Abstract
Ascaris suum eggs were inactivated in distilled water and digested sludge by butanoic, pentanoic, and hexanoic acids. The fatty acids (short-chain fatty acids [SCFA]) were effective only when protonated and at sufficient concentrations. The conjugate bases were not effective at the concentrations evaluated. Predictions from an inhibition model (50% inhibitory concentration [IC50]) based on quantitative structure-activity relationships were congruent with inactivation data.
The nematode Ascaris lumbricoides releases highly resistant, unembryonated eggs into the environment, causing ∼1.3 billion illnesses worldwide (12). The swine parasite Ascaris suum is routinely used as a surrogate for the human parasite (22) and is often found in sludges. Due to its resistance to biocontrol mechanisms (6) Ascaris is a model organism for developing environmentally safe disinfection methods (7, 22). The eggs can be rendered nonviable through natural processes using extreme heat (>40°C) or with UV radiation (4). The use of short-chain fatty acids (SCFA) is another possible method of controlling Ascaris. The toxicity of SCFA to bacteria, e.g., Escherichia coli (9), Streptococcus, and Staphylococcus (20), fungi (14, 23), insects (15), and birds (13) has been reported. Izumi (17) reported that acetic acid had limited ability to permeate the shells of Ascaris eggs. SCFA have also been used as an additive to disinfect silage or food and have been used to sanitize animal carcasses by spraying or dipping (8). In some cases, the action of the SCFA has been attributed to diffusion across the membrane, disassociation, and acidification of the cell interior (1, 3, 11). The work reported herein presents an evaluation of the efficacy of SCFA type, concentration, and pH on inactivation of Ascaris eggs in water and sludge. A model was developed that can predict the toxicity of SCFA-based concentration and pH.
Unembryonated A. suum eggs were collected from the intestinal contents of naturally infected pigs and cleaned (10). One thousand eggs were mixed with SCFA (the SCFA stock solutions were at their maximum water solubility without the use of a cosolvent) to a final volume of 1.0 ml. The reaction mixture was statically incubated at 37°C for 20 h. The eggs were collected by centrifugation, washed three times with phosphate buffer (pH 7.0, 10 mM), mixed with 0.5% Formalin solution and water, placed in a 24-well culture plate, and incubated at 28°C for 20 or 21 days. The outer coating was removed with 0.6% hypochlorite solution, and the eggs were scored as larvated (viable) or nonlarvated (not viable). Unless otherwise stated, triplets were conducted. A. suum eggs (40,000) were mixed with untreated sludge collected from the final stage (prior to dewatering) of an anaerobic digester (Syracuse, NY) at pH 4 (100-ml final volume) and sludge amended with butanoic (1.35 M), propanoic (1.5 M), or pentanoic (0.2 M) acid. The sludge samples were incubated at 37°C for 20 h, 1 week, and 1 month. After the prescribed exposure period, 20 ml of sludge was removed and eggs were separated from the sludge by magnesium sulfate (specific gravity, 1.2) flotation and then washed and incubated at 28°C for 14 days as described above. After incubation, the eggs were decorticated and scored for viability. Unless otherwise stated, all experiments were conducted in triplicate.
Butanoic, pentanoic, and hexanoic acids all reduced the viability of the Ascaris eggs to below detectable limits (Table 1). The effect was most pronounced at pH values below the pKa of the SCFA, and none of the SCFA were effective at pH 7 (data not shown). Mixtures of butanoic and pentanoic acids were also effective only at a low pH and at relatively high concentrations (Table 1). In the presence of sludge the SCFA were still effective at inactivating Ascaris eggs (Table 2). However, in this case propanoic acid exhibited toxicity at 20 h and completely inactivated the eggs after 7 days. Acetic acid was tested at 0.1, 0.5, 1.0, and 1.5 M and pH 2, 4, 4.5, 5, 6, and 7, while propanoic acid was tested at 1.5 M and pH 4 and 7. Neither acid appeared to be capable of reducing the viability below the control levels (data not shown). Heptanoic acid (6.3−6, 1.0−5, 1.0−4, and 1.9−3 M and pH 3.7, 5.1, 6.1, 6.5, and 7.2), octanoic acid (4.72−4 M, pH 4.2), and nonanoic acid (1.0−4 M, pH 4.4) also did not appear capable of inactivating the eggs (data not shown).
TABLE 1.
Percent viability of Ascaris eggs after exposure to fatty acids for 20 h
| Acid(s) | pKa | Molarity | Percent viability ± SD (pH) of Ascaris eggs at pH rangea |
|||
|---|---|---|---|---|---|---|
| 2.0-4.0 | 4.0-4.5 | 4.5-5.0 | 5.0-6.0 | |||
| Butanoic | 4.76 | 0.1 | 91.6 ± 2.5 (2.0) | 88.4 ± 1.7 a (4.0) | 90.8 ± 1.1 (4.5) | 91.7 ± 1.3 (5.0) |
| 1 | 0.0 ± 0.0 a (2.0) | 0.0 ± 0.1 a (4.0) | 0.1 ± 0.1 a (4.5) | 1.2 ± 0.3 a (5.0) | ||
| 1.5 | 0.0 ± 0.0 a (2.0) | 0.0 ± 0.0 a (4.0) | 0.1 ± 0.1 a (4.5) | 0.0 ± 0.1 a (5.0) | ||
| Pentanoic | 4.78 | 6.3E-06 | 91.8 ± 1.6 (3.5) | 89.9 ± 1.1 | 91.9 ± 0.2 (4.5) | 93.6 ± 1.2 (5.0) |
| 0.01 | 88.4 ± 2.1 a (2.9) | 89.9 ± 1.7 (4.0) | 91.0 ± 2.6 (4.5) | 88.6 ± 2.2 (5.0) | ||
| 0.1 | 86.4 ± 1.0 a (2.7) | 92.7 ± 1.8 (4.0) | 90.1 ± 1.0 (4.5) | 87.1 ± 3.0 a (5.0) | ||
| 0.2 | 0.3 ± 0.3 (2.5) | 0.2 ± 0.1 (4.0) | 90.5 ± 1.4 (4.5) | 90.8 ± 0.5 (5.0) | ||
| Hexanoic | 4.85 | 6.6E-06 | NDb | ND | ND | 89.3 ± 0.5 (5.4) |
| 0.0001 | ND | 91.2 ± 0.7 (4.4) | 88.2 ± 1.4 (4.5) | 89.3 ± 0.5 (5.1) | ||
| 0.001 | 85.9 ± 1.0 (3.4) | 91.1 ± 1.2 (4.0) | 89.2 ± 2.0(4.5) | 90.8 ± 1.8 a (5.0) | ||
| 0.0378 | 0.1 ± 0.2 a (3.1) | 0.3 ± 0.1 a (4.0) | 5.3 ± 0.7 a (4.5) | 91.9 ± 1.9 a (5.0) | ||
| Butanoic and pentanoicc | 4.76/4.78d | 1.36/0.2d | ND | 0.0033 ± 0.0058 b (4.0) | ND | 90.0 ± 3.6 (6.0) |
| 0.136/0.2d | ND | 1.98 ± 0.24 b (4.0) | ND | ND | ||
| 1.36/0.02d | ND | 0.0033 ± 0.0058 b (4.0) | ND | ND | ||
| 0.136/0.02d | ND | 91.38 ± 2.2 (4.0) | ND | ND | ||
Letters after viability measurements denote statistically significant differences among the percentages of viable eggs when compared to the water controls which were conducted with each round of testing (data not shown). The overall average percent survival for the water controls was 90.3 ± 2.7%. Data marked “a” were tested with the Proc Mix of SAS (P < 0.01). Data marked “b” were tested with the Student t test (P < 0.05). SD, standard deviation.
ND, not done.
The concentrations tested were chosen to reflect high concentrations and low concentrations.
Values refer to butanoic acid and pentanoic acid, respectively.
TABLE 2.
Percent viability of Ascaris eggs after exposure to fatty acids in sludge and incubation at 37°C
| Acid or sludge type | Molarity | % of Ascaris eggs viablea (±SD) after: |
|
|---|---|---|---|
| 0.83 days | 7 days | ||
| Propanoic | 1.5 | 39.7 ± 3.1 a | 0 ± 0 b |
| Butanoic | 1.35 | 0 ± 0 a | 0 ± 0 b |
| Pentanoic | 0.2 | 0 ± 0 a | 0 ± 0 b |
| Sludge control | None | 92.3 ± 1.7 | 83.8 ± 0.4 |
| Sludge, pH 4 | None | 93.3 ± 1.8 | 82.8 ± 7.2 |
Letters a and b denote statistically significant differences among the percentage of viable eggs when compared to the sludge control at pH 4 as tested via the Student t test (P < 0.05). SD, standard deviation.
The toxicity of the SCFA is dependent on several factors, including carbon chain length, SCFA concentration, pH, and Kow (hydrophobicity). These factors, to determine nonreactive toxicity, were modeled with quantitative structure-activity relationships (QSAR). Nonreactive toxicity is related to the quantity of toxicant acting upon an organism and not directly associated with a specific mechanism (2). Blum and Speece (2) reported on numerous studies that successfully related aquatic toxicology on a surrogate organism to the logarithm of the octanol-water partitioning coefficient (log Kow). The inhibition of the acids on Ascaris was modeled using a surrogate organism (Photobacterium phosphoreum) according to the relationship reported by Kamlet et al. (18) as follows:
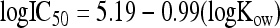 |
The 50% inhibitory concentration (IC50) is defined as the concentration of SCFA required to inactivate 50% of the Ascaris eggs. The values of Kow were determined according to the method of Lyman (19), and changes in Kow as a function of pH were determined as follows (16):
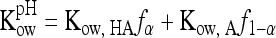 |
where fα and f1−α are the fractions of neutral and ionized SCFA species, respectively, in aqueous solution. Because a surrogate organism was used, Ascaris inactivation data were not required to calibrate the model, a significant advantage of the QSAR model (2).
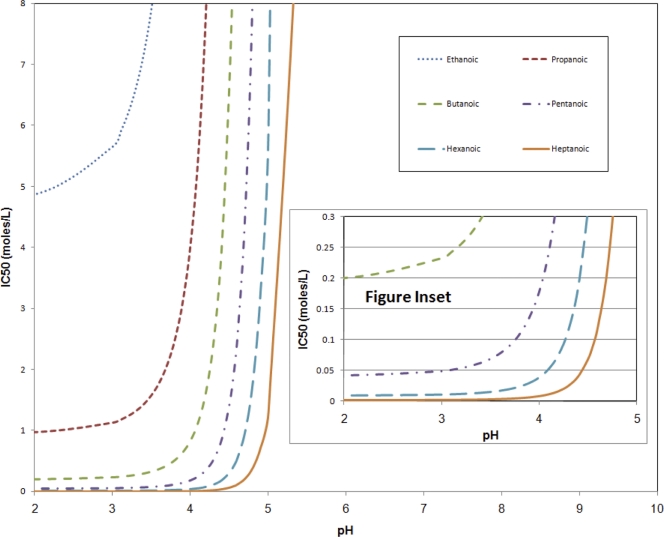
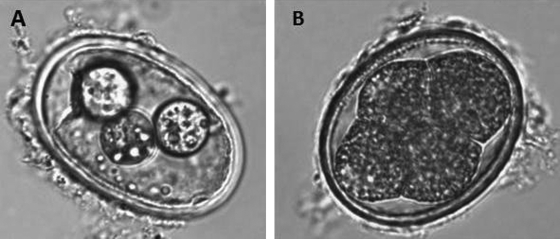
Figure 1 presents the required SCFA concentration to achieve inactivation of 50% (IC50) for Ascaris as a function of pH. The model predicts a clear concentration barrier that must be reached in order for the SCFA to be toxic, and that concentration increases substantially at pH values slightly above the pKa. The inactivation data in the tables and data not shown (e.g., octanoic acid) are congruent with the model predictions at pH values less than 4.5: all SCFA concentrations that were above and to the left of the model predictions resulted in a statistically significant inactivation greater than the IC50 of the Ascaris eggs, and concentrations below and to the right resulted in inactivation less than the IC50. Blum and Speece (2) reported that a reasonable goal for the accuracy of a QSAR in predicting toxicity to bacteria is approximately one order of magnitude (concentration), based on the variability of toxicity tests and applying those tests to a field application. The performance of the model is consistent with this goal at a pH less than 4.5. The model predictions are also consistent with inactivation data for pH values above 4.5 with the exception of butanoic acid. In addition, mixtures of butanoic and pentanoic acids were not effective until they passed the threshold concentration (Fig. 1 and Table 1). These findings are consistent with those of Paggi and Fay (21), where acetic acid was less effective than propanoic and butanoic acids against Streptococcus bovis. The authors speculated that it was due to the higher pKa and lower hydrophobicity of the former. The greater effect of the SCFA at lower pHs is likely due to the increased ability of the SCFA to permeate the membrane. The concentration of SCFA needed to decrease the growth by 50% decreased with increasing Kow. The interaction between the protonated fatty acids and the eggs is likely hydrophobic, since the eggs (with and without the outer coat) are hydrophobic and lack electron acceptors (5). This explains the lack of effect by the deprotonated conjugate base. In addition, visual examination of some eggs showed that some of the internal components were disrupted, which indicates the SCFA may have altered the cell membranes (Fig. 2). This phenomenon was not quantified.
FIG. 1.
Model of Ascaris suum inhibition (IC50, moles/liter) as a function of pH. Model predictions were based on a quantitative structure-activity model (QSAR) according to a relationship reported by Kamlet et al. (18).
FIG. 2.
(A) An Ascaris suum egg exposed to SCFA and exhibiting morphological damage to the interior cells. (B) A healthy A. suum egg.
Based on the results of this work, selected SCFA were effective in the inactivation of Ascaris eggs in the protonated form and at sufficient concentrations. QSAR models are capable of predicating the inhibitory effects of SCFA. Butanoic and pentanoic acids can be added to sludge as a beneficial treatment to reduce the survival of Ascaris eggs prior to land application.
Acknowledgments
We gratefully acknowledge the technical assistance of Jarod Ravich, Kathleen Danskin, and Ashleigh Davis, Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell University, Ithaca, NY.
This project was partially supported by a grant from the Defense Threat Reduction Agency.
Footnotes
Published ahead of print on 5 November 2010.
REFERENCES
- 1.Baronofsky, J. J., W. J. A. Schreurs, and E. R. Kashket. 1984. Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl. Environ. Microbiol. 48:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum, D. J. W., and R. E. Speece. 1990. Determining chemical toxicity to aquatic species. Environ. Sci. Technol. 24:284-293. [Google Scholar]
- 3.Booth, I. R. 1985. Regulation of cytoplasmic pH in bacteria. Microbiol. Mol. Biol. Rev. 49:359-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownell, S. A., and K. L. Nelson. 2006. Inactivation of single-celled Ascaris suum eggs by low-pressure UV radiation. Appl. Environ. Microbiol. 72:2178-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capizzi, S., and J. Schwartzbrod. 2001. Surface properties of Ascaris suum eggs: hydrophobic potential and Lewis acid-base interactions. Colloids and Surfaces B: Biointerfaces 22:99-105. [DOI] [PubMed] [Google Scholar]
- 6.Capizzi-Banas, S., M. Deloge, M. Remy, and J. Schwartzbrod. 2004. Liming as an advanced treatment for sludge sanitisation: helminth eggs elimination—Ascaris eggs as model. Water Res. 38:3251-3258. [DOI] [PubMed] [Google Scholar]
- 7.Capizzi-Banas, S., and J. Schwartzbrod. 2001. Irradiation of Ascaris ova in sludge using an electron beam accelerator. Water Res. 35:2256-2260. [DOI] [PubMed] [Google Scholar]
- 8.Cherrington, C. A., M. Hinton, G. C. Mead, and I. Chopra. 1991. Organic acid: chemistry, antibacterial activity and practical applications. Adv. Microbial Physiol. 32:87-108. [DOI] [PubMed] [Google Scholar]
- 9.Cherrington, C. A. M. H., G. R. Pearsonand, and I. Chopra. 1991. Inhibition of Escherichia coli K12 by short-chain organic acids: lack of evidence for induction of the SOS response. J. Appl. Bacteriol. 70:156-160. [DOI] [PubMed] [Google Scholar]
- 10.Collick, A. S., S. Inglis, P. Wright, T. S. Steenhuis, and D. D. Bowman. 2007. Inactivation of Ascaris suum in a biodrying compost system. J. Environ. Qual. 36:1528-1533. [DOI] [PubMed] [Google Scholar]
- 11.Dashper, S. G., and E. C. Reynolds. 2000. Effects of organic acid anions on growth, glycolysis, and intracellular pH of oral streptococci. J. Dent. Res. 79:90-96. [DOI] [PubMed] [Google Scholar]
- 12.de Silva, N. R., M. S. Chan, and D. A. P. Bundy. 1997. Morbidity and mortality due to ascariasis: re-estimation and sensitivity analysis of global numbers at risk. Trop. Med. Int. Health 2:519-528. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson, W. E., and B. L. Fites. 1970. Embryo mortality in quail induced by cyclopropene fatty acids: reduction by maternal diets high in unsaturated fatty acids. J. Nutr. 100:605-610. [DOI] [PubMed] [Google Scholar]
- 14.Hatton, P. V., and J. L. Kinderlerer. 1991. Toxicity of medium chain fatty acids to Penicillium crustosum Thom and their detoxification to methyl ketones. J. Appl. Microbiol. 70:401-407. [Google Scholar]
- 15.House, H. L. 1967. The nutritional status and larvicidal activities of C6- to C14-saturated fatty acids in Pseudosarcophaga affinis (Diptera: Sarcophagidae). Can. Entomol. 99:384-392. [Google Scholar]
- 16.Hyun, S., and L. S. Lee. 2004. Hydrophilic and hydrophobic sorption of organic acids by variable-charge soils: effect of chemical acidity and acidic functional group. Environ. Sci. Technol. 38:5413-5419. [DOI] [PubMed] [Google Scholar]
- 17.Izumi, S. 1952. Biological studies on Ascaris eggs. Jpn. Med. J. 5:21-36. [DOI] [PubMed] [Google Scholar]
- 18.Kamlet, M. J., R. M. Doherty, G. D. Veith, R. W. Taft, and M. H. Abraham. 1986. Solubility properties in polymers and biological media. 7. An analysis of toxicant properties that influence inhibition of bioluminescence in Photobacterium phosphoreum (the Microtox test). Environ. Sci. Technol. 20:690-695. [DOI] [PubMed] [Google Scholar]
- 19.Lyman, W. J. 1982. Octanol/water partition coefficient, p. 1-51. In W. J. Lyman, W. F. Reehl, and D. H. Rosenblatt (ed.), Handbook of chemical property estimation methods: environmental behavior of organic compounds. McGraw Hill, New York, NY.
- 20.Nair, M. K. M., J. Joy, P. Vasudevan, L. Hinckley, T. A. Hoagland, and K. S. Venkitanarayanan. 2005. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J. Dairy Sci. 88:3488-3495. [DOI] [PubMed] [Google Scholar]
- 21.Paggi, R. A., and J. P. Fay. 1996. Effect of short-chain fatty acids on growth of the ruminal bacterium Streptococcus bovis. J. Gen. Appl. Microbiol. 42:393-400. [Google Scholar]
- 22.Paulsrud, B., B. Gjerde, and A. Lundar. 2004. Full scale validation of helminth ova (Ascaris suum) inactivation by different sludge treatment processes. Water Sci. Technol. 49:139-146. [PubMed] [Google Scholar]
- 23.Teh, J. S. 1974. Toxicity of short-chain fatty acids and alcohols towards Cladosporium resinae. Appl. Environ. Microbiol. 28:840-844. [DOI] [PMC free article] [PubMed] [Google Scholar]