dosages conducted by Pappas et al. (28) clearly demonstrated that candidemic patients treated with high doses of micafungin (150 mg/day) did not show any improvement compared to patients treated with a regular dosage (100 mg/day). Those authors reported that 14 of 30 patients (47%) with deep-seated infections treated with 150 mg/day micafungin had treatment failure compared to only 6 of 28 patients (21%) in the 100 mg/day micafungin group and suggested that the lower rate of success among patients treated with 150 mg/day micafungin could possibly be explained by the PG effect. Further studies using noncandidemic models will be needed to determine whether PG contributes to human disease in vivo.
Although the clinical significance of PG remains unclear, the elucidation of the morphological, biochemical, and molecular pathways underlying this phenomenon is likely to contribute to a better understanding of novel cellular mechanisms that may be involved in rescue against echinocandin antifungal activity.
Acknowledgments
This study was sponsored by the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil; grant 2007/04925-8). A.L.C. received a grant from CNPq (304802/2007-7).
Caspofungin was kindly provided by Merck Sharp & Dohme.
Footnotes
Published ahead of print on 8 November 2010.
REFERENCES
- 1.Balashov, S. V., S. Park, and D. S. Perlin. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob. Agents Chemother. 50:2058-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi, F., E. Spreghini, S. Tomassetti, D. Arzeni, D. Giannini, and G. Scalise. 2005. Comparison of the fungicidal activities of caspofungin and amphotericin B against Candida glabrata. Antimicrob. Agents Chemother. 49:4989-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barchiesi, F., E. Spreghini, S. Tomassetti, A. Della Vittoria, D. Arzeni, E. Manso, and G. Scalise. 2006. Effects of caspofungin against Candida guilliermondii and Candida parapsilosis. Antimicrob. Agents Chemother. 50:2719-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizerra, F. C., C. V. Nakamura, C. de Poersch, T. I. Estivalet Svidzinski, R. M. Borsato Quesada, S. Goldenberg, M. A. Krieger, and S. F. Yamada-Ogatta. 2008. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 8:442-450. [DOI] [PubMed] [Google Scholar]
- 5.Canton, E., J. Peman, M. Romero, A. Valentin, and M. Gobernado. 2007. The fungicidal activity and paradoxical effect of caspofungin against yeast. Influence of culture medium and incubation time. Rev. Esp. Quimioter. 20:433-441. (In Spanish.) [PubMed] [Google Scholar]
- 6.Chaffin, W. L., J. L. Lopez-Ribot, M. Casanova, D. Gozalbo, and J. P. Martinez. 1998. Cell wall and secreted proteins of Candida albicans: identification, function, and expression. Microbiol. Mol. Biol. Rev. 62:130-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons, K. V., M. Espiritu, R. Parmar, and D. A. Stevens. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI). 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, third ed. Approved standard, M27-A3. CLSI, Wayne, PA.
- 11.da Matta, D. A., L. P. de Almeida, A. M. Machado, A. C. Azevedo, E. J. Kusano, N. F. Travassos, R. Salomao, and A. L. Colombo. 2007. Antifungal susceptibility of 1000 Candida bloodstream isolates to 5 antifungal drugs: results of a multicenter study conducted in Sao Paulo, Brazil, 1995-2003. Diagn. Microbiol. Infect. Dis. 57:399-404. [DOI] [PubMed] [Google Scholar]
- 12.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 13.Douglas, C. M. 2001. Fungal beta(1,3)-d-glucan synthesis. Med. Mycol. 39(Suppl. 1):55-66. [DOI] [PubMed] [Google Scholar]
- 14.Dubois, M., K. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1951. A colorimetric method for the determination of sugars. Nature 168:167. [DOI] [PubMed] [Google Scholar]
- 15.Fleischhacker, M., C. Radecke, B. Schulz, and M. Ruhnke. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127-131. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Effron, G., D. P. Kontoyiannis, R. E. Lewis, and D. S. Perlin. 2008. Caspofungin-resistant Candida tropicalis strains causing breakthrough fungemia in patients at high risk for hematologic malignancies. Antimicrob. Agents Chemother. 52:4181-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncalves, S. S., C. S. Amorim, M. Nucci, A. C. Padovan, M. R. Briones, A. S. Melo, and A. L. Colombo. 2010. Prevalence rates and antifungal susceptibility profiles of the Candida parapsilosis species complex: results from a nationwide surveillance of candidaemia in Brazil. Clin. Microbiol. Infect. 16:885-887. [DOI] [PubMed] [Google Scholar]
- 19.Kahn, J. N., G. Garcia-Effron, M. J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 51:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letscher-Bru, V., and R. Herbrecht. 2003. Caspofungin: the first representative of a new antifungal class. J. Antimicrob. Chemother. 51:513-521. [DOI] [PubMed] [Google Scholar]
- 21.Marine, M., F. J. Pastor, I. H. Sahand, J. Ponton, G. Quindos, and J. Guarro. 2009. Paradoxical growth of Candida dubliniensis does not preclude in vivo response to echinocandin therapy. Antimicrob. Agents Chemother. 53:5297-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melo, A. S., A. L. Colombo, and B. A. Arthington-Skaggs. 2007. Paradoxical growth effect of caspofungin observed on biofilms and planktonic cells of five different Candida species. Antimicrob. Agents Chemother. 51:3081-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messer, S. A., D. J. Diekema, L. Boyken, S. Tendolkar, R. J. Hollis, and M. A. Pfaller. 2006. Activities of micafungin against 315 invasive clinical isolates of fluconazole-resistant Candida spp. J. Clin. Microbiol. 44:324-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan, W. T., and L. A. Elson. 1934. A colorimetric method for the determination of N-acetylglucosamine and N-acetylchrondrosamine. Biochem. J. 28:988-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munro, C. A., S. Selvaggini, I. de Bruijn, L. Walker, M. D. Lenardon, B. Gerssen, S. Milne, A. J. Brown, and N. A. Gow. 2007. The PKC, HOG and Ca2+ signalling pathways co-ordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63:1399-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nett, J., L. Lincoln, K. Marchillo, R. Massey, K. Holoyda, B. Hoff, M. VanHandel, and D. Andes. 2007. Putative role of beta-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama, Y., K. Uchida, and H. Yamaguchi. 2002. Morphological changes of Candida albicans induced by micafungin (FK463), a water-soluble echinocandin-like lipopeptide. J. Electron Microsc. (Tokyo) 51:247-255. [DOI] [PubMed] [Google Scholar]
- 28.Pappas, P. G., C. M. Rotstein, R. F. Betts, M. Nucci, D. Talwar, J. J. De Waele, J. A. Vazquez, B. F. Dupont, D. L. Horn, L. Ostrosky-Zeichner, A. C. Reboli, B. Suh, R. Digumarti, C. Wu, L. L. Kovanda, L. J. Arnold, and D. N. Buell. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883-893. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., L. Boyken, R. J. Hollis, J. Kroeger, S. A. Messer, S. Tendolkar, and D. J. Diekema. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, K. P. Ng, A. Colombo, J. Finquelievich, R. Barnes, and J. Wadula. 2008. Geographic and temporal trends in isolation and antifungal susceptibility of Candida parapsilosis: a global assessment from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 46:842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., S. A. Messer, L. Boyken, C. Rice, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2003. Caspofungin activity against clinical isolates of fluconazole-resistant Candida. J. Clin. Microbiol. 41:5729-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popolo, L., D. Gilardelli, P. Bonfante, and M. Vai. 1997. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1delta mutant of Saccharomyces cerevisiae. J. Bacteriol. 179:463-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soczo, G., G. Kardos, I. Varga, B. Kelentey, R. Gesztelyi, and L. Majoros. 2007. In vitro study of Candida tropicalis isolates exhibiting paradoxical growth in the presence of high concentrations of caspofungin. Antimicrob. Agents Chemother. 51:4474-4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker, L. A., C. A. Munro, I. de Bruijn, M. D. Lenardon, A. McKinnon, and N. A. Gow. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiederhold, N. P. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr. Opin. Infect. Dis. 20:574-578. [DOI] [PubMed] [Google Scholar]
- 38.Wiederhold, N. P., D. P. Kontoyiannis, R. A. Prince, and R. E. Lewis. 2005. Attenuation of the activity of caspofungin at high concentrations against Candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 49:5146-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]