Abstract
The microaerophilic bacterium Campylobacter jejuni is the most common cause of bacterial food-borne infections in the developed world. Tolerance to environmental stress relies on proteases and chaperones in the cell envelope, such as HtrA and SurA. HtrA displays both chaperone and protease activities, but little is known about how each of these activities contributes to stress tolerance in bacteria. In vitro experiments showed temperature-dependent protease and chaperone activities of C. jejuni HtrA. A C. jejuni mutant lacking only the protease activity of HtrA was used to show that the HtrA chaperone activity is sufficient for growth at high temperature or under oxidative stress, whereas the HtrA protease activity is essential only under conditions close to the growth limit for C. jejuni. However, the protease activity was required to prevent induction of the cytoplasmic heat shock response even under optimal growth conditions. Interestingly, the requirement of HtrA at high temperatures was found to depend on the oxygen level, and our data suggest that HtrA may protect oxidatively damaged proteins. Finally, protease activity stimulates HtrA production and oligomer formation, suggesting that a regulatory role depends on the protease activity of HtrA. Studying a microaerophilic organism encoding only two known periplasmic chaperones (HtrA and SurA) revealed an efficient HtrA chaperone activity and proposed multiple roles of the protease activity, increasing our understanding of HtrA in bacterial physiology.
The Gram-negative pathogenic bacterium Campylobacter jejuni is the most common cause of bacterial food-borne infections in developed countries (1), and handling and consumption of contaminated poultry meat products are recognized as the most frequent sources of infection (22). The symptoms of human campylobacter infections are diarrhea, abdominal pain, and fever, which in rare cases are followed by development of more serious complications. C. jejuni colonizes the intestinal tract of mammals and birds, but in contrast to infected humans, no apparent symptoms are observed in colonized poultry or other avian species. C. jejuni is a microaerophilic bacterium having an optimal growth temperature of 42°C, which may reflect that C. jejuni is highly adapted to a commensal lifestyle in the avian cecum. During transmission from the avian host to the intestinal tract of humans, C. jejuni is exposed to various stressful conditions, such as temperature and pH fluctuations, osmotic stress, and atmospheric oxygen concentrations. C. jejuni is generally considered a fragile organism, because it is killed more readily by environmental stress conditions than other food-borne pathogens (46) and because of its fastidious growth requirements. C. jejuni grows only between 30°C and 45°C (16, 59) in a microaerobic atmosphere containing carbon dioxide (5, 30) and fails to grow on sugars and by fermentation (47). Despite these growth limitations, C. jejuni is able to survive in the food production chain and is a frequent cause of human disease.
A number of vital processes take place in the periplasmic space of Gram-negative bacteria. In C. jejuni, these processes include nutrient transport (33, 39), protein glycosylation (66), protection against oxidative stress (4), and respiration (14). Because the outer membrane allows free diffusion of small molecules (29), periplasmic proteins are more highly exposed to chemical variations in the extracellular environment than cytoplasmic proteins, which are protected by a tight inner membrane. Periplasmic proteins and proteins targeted for the outer membrane are synthesized in the cytoplasm but folded in the periplasm (57). Since protein misfolding and aggregation are accelerated by stress, the native state of periplasmic proteins is constantly challenged by the fluctuating environmental conditions encountered by C. jejuni during its transmission to the human host. Therefore, C. jejuni, and Gram-negative bacteria in general, depends on periplasmic proteases and chaperones to degrade misfolded or damaged proteins and to promote their folding by protecting them from interactions that lead to aggregation, respectively.
In Gram-negative bacteria, such as Salmonella and Escherichia coli, the periplasmic chaperones HtrA (DegP), SurA, Skp (OmpH), and FkpA have partly overlapping functions (23, 55) and provide these bacteria with a robust system for protein folding in the periplasm. In contrast, C. jejuni carries homologs of only HtrA and SurA and thus depends on these two chaperones for protein folding in the periplasm. A few bacterial HtrA homologs have been investigated in vitro, and these experiments have shown that HtrA is a bifunctional protein that, in addition to the chaperone activity, possesses proteolytic activity (24, 28, 63). In general, HtrA homologs are characterized by the presence of a trypsin-like serine protease domain and one or two PDZ domains that recognize substrates and activate the protease function (25, 31). Upon binding of unfolded proteins to the PDZ domains, HtrA organizes into larger active oligomers, while reverting to a hexameric resting state after substrate degradation is completed (32). We previously showed that HtrA is required for growth of C. jejuni at elevated oxygen tensions and under conditions that induce protein misfolding, such as high temperature and puromycin (6). While bacterial htrA mutants show different stress-sensitive phenotypes, all htrA mutants examined so far are sensitive to high temperature (9, 10, 34, 35, 48, 69). In addition, several studies have suggested an important role of HtrA in virulence of C. jejuni, as an htrA mutant invades epithelial cells (6, 44) and kills infected Galleria mellonella larvae less efficiently than the wild type (8). However, despite a detailed mechanistic insight into HtrA function (26, 31, 32), only few studies have addressed how the individual HtrA activities contribute to stress tolerance in bacteria. These studies have shown that plasmid-mediated expression of an HtrA mutant protein that lacks protease activity but retains chaperone activity allows growth of E. coli and Salmonella htrA deletion mutants at high temperature (34, 61, 63). Such experiments indicate that the protease activity may be dispensable if the HtrA chaperone activity is present in elevated amounts; however, they do not show how each of these activities contributes to stress tolerance in bacteria.
With the aim of determining the contribution of the HtrA protease and chaperone activities to heat and oxidative stress tolerance of C. jejuni, we used a method described by Hendrixson et al. (18), which employs counterselection for site-directed mutagenesis in C. jejuni, to construct a mutant that lacks HtrA protease activity but retains HtrA chaperone activity. Using this mutant, we show that the chaperone activity of HtrA is sufficient for growth under a wide range of heat and oxidative stress conditions, whereas the protease activity is essential only under severe stress conditions. Furthermore, we find that the heat sensitivity of an htrA mutant depends on the level of oxidative stress, emphasizing an important role of HtrA in tolerance to oxidative stress. In this study, we provide molecular and phenotypic evidence demonstrating that HtrA chaperone and protease activities participate in both stress tolerance and protein homeostasis in C. jejuni, thereby contributing to the success of this apparently fragile zoonotic pathogen. By studying a conserved protein in a new context, namely, a microaerophilic bacterium that encodes a limited number of periplasmic chaperones and regulatory factors, we have shown the chaperone activity of HtrA to play a significant role in stress tolerance, and we propose a regulatory function of the HtrA protease activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. jejuni NCTC11168 (National Collection of Type Cultures) and derivatives thereof were routinely grown on blood agar base II (Oxoid) supplemented with 5% (vol/vol) bovine blood or in brain heart infusion (BHI) broth (Oxoid) at 37°C in a microaerobic environment (6% O2, 6% CO2, 4% H2, and 84% N2). E. coli strains were routinely grown in Luria broth (LB) or on Luria agar. When appropriate, media were supplemented with 100 μg ml−1 ampicillin, 50 μg ml−1 kanamycin, 50 μg ml−1 streptomycin, or 20 μg ml−1 chloramphenicol.
Construction of C. jejuni htrA(S197A) mutant.
Streptomycin counterselection (18) was used to construct a C. jejuni htrA(S197A) mutant. Plasmid pLB252 (ΔhtrA::cat-rpsL) was constructed by cloning a cat-rpsL SmaI fragment from pDRH265 (18) into the PstI site of pLB225, containing an in-frame deletion in the htrA gene with an internal PstI site (6). A PCR fragment containing a site-specific mutation in the htrA gene was obtained by the following procedure. First, two PCR fragments were amplified from C. jejuni NCTC11168 using primers HT1 (5′-AATTTAATGGTTTCGCCTTG-3′) and HT2 (5′-ATCCACCAAAGCTCCGCCGGCATTTCCTGGATTGAT-3′) or HT3 (5′-ATCAATCCAGGAAATGCCGGCGGAGCTTTGGTGGAT-3′) and HT4 (5′-AGCTTATAACCTATTCCACG-3′). Primers HT2 and HT3 are overlapping and contain substitutions that change HtrA serine 197 to alanine 197. The PCR fragments were joined by the splicing-by-overlap-extension PCR method (21) using HT1 and HT4 as primers, and the resulting fragment carrying htrA(S197A) was cloned into pSC-A (Stratagene), resulting in pKB1002. A spontaneous streptomycin-resistant mutant of C. jejuni NCTC11168 was isolated by streaking on streptomycin plates. A 0.8-kb fragment containing the mutated rpsL gene (rpsLSm) was amplified by PCR using primers 5′-GGGGGCAGCTGCATGGTCTTGAAGAATATTTAGC-3′ and 5′-GGGGGCAGCTGCTAACGGATTTGTCTG-3′, cloned into vector pCR2.1 (Invitrogen), and transferred to NCTC11168 by electroporation (7), thereby creating LB1238 (rpsLSm). A C. jejuni ΔhtrA::cat-rpsL mutant (KB1022) was obtained by transformation of LB1238 (rpsLSm) with pLB252 (ΔhtrA::cat-rpsL) and selection for chloramphenicol resistance. KB1022 was then transformed with pKB1002 [htrA(S197A)], and ΔhtrA::cat-rpsL located on the chromosome was replaced by htrA(S197A) by selection for streptomycin resistance. Smr colonies were tested for sensitivity to chloramphenicol, and one sensitive clone [KB1025; C. jejuni htrA(S197A)] was isolated.
Cloning of HtrA expression plasmids.
PCR fragments containing htrA or htrA(S197A) were amplified using C. jejuni NCTC11168 chromosomal DNA or pKB1002 DNA, respectively, as the template and 5′-TATACCATGGGCGCAAGTATTAATTTTAAC-3′ (HtrA-his-F) and 5′-GTGCTCGAGTTTAAGCACAAGCAAAGTCGC-3′ (HtrA-his-R) as primers. The resulting fragment contains no htrA signal peptide and carries an NcoI site and Met-Gly codons upstream of codon 17 of the htrA gene as well as an XhoI site and Leu-Glu codons upstream of the htrA stop codon. The NcoI and XhoI sites were used to clone the PCR fragments in frame and upstream of six C-terminal His codons in the pET28a+ vector (Novagen) to give pKB1004 (HtrA-His) and pKB1005 (HtrAS197A-His). To express HtrA-His to the periplasm, an NcoI-XhoI fragment encoding HtrA-His was cloned in the pET26b+ expression vector (Novagen), giving rise to pKB1012. Blunt-end-forming DNA polymerase was used to amplify a fragment containing htrA(S197A) and the six C-terminal His codons by PCR using pKB1005 as the template and the primers 5′-TAGTTATTGCTCAGCGGTGG-3′ and 5′-TTGTGAGCGGATCCCAATTC-3′. This PCR fragment was digested with BamHI and ligated into pJS17 (62), which had been opened with HindIII, blunt ended with T4 DNA polymerase, and redigested with BamHI to give pKB1014, containing htrA(S197A)-His6 controlled by the T5 promoter.
Expression and purification of HtrA.
Wild-type HtrA-His was expressed from pKB1012 in E. coli BL21(DE3) by growing the cells at 37°C to an optical density at 600 nm (OD600) of 0.9, and expression was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h. HtrAS197A-His was expressed from pKB1014 in E. coli htrA strain BL20 (35) by growth at 30°C to an OD600 of 0.9, and expression was induced by addition of 1 mM IPTG for 16 h. After induction, cells were lysed on ice in 20 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, 10% [vol/vol] glycerol) by addition of 1 mg ml−1 lysozyme, followed by sonication. Lysates were cleared by centrifugation at 35,000 × g for 30 min. His-tagged HtrA proteins were purified by nickel affinity chromatography using Ni-nitrilotriacetic acid (NTA) resin (Qiagen) equilibrated with lysis buffer. Unspecifically bound proteins were removed with 50 mM imidazole, and HtrA was eluted with 250 mM imidazole. Eluted proteins were concentrated and dialyzed against 25 mM HEPES, pH 7.5, 50 mM NaCl, 10% (vol/vol) glycerol. Protein bands were excised from the gel and prepared for mass spectrometry (MS) as described previously (12).
Proteolytic activity of HtrA.
Proteolytic activity of HtrA or HtrAS197A was determined using casein as the substrate in reaction mixtures containing 50 mM Tris-HCl (pH 8.0), 0.4 mg ml−1 β-casein, and 20 μg ml−1 HtrA or HtrAS197A at 37°C. At different time points, aliquots were transferred to SDS sample buffer containing dithiothreitol (DTT) and immediately frozen in −80°C ethanol. The samples were separated by SDS-PAGE and stained with Coomassie brilliant blue. Protease activity was also tested using an EnzChek protease activity kit (Molecular Probes) according to the manufacturer's instructions. Briefly, reaction mixtures containing casein labeled with the fluorescent dye BODIPY FL were incubated in a quartz cuvette at different temperatures, and fluorescence was measured using a Perkin-Elmer LS55 luminescence spectrophotometer. Upon hydrolysis of the labeled casein by HtrA, quenching is relieved and the increase in fluorescence is proportional to substrate degradation. Fluorescence was followed upon addition of 4 μg ml−1 HtrA, and the slope of the initial linear increase in fluorescence was a measure of proteolytic activity.
Light scattering.
Assays were performed essentially as previously described (61). Reaction mixtures containing 40 μM lysozyme, 20 μM HtrAS197A, 25 mM HEPES, pH 7.5, and 50 mM NaCl were incubated at different temperatures in quartz cuvettes in a thermostated cell holder to record the light scattering baseline. Then, 4 mM DTT was added and light scattering was measured immediately. Protein aggregation was monitored using a Perkin-Elmer LS55 luminescence spectrophotometer, with excitation and emission wavelengths set to 360 nm.
Lysozyme aggregation.
Measurements were performed as previously described (61) in reaction mixtures containing 80 μM lysozyme, 40 μM HtrAS197A, 25 mM HEPES, pH 7.5, and 50 mM NaCl incubated at different temperatures. DTT was added to 4 mM, and aliquots were withdrawn at selected time points and centrifuged immediately at 21,000 × g for 10 min. Pellets were dissolved in 8 M urea, 100 mM NaPO4, pH 7.5, and protein concentration was determined by staining with amido black. Relative values of protein content were obtained by densitometry. Absolute protein concentration was estimated by spectrophotometric measurements of amido black-stained samples, using native lysozyme as the standard.
HtrA polyclonal antibody preparation.
Wild-type HtrA (HtrAwt) was purified as described above and separated by SDS-PAGE. The band containing full-length HtrA (determined by mass spectrometry) was cut out and used for the production of rabbit polyclonal antibodies (CovalAB, Lyon, France). Final serum was acquired 67 days after the initial immunization.
Protein analysis and Western blotting.
For denatured protein extracts, C. jejuni cells were grown in BHI broth at 37°C or 42°C under microaerobic conditions, and proteins were extracted as described by Brøndsted et al. (6), separated by SDS-PAGE, Coomassie stained or blotted onto a polyvinylidene difluoride (PVDF) membrane, and probed with HtrA antibody. For the native protein extracts, C. jejuni cells were grown in BHI broth at 37°C under microaerobic conditions to the exponential phase (OD600 of 0.1). Native lysates were prepared by washing the harvested cells in 0.9% (wt/vol) NaCl, resuspension in NativPAGE buffer (Invitrogen), and sonication. Native samples were separated by blue native PAGE (58) on a NativPAGE bis-Tris gel (Invitrogen), blotted onto a PVDF membrane, and probed with HtrA antibody. Bound antibody was detected with a WesternBreeze chemiluminescence detection kit (Invitrogen) and quantified densitometrically with GeneTools software (PerkinElmer).
Growth experiments on solid medium.
C. jejuni cells were grown overnight on base II, 5% (vol/vol) blood agar plates at 37°C under microaerobic conditions and harvested in 0.9% (wt/vol) NaCl, and the OD600 was adjusted to 0.1. The cell suspension was serially diluted, and 10 μl of the 10−1 to 10−5 dilutions was spotted on base II, 5% blood agar plates and incubated for 3 days at 37°C, 42°C, or 44°C under the following conditions. The microaerobic atmosphere was generated by CampyGen (Oxoid), the ca. 18% O2 atmosphere was generated by the presence of a lit candle in a closed jar (37), and the 1% O2 atmosphere was obtained in an incubator with 6% CO2, 4% H2, and 89% N2. A mixture of ferrous sulfate, sodium bisulfate, and pyruvate (FBP) was added to 0.025% (wt/vol) each by addition of campylobacter growth supplement (Oxoid).
Disc diffusion assays.
Approximately 109 C. jejuni stationary-phase cells were added to 0.6% Mueller-Hinton top agar and poured on Mueller-Hinton agar plates. Filter discs (13 mm; Whatman) were placed on the top agar, and 10 μl 30% hydrogen peroxide, 5 μl 80% cumene hydroperoxide, or 20 μl 98% paraquat (all from Sigma) was added to the discs. Plates were incubated microaerobically at 37°C or 42°C for 3 days, and inhibition zones were measured.
Protein carbonylation.
C. jejuni cells were grown in 50 ml BHI broth in 300-ml Erlenmeyer flasks at 42°C under microaerophilic conditions until stationary phase (OD600 of 0.7 to 1.4), transferred to aerobic conditions, and shaken at 300 rpm for 1 h. Cells were pelleted at 6,000 × g for 10 min, washed in 50 mM Tris-HCl, pH 7.8, and split into two portions. One portion, used for whole-cell extracts, was lysed for 1 h at room temperature in phosphate-buffered saline (PBS) buffer containing 8 M urea, 1% (vol/vol) Triton X-100, 1% (wt/vol) SDS, and 1% (vol/vol) β-mercaptoethanol in order to solubilize proteins and subsequently sonicated briefly to shear DNA and centrifuged (10,000 × g for 10 min) to remove cell debris. The second portion was used to extract outer membrane proteins essentially as described previously (38). Briefly, the pellet was resuspended in 0.2 M glycine-HCl, pH 2.2, to an OD600 of 10 and incubated at room temperature for 10 min, followed by removal of cells at 6,000 × g for 5 min. The protein content in the extracts was quantified by densitometry of amido black-stained proteins, with bovine serum albumin (BSA) as the standard. Carbonylated proteins were derivatized with 2,4-dinitrophenylhydrazine (DNPH), and equal amounts of protein were separated by SDS-PAGE. DNPH-derivatized proteins were detected by immunoblotting with anti-2,4-dinitrophenol (anti-DNP) antibody using an OxyBlot protein oxidation detection kit (Chemicon).
Northern blotting.
C. jejuni cells were grown under microaerobic conditions to an OD600 of 0.4 or 0.7. RNA extraction was performed essentially as described previously (42). Briefly, 1 volume of cells was added to 2 volumes of RNAprotect bacterial reagent (Qiagen), and the mixture was incubated for 5 min at room temperature before the cells were pelleted at 8,000 × g for 2 min at 0°C. Subsequently, cells were lysed in Tris-EDTA (TE) buffer containing 1 mg ml−1 lysozyme at room temperature for 10 min, and RNA was extracted using an RNeasy kit (Qiagen). Equal amounts of RNA (3 to 5 μg) were denatured at 75°C for 10 min and separated in a 1% agarose gel in 10 mM NaPO4 buffer as described by Pelle and Murphy (50). RNA was blotted onto a positively charged nylon membrane (Hybond-N; GE Healthcare) and subsequently fixed to the membrane by UV light exposure. A gel-purified PCR fragment internal to the htrA gene was used as a probe and labeled with [32P]dCTP using Ready-to-Go labeling beads (GE Healthcare), and unincorporated nucleotides were removed using ProbeQuant G-50 microcolumns (GE Healthcare). Probes were hybridized to the membrane overnight at 65°C in 0.5 M NaPO4 (pH 7.2), 7% (wt/vol) SDS after 1 h of prehybridization under the same conditions. To remove unspecifically bound probe, membranes were washed twice in 20 mM NaPO4 (pH 7.2), 1% (wt/vol) SDS. The washed membrane was used to expose a phosphorimage screen at room temperature, and the screen was scanned in a phosphorimager (Cyclone Plus; PerkinElmer).
RESULTS
C. jejuni HtrA has both protease and chaperone activities.
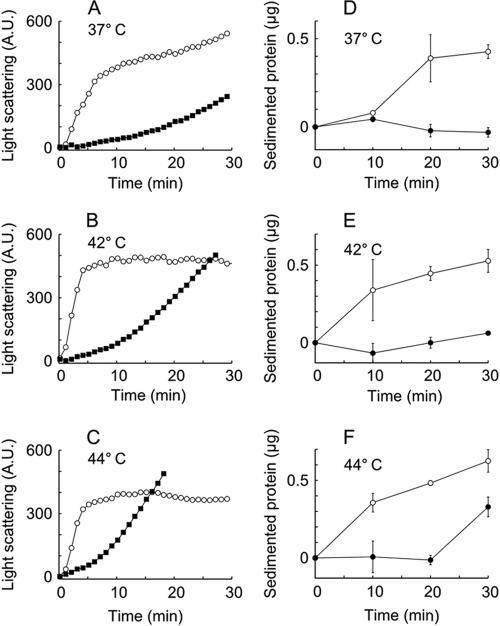
A bioinformatic analysis suggested that HtrA of C. jejuni is a bifunctional protein possessing protease as well as chaperone activity, since the protein contains the conserved active-site residues characteristic of serine proteases (His92-Asp123-Ser197) as well as two PDZ domains. To explore this predicted dual functionality of HtrA, we constructed a protease-negative mutant protein (HtrAS197A) by replacing the serine in the active proteolytic site with alanine. His-tagged HtrAS197A or HtrAwt protein was subsequently expressed in E. coli and purified. The protease activity of HtrA was demonstrated by degradation by HtrAwt of β-casein (Fig. 1 A), a substrate commonly used to investigate proteolytic activity of HtrA homologs (26, 36, 63). HtrAS197A, on the other hand, was unable to degrade this substrate (Fig. 1A), showing that HtrA of C. jejuni is a protease, whose proteolytic activity depends on serine 197. Furthermore, the protease activity was temperature dependent (Fig. 1B). The chaperone activity was measured as the ability of purified HtrAS197A to prevent aggregation of denatured lysozyme at various temperatures. Lysozyme aggregation was determined by light scattering and by quantification of large insoluble aggregates, and we found that HtrAS197A decreased the rate of lysozyme aggregation in a dose-dependent manner at all tested temperatures within the growth range of C. jejuni (Fig. 2 and data not shown). The ability of HtrAS197A to prevent aggregation was slightly reduced at higher temperatures, especially at 44°C, which may be explained by a lower intrinsic chaperone activity of HtrA at this temperature. In conclusion, these results show that HtrA of C. jejuni has both protease and chaperone activities.
FIG. 1.
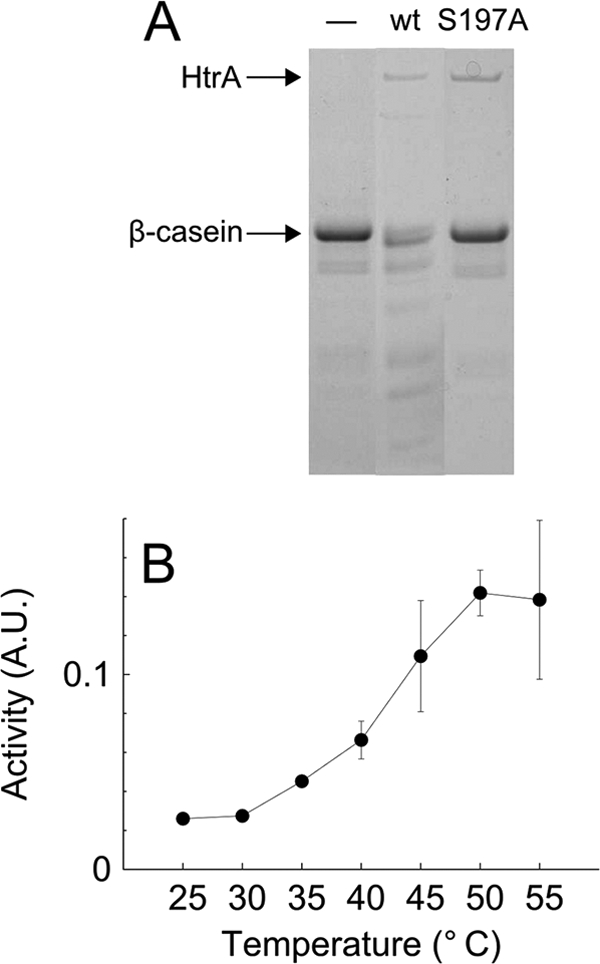
Protease activity of HtrA in vitro. Wild-type HtrA and HtrAS197A were expressed from plasmids pKB1012 and pKB1014, respectively, in E. coli and purified. (A) Degradation of β-casein at 37°C in the absence (−) or presence of wild-type HtrA or HtrAS197A. (B) Degradation of BODIPY-labeled β-casein at different temperatures in the presence of wild-type HtrA. The activity is the degradation rate measured fluorimetrically at 511 nm in triplicate. The averages from three independent measurements are shown. Error bars indicate the standard deviations. A.U., arbitrary units.
FIG. 2.
Chaperone activity of HtrA in vitro. HtrAS197A and lysozyme were mixed in a molar ratio of 1:2, and DTT was added at time zero. (A to C) Aggregation of denatured lysozyme in the presence (filled squares) or absence (open circles) of HtrAS197A, measured by light scattering at 300 nm. (D to F) Aggregation of denatured lysozyme in the presence (filled circles) or absence (open circles) of HtrAS197A, measured by sedimentation of insoluble aggregates at 21,000 × g for 10 min. Sedimented protein was quantified as described in Materials and Methods. The values shown are averages from two individual experiments, normalized to the value at time zero. Error bars indicate the standard deviations.
Function of HtrA chaperone and protease activities in heat and oxygen tolerance of C. jejuni.
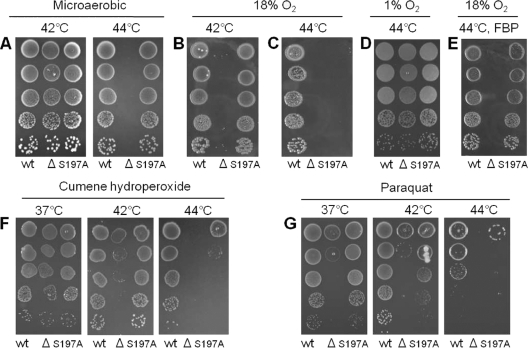
We previously used a ΔhtrA mutant to show that C. jejuni exposed to high temperature (44°C) or near-aerobic conditions (18% O2 at 42°C) requires HtrA to form colonies (6). To investigate the contribution of the HtrA chaperone and protease activities to stress tolerance of C. jejuni, we constructed a C. jejuni htrA(S197A) mutant by replacing the wild-type htrA allele with a mutated htrA(S197A) allele. Thus, the C. jejuni htrA(S197A) mutant lacks HtrA protease activity but retains HtrA chaperone activity. We compared the heat and oxygen tolerance of the htrA(S197A) mutant with that of the wild type and the ΔhtrA mutant and found that protease activity of HtrA was not required for C. jejuni to form colonies either at high temperature (44°C) (Fig. 3 A) or at high oxygen tension (18% at 42°C) (Fig. 3B). In contrast, the protease activity of HtrA was required for growth of C. jejuni if the two stress conditions were combined (18% O2 at 44°C), under which condition the htrA(S197A) mutant failed to form colonies (Fig. 3C). These findings show that HtrA chaperone activity is sufficient to allow growth under mild stress, whereas the protease activity of HtrA is required for growth only when the stress is severe. In addition, the requirement for HtrA at high temperature could be eliminated completely by significantly reducing the oxygen tension, as HtrA was not required for colony formation in a 1% O2 atmosphere at 44°C (Fig. 3D). Thus, the requirement of HtrA for growth of C. jejuni at high temperature depends on the amount of oxygen in the atmosphere.
FIG. 3.
Effect of temperature and oxidative stress on growth of C. jejuni htrA mutants on solid medium. Serial dilutions (10−1 to 10−5) of C. jejuni NCTC11168 (wild type; wt), LB1281 (ΔhtrA mutant; Δ), and KB1025 [htrA(S197A) mutant; S197A] at an OD600 of 0.1 were spotted in 10-μl volumes on base II, 5% blood agar plates. The plates were incubated under microaerobic conditions (A, F, and G), in an 18% O2 atmosphere (candle jar) (B, C, and E), or in a 1% O2 atmosphere (D). Ferrous sulfate, sodium bisulfate, and pyruvate (FBP), cumene hydroperoxide, or paraquat was added as indicated.
HtrA is required for tolerance to oxidative stress.
To determine if the increased oxygen sensitivity of the htrA mutants was mediated by reactive oxygen species (ROS), we tested growth of the wild type and the ΔhtrA and htrA(S197A) mutants on solid medium containing ferrous sulfate, sodium bisulfate, and pyruvate (FBP). These compounds act as scavengers that destroy H2O2 and O2− generated in the growth medium (20, 67). In the presence of these scavengers, the htrA(S197A) mutant could form colonies at 44°C in an 18% O2 atmosphere, while the ΔhtrA mutant failed to form colonies under this condition (Fig. 3E). In contrast, both the ΔhtrA and htrA(S197A) mutants were able to form colonies when the temperature was lowered to 42°C (data not shown). Therefore, the increased oxygen sensitivity of C. jejuni lacking HtrA must be mediated, at least in part, by exogenous H2O2 or O2−. To more directly investigate the sensitivity of the htrA mutants to oxidative stress induced by reactive oxygen species, cumene hydroperoxide or the intracellular O2− generator paraquat (15) was added to the growth medium, and the formation of colonies under microaerobic conditions was investigated. In the presence of these compounds, the ability of ΔhtrA cells and htrA(S197A) cells to form colonies was reduced compared to that of the wild type in a temperature-dependent manner (Fig. 3F and G). In agreement with this, a disc diffusion assay with H2O2, cumene hydroperoxide, and paraquat showed that the ΔhtrA and htrA(S197A) mutants were significantly more sensitive to reactive oxygen species than the wild type and that the difference was more pronounced at 42°C than at 37°C (Table 1). In conclusion, these data show that HtrA plays an essential role in protecting C. jejuni against reactive oxygen species.
TABLE 1.
Effects of oxidative stress agents on wild-type and htrA mutant strains, as measured by disc diffusion assays
| Temp (°C) | Oxidative stress agent | Mean diam (mm) of inhibition zone ± SD fora: |
No. of samples | ||
|---|---|---|---|---|---|
| Wild type | ΔhtrA mutant | htrA(S197A) mutant | |||
| 37 | H2O2 | 68 ± 5.3 | 81 ± 7.1** | 79 ± 11* | 7 |
| Cumene hydroperoxide | 76 ± 6.9 | 88 ± 10* | 88 ± 8.2* | 7 | |
| Paraquat | 58 ± 4.6 | 70 ± 2.2** | 66 ± 5.2 | 4 | |
| 42 | H2O2 | 59 ± 5.9 | 77 ± 5.7*** | 72 ± 7.0** | 7 |
| Cumene hydroperoxide | 73 ± 6.6 | 91 ± 6.9*** | 91 ± 11** | 7 | |
| Paraquat | 50 ± 2.4 | 64 ± 1.4*** | 63 ± 2.0*** | 4 | |
Asterisks indicate significant differences from results for the wild type, based on Student's t test, as follows. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The role of HtrA in oxidative stress tolerance of C. jejuni suggests that HtrA degrades, binds, or refolds oxidatively damaged proteins in the periplasm. To investigate whether the lack of HtrA affects the level of oxidatively damaged proteins, we assayed protein carbonylation in the wild type and the ΔhtrA and htrA(S197A) mutants. Carbonylation is an irreversible modification of proteins exposed to oxidative stress (45), and the level of protein carbonylation can be used as an indicator of the degree of oxidative protein damage. We found no overall increase in the level of carbonylated proteins from ΔhtrA or htrA(S197A) cells compared to that from the wild type under microaerobic growth conditions (data not shown) or upon exposure to aerobic growth conditions (Fig. 4). However, since HtrA is a periplasmic protein and since envelope proteins constitute only a small fraction of all cellular protein, a difference in level of protein carbonylation is difficult to detect in whole-cell extracts. Therefore, we performed the analysis of carbonylation with glycine extracts, which contain periplasmic and surface-located proteins (49). Interestingly, we consistently observed a different band pattern with these proteins from the ΔhtrA mutant than with those from the wild type and the htrA(S197A) mutant (Fig. 4); however, the specific band differences varied slightly between repeated experiments. These results indicate that HtrA does not lower the level of carbonylated proteins but rather degrades some carbonylated proteins while protecting others in a protease-independent manner.
FIG. 4.
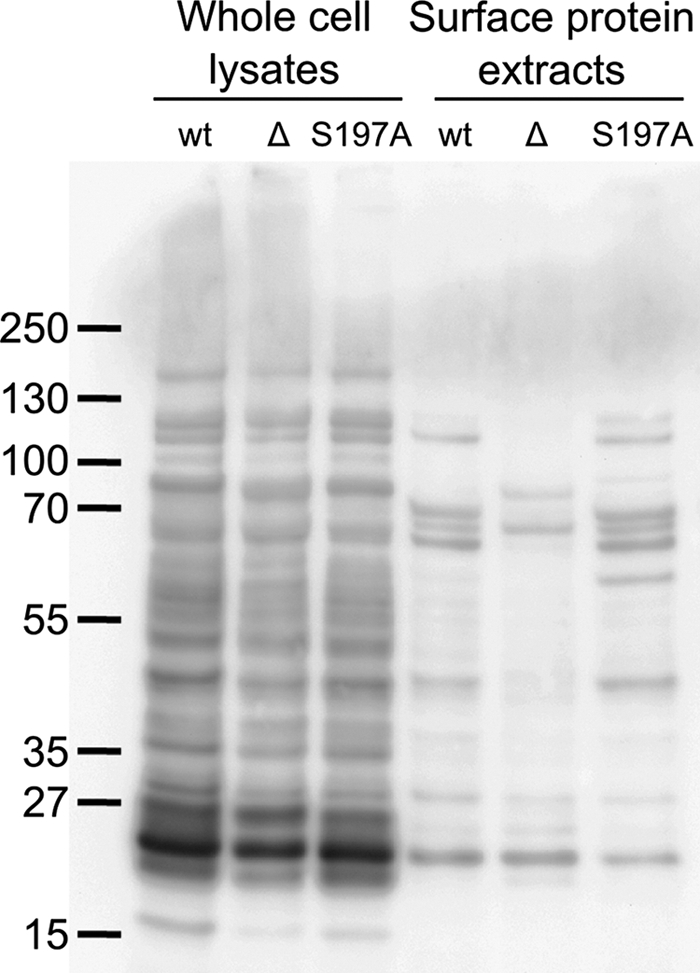
Protein carbonylation. C. jejuni NCTC11168 (wt), LB1281 (ΔhtrA mutant; Δ), and KB1025 [htrA(S197A) mutant; S197A] were grown microaerobically overnight at 42°C to stationary phase and then exposed to vigorous shaking for 1 h. Carbonylation of proteins in whole-cell lysates and surface protein extracts was determined as described in Materials and Methods. Equal amounts of protein were loaded in each lane for the lysates and surface extracts. The positions of molecular size standards are indicated on the left (in kilodaltons).
The protease activity of HtrA is important for protein homeostasis under optimal growth conditions.
In the absence of HtrA, the cytoplasmic heat shock chaperones ClpB and DnaK are upregulated in C. jejuni (6), suggesting that increased amounts of ClpB and DnaK in htrA mutants can be used as an indicator of disturbed protein homeostasis in the cell envelope. The amounts of ClpB and DnaK were increased only slightly in the htrA(S197A) mutant compared to those in the wild type at 37°C, whereas at 42°C the amounts of ClpB and DnaK in the htrA(S197A) mutant approached the amounts observed in ΔhtrA cells (Fig. 5). This indicates that the chaperone activity of HtrA is sufficient to prevent any accumulation of misfolded substrates that may lead to increased amounts of ClpB and DnaK at 37°C. However, at 42°C, misfolded HtrA substrates must be degraded by HtrA to prevent upregulation of ClpB and DnaK. Furthermore, ClpB and DnaK were upregulated in the ΔhtrA mutant even at low oxidative stress (1% O2 or added FBP) (data not shown). Thus, a lack of HtrA caused increased levels of ClpB and DnaK, regardless of whether there was oxidative stress. Exposure to 18% O2 or ROS at 42°C led to an even greater increase in the amounts of ClpB and DnaK in the htrA(S197A) mutant (data not shown), indicating an increased requirement for protease activity in the presence of oxidative stress. Protein levels of the ΔhtrA mutant could not be examined at 42°C or under oxidative stress, due to a lack of growth under these conditions (Fig. 3B, F, and G). In conclusion, with ClpB and DnaK levels used as indicators of disturbed protein homeostasis in the cell envelope, the results show that HtrA is important to maintain protein homeostasis in the entire growth interval of C. jejuni and that the chaperone activity alone is sufficient to fulfill this role at low temperature.
FIG. 5.
Effect of lack of HtrA protease activity on induction of cytoplasmic heat shock response. C. jejuni NCTC11168 (wt), LB1281 (ΔhtrA mutant; Δ), and KB1025 [htrA(S197A) mutant; S197A] were grown in BHI broth at the indicated temperatures in a microaerobic atmosphere. Extracted proteins were separated by SDS-PAGE and stained with Coomassie brilliant blue. ClpB and DnaK were identified previously (6).
HtrA protease activity stimulates HtrA production and oligomer disassembly.
Because a lack of HtrA protease activity results in the upregulation of ClpB and DnaK at 42°C (Fig. 5), we hypothesized that htrA expression may also be upregulated under this condition. We analyzed htrA expression in the htrA(S197A) mutant grown microaerobically at 42°C and found that the amounts of htrA mRNA were identical in the wild type and the htrA(S197A) mutant (Fig. 6 A). Thus, C. jejuni does not compensate for a loss of HtrA protease activity by increasing the transcription of htrA. To examine HtrA protein levels, a polyclonal antibody against HtrA was prepared, and protein extracts from wild-type and htrA(S197A) cells were analyzed by immunoblotting. Two bands were detected in the wild type by the HtrA antibody: one band corresponding in size to the HtrA monomer (full-length HtrA) and one band corresponding in size to a shorter fragment of HtrA (s-HtrA) (Fig. 6B). Both bands were identified as HtrA by mass spectrometry of the purified wild-type HtrA (data not shown). In contrast, only the full-length HtrA band was detected in protein extracts from htrA(S197A) cells (Fig. 6B), and no bands were detected in the ΔhtrA mutant (data not shown). Furthermore, when purified wild-type HtrA was incubated alone at either 37°C or 45°C, a steady increase in the amount of the short HtrA fragment was observed over the course of 3 h (data not shown). These observations imply that the short HtrA fragment is an autocleavage product, a feature also observed for the E. coli HtrA ortholog (27, 62). The amounts of full-length HtrA in the wild type and the htrA(S197A) mutant were identical, as measured by densitometry; however, the wild type contained a substantial amount of s-HtrA (1.2-fold the amount of full-length HtrA) (Fig. 6B). Since HtrAS197A and s-HtrA were equally stable for several cell generations (10 h [data not shown]), this indicates that, overall, more HtrA is produced in the wild type than in the htrA(S197A) mutant.
FIG. 6.
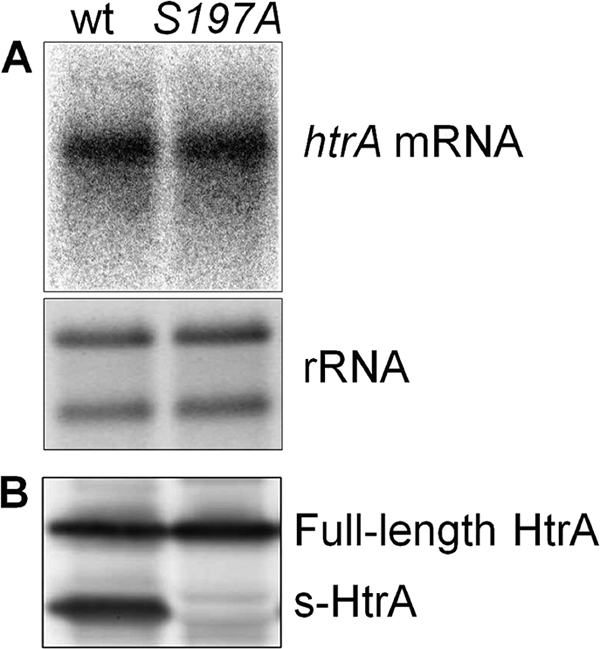
Effect of HtrA protease activity on HtrA content and htrA expression. (A) htrA mRNA in exponential-phase-growing C. jejuni NCTC11168 (wt) and KB1025 [htrA(S197A) mutant; S197A] cultures, detected by Northern blotting with an htrA-specific DNA probe. (B) Full-length and short HtrA (s-HtrA) in exponential-phase-growing C. jejuni NCTC11168 (wt) and KB1025 [htrA(S197A) mutant; S197A], detected by immunoblotting with an HtrA antibody.
Purified HtrA from E. coli organizes into large 12- and 24-mers upon binding of substrate and reverts to a hexameric state after substrate degradation is complete (32). We analyzed native protein extracts from the wild type and the htrA(S197A) mutant by immunoblotting to investigate whether such HtrA oligomers were present in C. jejuni (Fig. 7). HtrA from the wild type was found as oligomers corresponding in size to trimers, hexamers, and 12-mers, while HtrA from the htrA(S197A) mutant was found only as larger oligomers. This result shows that the HtrA oligomerization observed in vitro also takes place in vivo and that disassembly of the large oligomers depends on HtrA protease activity.
FIG. 7.
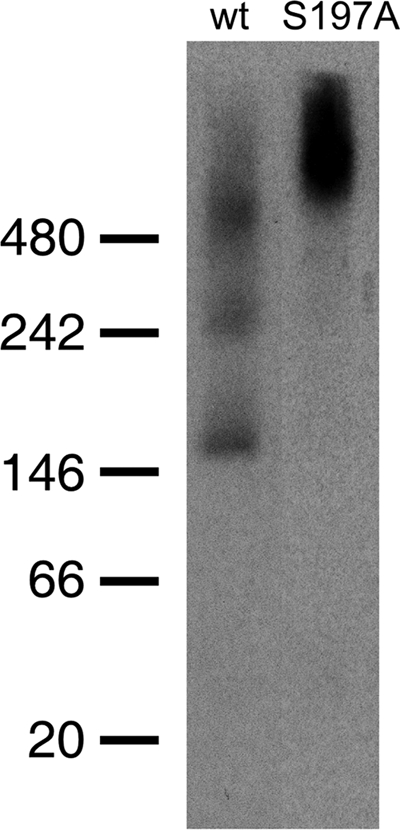
Visualization of HtrA oligomers in exponential-phase-growing C. jejuni NCTC11168 (wt) and KB1025 [htrA(S197A); S197A] by blue native PAGE followed by immunoblotting with HtrA antibody. The positions of molecular size standards (NativeMark; Invitrogen) are indicated on the left (in kilodaltons).
DISCUSSION
C. jejuni encodes the periplasmic chaperones HtrA and SurA for folding of periplasmic and outer membrane proteins. HtrA is a conserved protein that contains both protease and chaperone activities, and we previously showed that HtrA is essential for growth of C. jejuni in a near-aerobic environment and for prevention of accumulation of misfolded proteins caused by heat (6). In this study, we examined the in vitro proteolytic and chaperone activities of HtrA and furthermore explored how these activities contribute to C. jejuni stress tolerance in vivo by analysis of a defined htrA mutant that lacks proteolytic activity.
We show that purified HtrA of C. jejuni possesses both serine protease and molecular chaperone activities in the entire temperature growth range of C. jejuni. Consistent with findings in E. coli (63), the protease activity of C. jejuni HtrA is more efficient at high temperature, while the chaperone activity of HtrAS197A is slightly reduced at 44°C. Interestingly, we found that chaperone activity alone is sufficient for growth of C. jejuni at high temperature, while E. coli and Salmonella require plasmid-mediated overexpression of protease-negative HtrA to suppress the temperature-sensitive phenotype of an htrA deletion mutant (34, 61), suggesting that the chaperone activity of C. jejuni is more efficient. Interestingly, only when the temperature and oxygen stresses are combined is protease activity essential for growth of C. jejuni. This observation suggests that severe stress increases the rate of protein damage to a point at which chaperone activity is not sufficient to prevent accumulation of toxic protein aggregates and that, therefore, proteolytic activity is needed to remove the damaged proteins from the HtrA oligomer. However, the observation that the chaperone activity is slightly less efficient in preventing aggregation at 44°C in vitro may also explain the requirement for protease activity during severe stress. It is noteworthy that HtrA protease activity is required for growth of C. jejuni only under rather extreme conditions, as 44°C is only 1°C below the upper temperature limit for growth of the wild type (16, 59) and 18% oxygen is far from the microaerobic environment ideal for C. jejuni (5). Consequently, HtrA chaperone activity may be sufficient to support growth of C. jejuni in the gastrointestinal tract of both avian and human hosts, due to the temperatures (42°C and 37°C, respectively) and the low oxygen concentration found in these environments (17).
Interestingly, our analyses of the requirement of HtrA for stress tolerance of C. jejuni showed that heat sensitivity of the htrA mutant depends on the level of oxidative stress, demonstrating that protein damage caused by increased temperature per se does not harm the bacterium enough to require HtrA for degradation or repair. While loss of htrA has been shown to cause sensitivity to high temperature in all examined bacteria (9, 11, 35, 41, 48), conditional heat sensitivity has not previously been reported, but revisiting the phenotypes of these mutants will reveal whether oxidative-stress-dependent thermosensitivity is a general feature of htrA mutants.
The data presented here show that HtrA plays a central role in protecting C. jejuni during oxidative stress, since an htrA mutant has increased sensitivity to oxygen, H2O2, cumene hydroperoxide, and paraquat. Brøndsted et al. previously reported no difference in sensitivity between the wild type and the ΔhtrA mutant in a similar disc diffusion assay at 37°C (6). However, the present data show that the sensitivity to these oxidants is most pronounced at 42°C, which may explain the discrepancy between the results. Reactive oxygen species can be generated by oxidation of growth medium constituents (7, 19) or by cellular respiration (65). The oxygen-sensitive phenotypes of the htrA mutants are mediated partly by H2O2 or O2− generated in the growth medium. However, H2O2 or O2− scavengers fail to rescue growth of the ΔhtrA mutant at 44°C and 18% O2, either because they cannot quench H2O2 and O2− completely or because HtrA also protects against reactive oxygen species arising from cellular respiration. The latter explanation seems likely, since C. jejuni encodes a cytochrome bc1 complex (47), which is a possible source of O2− in the periplasm (43), and a formate dehydrogenase, which generates periplasmic H2O2 (13, 19). HtrA may act in the oxidative stress defense of C. jejuni by degradation or refolding of oxidatively damaged proteins or, alternatively, by assisting the folding of periplasmic proteins that are part of the oxidative stress defense, such as the cytochrome c peroxidases (4, 47). Indeed, analysis of carbonylated surface proteins indicates that degradation of oxidatively damaged proteins by HtrA is limited, since the levels and the band patterns of carbonylated proteins were almost identical in the wild type and the htrA(S197A) mutant. In contrast, several carbonylated protein bands were absent in the ΔhtrA mutant, indicating that HtrA may capture oxidatively damaged proteins and thereby protect them from degradation by other proteases. A similar mechanism has been demonstrated in vitro with E. coli HtrA, which is able to protect a substrate from degradation by an added protease (32). Our data suggest that this role of HtrA is physiologically important in vivo, and we propose that HtrA may be responsible for rescuing oxidatively damaged proteins in the periplasm.
Our data suggest that both the chaperone and protease activities of HtrA are essential for maintaining periplasmic protein homeostasis in C. jejuni even under optimal growth conditions, which is indicated by increased amounts of DnaK and ClpB in the htrA mutants. Thus, ClpB and DnaK are upregulated in response to even low rates of protein misfolding occurring in the htrA(S197A) mutant at 42°C, while the growth defect is observed only under stress conditions that increase the rate of protein misfolding. In E. coli and Shigella flexneri, the HtrA chaperone is important for folding of outer membrane proteins and ensures their safe transit across the periplasmic space (32, 51). A similar function in C. jejuni would explain the need for HtrA chaperone activity under all growth conditions; however, so far we have not been able to determine if such a function applies to HtrA in C. jejuni. The proteolytic HtrA activity affects the structural arrangement of HtrA oligomers found in C. jejuni, as the wild type and the htrA(S197A) mutant contain large HtrA oligomers while the smaller and presumably resting oligomers are present only in the wild type. Consistent with this finding, the turnover of the active E. coli HtrA multimers into the resting hexameric conformation takes place after a substrate has been degraded (32). These findings suggest that proteolytically inactive HtrA may become occupied with unfoldable proteins. We therefore propose that the role of HtrA-mediated proteolysis under nonstress conditions in C. jejuni is simply to empty the HtrA cavity of unfoldable proteins, which otherwise hinder the proper folding of periplasmic proteins or the transfer of outer membrane proteins across the periplasmic space.
The proteolytic activity of HtrA also mediates autodegradation, which was observed in vivo under all tested conditions (37 to 44°C, 1% to 18% O2), as well as in vitro, with purified wild-type HtrA. In contrast, HtrA autodegradation occurs only under reducing conditions in E. coli, most likely because E. coli HtrA is stabilized by a disulfide bridge in the main regulatory loop (62). However, C. jejuni HtrA contains no disulfide bridge, supporting our observation that this protein is more prone to autodegradation. Our results furthermore indicate that the proteolytic activity may be important for regulation of HtrA expression, since, overall, more HtrA is produced in the wild type than in the htrA(S197A) mutant. However, the increased HtrA production is not controlled at the transcriptional level, as the amounts of htrA mRNA are similar in the wild type and the htrA(S197A) mutant. Expression studies using microarrays have shown that transcription of htrA in C. jejuni is induced moderately by heat (64) but downregulated in a low-oxygen environment (68). However, it is not known how the synthesis of HtrA is controlled in C. jejuni, as the genome does not encode homologs of the σE sensing system for extracytoplasmic stress, which regulates periplasmic chaperones in E. coli and Salmonella (6, 52). In particular, C. jejuni does not encode the HtrA paralog DegS, which cleaves an inner membrane protein during stress and initiates a signal cascade resulting in increased htrA expression in E. coli. It is tempting to speculate that, in the absence of a DegS-like protein, C. jejuni employs another mechanism to sense periplasmic stress that combines the regulatory proteolysis and the quality control proteolysis in the same protein. Future work will show whether C. jejuni HtrA is involved in such a regulatory mechanism.
C. jejuni is dependent on the HtrA and SurA chaperones to fold outer membrane proteins and maintain periplasmic protein homeostasis important for tolerating stress, since it lacks homologs of the Skp and FkpA chaperones (47) found in other Gram-negative bacteria (2, 60). While SurA does not possess protease activity, the chaperone activities of SurA and HtrA may functionally overlap in C. jejuni, as HtrA is upregulated in a surA mutant (3). SurA facilitates outer membrane protein biogenesis in E. coli (60), and consistent with this function, the outer membrane profile of C. jejuni is altered by a surA mutation (54). However, HtrA may have a more dominant role than SurA in maintaining periplasmic protein homeostasis, since a lack of SurA does not result in upregulation of DnaK and ClpB (53). Thus, both HtrA and SurA chaperone activities may be involved in folding of envelope proteins in C. jejuni, but the mechanism that determines the substrate specificity is so far unknown. However, HtrA may specifically be involved in degradation and folding of heat-damaged proteins, since expression data indicate that only HtrA is important for heat tolerance of C. jejuni, as surA is downregulated at high temperature (53), while htrA is upregulated (64). Thus, in summary, we here present data showing that the HtrA chaperone activity alone is sufficient to protect against either heat or oxidative stress in C. jejuni, while the protease activity of HtrA is essential only under severe stress conditions. Finally, other chaperones display additional enzymatic activity (40, 56), and for HtrA, the proteolytic activity may very well be crucial for the overall performance of HtrA as a chaperone.
Acknowledgments
We thank David R. Hendrixson for providing plasmid pDRH265. We sincerely appreciate the expert technical assistance of Lisbeth Schade Hansen and Birgit Andersen.
This study was financially supported by the Faculty of Life Sciences, University of Copenhagen.
Footnotes
Published ahead of print on 12 November 2010.
REFERENCES
- 1.Allos, B. M. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 2.Arie, J. P., N. Sassoon, and J. M. Betton. 2001. Chaperone function of FkpA, a heat shock prolyl isomerase, in the periplasm of Escherichia coli. Mol. Microbiol. 39:199-210. [DOI] [PubMed] [Google Scholar]
- 3.Asakura, H., M. Yamasaki, S. Yamamoto, and S. Igimi. 2007. Deletion of peb4 gene impairs cell adhesion and biofilm formation in Campylobacter jejuni. FEMS Microbiol. Lett. 275:278-285. [DOI] [PubMed] [Google Scholar]
- 4.Atack, J. M., and D. J. Kelly. 2009. Oxidative stress in Campylobacter jejuni: responses, resistance and regulation. Future Microbiol. 4:677-690. [DOI] [PubMed] [Google Scholar]
- 5.Bolton, F. J., and D. Coates. 1983. A study of the oxygen and carbon dioxide requirements of thermophilic campylobacters. J. Clin. Pathol. 36:829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brøndsted, L., M. T. Andersen, M. Parker, K. Jorgensen, and H. Ingmer. 2005. The HtrA protease of Campylobacter jejuni is required for heat and oxygen tolerance and for optimal interaction with human epithelial cells. Appl. Environ. Microbiol. 71:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, J., G. Nyberg, and J. Wrethen. 1978. Hydrogen peroxide and superoxide radical formation in anaerobic broth media exposed to atmospheric oxygen. Appl. Environ. Microbiol. 36:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion, O. L., A. V. Karlyshev, N. J. Senior, M. Woodward, R. La Ragione, S. L. Howard, B. W. Wren, and R. W. Titball. 2010. Insect infection model for Campylobacter jejuni reveals that O-methyl phosphoramidate has insecticidal activity. J. Infect. Dis. 201:776-782. [DOI] [PubMed] [Google Scholar]
- 9.Cortes, G., B. de Astorza, V. J. Benedi, and S. Alberti. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high temperature A (htrA) deletion mutant. Cancer Res. 62:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flannagan, R. S., D. Aubert, C. Kooi, P. A. Sokol, and M. A. Valvano. 2007. Burkholderia cenocepacia requires a periplasmic HtrA protease for growth under thermal and osmotic stress and for survival in vivo. Infect. Immun. 75:1679-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobom, J., E. Nordhoff, E. Mirgorodskaya, R. Ekman, and P. Roepstorff. 1999. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34:105-116. [DOI] [PubMed] [Google Scholar]
- 13.Goodhew, C. F., A. B. elKurdi, and G. W. Pettigrew. 1988. The microaerophilic respiration of Campylobacter mucosalis. Biochim. Biophys. Acta 933:114-123. [DOI] [PubMed] [Google Scholar]
- 14.Guccione, E., A. Hitchcock, S. J. Hall, F. Mulholland, N. Shearer, A. H. van Vliet, and D. J. Kelly. 2009. Reduction of fumarate, mesaconate and crotonate by Mfr, a novel oxygen-regulated periplasmic reductase in Campylobacter jejuni. Environ. Microbiol. 12:576-591. [DOI] [PubMed] [Google Scholar]
- 15.Hassan, H. M., and I. Fridovich. 1978. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J. Biol. Chem. 253:8143-8148. [PubMed] [Google Scholar]
- 16.Hazeleger, W. C., J. A. Wouters, F. M. Rombouts, and T. Abee. 1998. Physiological activity of Campylobacter jejuni far below the minimal growth temperature. Appl. Environ. Microbiol. 64:3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, G., R. A. Shankar, M. Chzhan, A. Samouilov, P. Kuppusamy, and J. L. Zweier. 1999. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. U. S. A. 96:4586-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrixson, D. R., B. J. Akerley, and V. J. DiRita. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol. Microbiol. 40:214-224. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman, P. S., H. A. George, N. R. Krieg, and R. M. Smibert. 1979. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. 2. Role of exogenous superoxide anions and hydrogen-peroxide. Can. J. Microbiol. 25:8-16. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman, P. S., N. R. Krieg, and R. M. Smibert. 1979. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. 1. Physiological aspects of enhanced aerotolerance. Can. J. Microbiol. 25:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 22.Humphrey, T., S. O'Brien, and M. Madsen. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int. J. Food Microbiol. 117:237-257. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys, S., G. Rowley, A. Stevenson, W. J. Kenyon, M. P. Spector, and M. Roberts. 2003. Role of periplasmic peptidylprolyl isomerases in Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 71:5386-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huston, W. M., J. E. Swedberg, J. M. Harris, T. P. Walsh, S. A. Mathews, and P. Timms. 2007. The temperature activated HtrA protease from pathogen Chlamydia trachomatis acts as both a chaperone and protease at 37 degrees C. FEBS Lett. 581:3382-3386. [DOI] [PubMed] [Google Scholar]
- 25.Iwanczyk, J., D. Damjanovic, J. Kooistra, V. Leong, A. Jomaa, R. Ghirlando, and J. Ortega. 2007. Role of the PDZ domains in Escherichia coli DegP protein. J. Bacteriol. 189:3176-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jomaa, A., D. Damjanovic, V. Leong, R. Ghirlando, J. Iwanczyk, and J. Ortega. 2007. The inner cavity of Escherichia coli DegP protein is not essential for molecular chaperone and proteolytic activity. J. Bacteriol. 189:706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jomaa, A., J. Iwanczyk, J. Tran, and J. Ortega. 2009. Characterization of the autocleavage process of the Escherichia coli HtrA protein: implications for its physiological role. J. Bacteriol. 191:1924-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim, D. Y., D. R. Kim, S. C. Ha, N. K. Lokanath, C. J. Lee, H. Y. Hwang, and K. K. Kim. 2003. Crystal structure of the protease domain of a heat-shock protein HtrA from Thermotoga maritima. J. Biol. Chem. 278:6543-6551. [DOI] [PubMed] [Google Scholar]
- 29.Koebnik, R., K. P. Locher, and P. Van Gelder. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239-253. [DOI] [PubMed] [Google Scholar]
- 30.Krieg, N. R., and P. S. Hoffman. 1986. Microaerophily and oxygen toxicity. Annu. Rev. Microbiol. 40:107-130. [DOI] [PubMed] [Google Scholar]
- 31.Krojer, T., K. Pangerl, J. Kurt, J. Sawa, C. Stingl, K. Mechtler, R. Huber, M. Ehrmann, and T. Clausen. 2008. Interplay of PDZ and protease domain of DegP ensures efficient elimination of misfolded proteins. Proc. Natl. Acad. Sci. U. S. A. 105:7702-7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krojer, T., J. Sawa, E. Schafer, H. R. Saibil, M. Ehrmann, and T. Clausen. 2008. Structural basis for the regulated protease and chaperone function of DegP. Nature 453:885-890. [DOI] [PubMed] [Google Scholar]
- 33.Leon-Kempis, M. D. R., E. Guccione, F. Mulholland, M. P. Williamson, and D. J. Kelly. 2006. The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol. Microbiol. 60:1262-1275. [DOI] [PubMed] [Google Scholar]
- 34.Lewis, C., H. Skovierova, G. Rowley, B. Rezuchova, D. Homerova, A. Stevenson, J. Spencer, J. Farn, J. Kormanec, and M. Roberts. 2009. Salmonella enterica serovar Typhimurium HtrA: regulation of expression and role of the chaperone and protease activities during infection. Microbiology (Reading, Engl.) 155:873-881. [DOI] [PubMed] [Google Scholar]
- 35.Lipinska, B., O. Fayet, L. Baird, and C. Georgopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipinska, B., M. Zylicz, and C. Georgopoulos. 1990. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J. Bacteriol. 172:1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luechtefeld, N. W., L. B. Reller, M. J. Blaser, and W.-L. L. Wang. 1982. Comparison of atmospheres of incubation for primary isolation of Campylobacter fetus subsp. jejuni from animal specimens: 5% oxygen versus candle jar. J. Clin. Microbiol. 15:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCoy, E. C., D. Doyle, K. Burda, L. B. Corbeil, and A. J. Winter. 1975. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect. Immun. 11:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, C. E., P. H. Williams, and J. M. Ketley. 2009. Pumping iron: mechanisms for iron uptake by Campylobacter. Microbiology 155:3157-3165. [DOI] [PubMed] [Google Scholar]
- 40.Missiakas, D., J. M. Betton, and S. Raina. 1996. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol. Microbiol. 21:871-884. [DOI] [PubMed] [Google Scholar]
- 41.Mo, E., S. E. Peters, C. Willers, D. J. Maskell, and I. G. Charles. 2006. Single, double and triple mutants of Salmonella enterica serovar Typhimurium degP (htrA), degQ (hhoA) and degS (hhoB) have diverse phenotypes on exposure to elevated temperature and their growth in vivo is attenuated to different extents. Microb. Pathog. 41:174-182. [DOI] [PubMed] [Google Scholar]
- 42.Moen, B., A. Oust, O. Langsrud, N. Dorrell, G. L. Marsden, J. Hinds, A. Kohler, B. W. Wren, and K. Rudi. 2005. Explorative multifactor approach for investigating global survival mechanisms of Campylobacter jejuni under environmental conditions. Appl. Environ. Microbiol. 71:2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller, F. L., Y. Liu, and H. Van Remmen. 2004. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 279:49064-49073. [DOI] [PubMed] [Google Scholar]
- 44.Novik, V., D. Hofreuter, and J. E. Galan. 2010. Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect. Immun. 78:3540-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyström, T. 2005. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park, S. F. 2002. The physiology of Campylobacter species and its relevance to their role as foodborne pathogens. Int. J. Food Microbiol. 74:177-188. [DOI] [PubMed] [Google Scholar]
- 47.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. Van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen, L. L., M. Radulic, M. Doric, and Y. Abu Kwaik. 2001. HtrA homologue of Legionella pneumophila: an indispensable element for intracellular infection of mammalian but not protozoan cells. Infect. Immun. 69:2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pei, Z. H., R. T. Ellison III, and M. J. Blaser. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 266:16363-16369. [PubMed] [Google Scholar]
- 50.Pelle, R., and N. B. Murphy. 1993. Northern hybridization: rapid and simple electrophoretic conditions. Nucleic Acids Res. 21:2783-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Purdy, G. E., C. R. Fisher, and S. M. Payne. 2007. IcsA surface presentation in Shigella flexneri requires the periplasmic chaperones DegP, Skp, and SurA. J. Bacteriol. 189:5566-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raivio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 53.Rathbun, K. M., J. E. Hall, and S. A. Thompson. 2009. Cj0596 is a periplasmic peptidyl prolyl cis-trans isomerase involved in Campylobacter jejuni motility, invasion, and colonization. BMC Microbiol. 9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rathbun, K. M., and S. A. Thompson. 2009. Mutation of PEB4 alters the outer membrane protein profile of Campylobacter jejuni. FEMS Microbiol. Lett. 300:188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizzitello, A. E., J. R. Harper, and T. J. Silhavy. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794-6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rouviere, P. E., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz, N., D. Kahne, and T. J. Silhavy. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57-66. [DOI] [PubMed] [Google Scholar]
- 58.Schagger, H., W. A. Cramer, and G. von Jagow. 1994. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217:220-230. [DOI] [PubMed] [Google Scholar]
- 59.Skirrow, M. B., and J. Benjamin. 1980. ′1001′ campylobacters: cultural characteristics of intestinal campylobacters from man and animals. J. Hyg. (Lond.) 85:427-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sklar, J. G., T. Wu, D. Kahne, and T. J. Silhavy. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 21:2473-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skorko-Glonek, J., E. Laskowska, A. Sobiecka-Szkatula, and B. Lipinska. 2007. Characterization of the chaperone-like activity of HtrA (DegP) protein from Escherichia coli under the conditions of heat shock. Arch. Biochem. Biophys. 464:80-89. [DOI] [PubMed] [Google Scholar]
- 62.Skorko-Glonek, J., D. Zurawa, F. Tanfani, A. Scire, A. Wawrzynow, J. Narkiewicz, E. Bertoli, and B. Lipinska. 2003. The N-terminal region of HtrA heat shock protease from Escherichia coli is essential for stabilization of HtrA primary structure and maintaining of its oligomeric structure. Biochim. Biophys. Acta 1649:171-182. [DOI] [PubMed] [Google Scholar]
- 63.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 64.Stintzi, A. 2003. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 185:2009-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storz, G., and J. A. Imlay. 1999. Oxidative stress. Curr. Opin. Microbiol. 2:188-194. [DOI] [PubMed] [Google Scholar]
- 66.Szymanski, C. M., and B. W. Wren. 2005. Protein glycosylation in bacterial mucosal pathogens. Nat. Rev. Microbiol. 3:225-237. [DOI] [PubMed] [Google Scholar]
- 67.Verhoeff-Bakkenes, L., A. P. Arends, J. L. Snoep, M. H. Zwietering, and J. R. De. 2008. Pyruvate relieves the necessity of high induction levels of catalase and enables Campylobacter jejuni to grow under fully aerobic conditions. Lett. Appl. Microbiol. 46:377-382. [DOI] [PubMed] [Google Scholar]
- 68.Woodall, C. A., M. A. Jones, P. A. Barrow, J. Hinds, G. L. Marsden, D. J. Kelly, N. Dorrell, B. W. Wren, and D. J. Maskell. 2005. Campylobacter jejuni gene expression in the chick cecum: evidence for adaptation to a low-oxygen environment. Infect. Immun. 73:5278-5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto, T., T. Hanawa, S. Ogata, and S. Kamiya. 1996. Identification and characterization of the Yersinia enterocolitica gsrA gene, which protectively responds to intracellular stress induced by macrophage phagocytosis and to extracellular environmental stress. Microbiology 64:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]