Abstract
Cry11Ba is one of the most toxic proteins to mosquito larvae produced by Bacillus thuringiensis. It binds Aedes aegypti brush border membrane vesicles (BBMV) with high affinity, showing an apparent dissociation constant (Kd) of 8.2 nM. We previously reported that an anticadherin antibody competes with Cry11Ba binding to BBMV, suggesting a possible role of cadherin as a toxin receptor. Here we provide evidence of specific cadherin repeat regions involved in this interaction. Using cadherin fragments as competitors, a C-terminal fragment which contains cadherin repeat 7 (CR7) to CR11 competed with Cry11Ba binding to BBMV. This binding was also efficiently competed by the CR9, CR10, and CR11 peptide fragments. Moreover, we show CR11 to be an important region of interaction with Cry11Ba toxin. An alkaline phosphatase (AaeALP1) and an aminopeptidase-N (AaeAPN1) also competed with Cry11Ba binding to Ae. aegypti BBMV. Finally, we found that Cry11Ba and Cry4Ba share binding sites. Synthetic peptides corresponding to loops α8, β2-β3 (loop 1), β8-β9, and β10-β11 (loop 3) of Cry4Ba compete with Cry11Ba binding to BBMV, suggesting Cry11Ba and Cry4Ba have common sites involved in binding Ae. aegypti BBMV. The data suggest that three different Ae. aegypti midgut proteins, i.e., cadherin, AaeALP1, and AaeAPN1, are involved in Cry11Ba binding to Ae. aegypti midgut brush border membranes.
Microbiological control strategies involving Bacillus thuringiensis subsp. israelensis or Bacillus sphaericus are increasingly used worldwide for the control of insect vectors. B. thuringiensis subsp. israelensis produces four major insecticidal Cry proteins (Cry4Aa, Cry4Ba, Cry10Aa, and Cry11Aa) and three cytolytic proteins (Cyt1Aa, Cyt2Ba, and Cyt1Ca) (6). Among them, Cry11Aa is the most active toxin against Aedes aegypti (13). However, B. thuringiensis strains producing other mosquitocidal Cry toxins have been identified, including B. thuringiensis subsp. jegathesan (27). Parasporal crystals of this species contain seven major proteins, one of which is a protein of 80 kDa designated Cry11Ba. Cry11Ba exhibits 58% identity with Cry11Aa at the amino acid level (15), and it is, to date, the toxin with the highest activity against mosquitoes, having about 6 to 40 times more activity (depending on the species of mosquito tested) than Cry11Aa of B. thuringiensis subsp. israelensis (15). Consequently, the Cry11Ba toxin is an alternative to those used in current control programs, since the use of this toxin may directly address the risk of development of resistance to B. thuringiensis subsp. israelensis or B. sphaericus toxins in mosquitoes.
The highly conserved structure of Cry toxins suggests that they may share a mode of action in which domains II and III, composed mainly of β sheets, are responsible for binding membrane receptors. A number of proteins have been identified as Cry toxin receptors, such as cadherin, alkaline phosphatases (ALPs), and aminopeptidase (APN), and these were first identified in different lepidopteran insects as Cry1A toxin binding proteins (4, 22, 23, 26, 29, 32, 36, 37, 42, 44). In mosquito larvae, similar proteins have been described. Cadherin-like proteins that bind Cry4Ba in Anopheles gambiae (24) and Cry11Aa in Ae. aegypti (11) were described. Moreover, glycosylphosphatidylinositol (GPI)-anchored proteins, such as APNs from Anopheles quadrimaculatus, An. gambiae, and Ae. aegypti (1, 12, 45, 46) and ALPs from Ae. aegypti and An. gambiae (18, 25), were also found to bind Cry11Aa and Cry11Ba toxins and were proposed as potential toxin receptors. After binding receptors, Cry toxins are believed to create pores that are permeable to small ions and solutes by use of membrane-embedded oligomeric Cry structures, therefore causing osmotic lysis of midgut cells in susceptible insects (16, 39, 40). However, little is known about Ae. aegypti midgut proteins that bind the Cry11Ba toxin. Here we identify and characterize these proteins.
MATERIALS AND METHODS
Cry11Ba preparation and toxin biotinylation.
A B. thuringiensis strain expressing only the Cry11Ba (15) was grown in nutrient broth sporulation medium with erythromycin (25 μg/ml) at 30°C (30). Following autolysis, spores and inclusions were harvested and washed three times with 1 M NaCl-10 mM EDTA, pH 8.0. The final pellet was resuspended in the same buffer (30 ml) and purified by use of NaBr gradients as previously described (10). Purified Cry11Ba inclusions were solubilized in 50 mM Na2CO3 (pH 10.0) and activated with trypsin (1:20, wt/wt). Ion exchange chromatography (MonoQ fast protein liquid chromatography [FPLC]) (AKTA; Amersham Biosciences) was used to further purify the activated-Cry11Ba toxin. The solubilized and activated Cry11Ba toxin was biotinylated using a protein biotinylation module kit (40 μl of reagent with 1 mg toxin; Amersham Biosciences), and a Sephadex G25 column was used to remove uncoupled biotin.
Preparation of BBMV.
Midguts were dissected from early fourth-instar Ae. aegypti larvae and kept at −80°C until use. Brush border membrane vesicles (BBMV) were prepared by the differential magnesium precipitation method (33). BBMV were resuspended in ice-cold buffer A (0.3 M mannitol, 0.5 M EGTA, 20 mM Tris-Cl, pH 8), and the concentration of total protein was measured with a bicinchoninic acid (BCA) kit (Pierce). Alkaline phosphatase and leucine aminopeptidase activities were determined as previously described (33). Freshly prepared BBMV were kept on ice and used the same day.
Western blotting and toxin overlay assay.
Purified cadherin repeats CR7 to CR11 (10 μg) were separated by 10% SDS-PAGE and electrotransferred, and the membranes were incubated first in blocking buffer (phosphate-buffered saline [PBS] and 0.1% Tween 20 [PBST] with 5% skim milk) for 1 h and then with 20 nM Cry11Ba toxins for 2 h. Unbound toxins were removed by washing the membrane four times with washing buffer (PBST) for 15 min each. Membranes were then incubated with rabbit anti-Cry11Ba polyclonal antibody (1:1,500 dilution) followed by a secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (HRP) (1:5,000 dilution), and bound toxin revealed using luminol (ECL; Amersham Biosciences).
Toxin binding assays.
The kinetics of Cry11Ba binding to BBMV were measured using a modified microplate assay. Briefly, 96-well plates coated with 4 μg BBMV were incubated overnight at 4°C and then in PBST for 1 h at room temperature. Increasing concentrations of biotinylated Cry11Ba toxin (0.01 to 200 nM) in 100 μl binding buffer (0.1% bovine serum albumin [BSA], 0.1% Tween 20, 1× PBS, pH 7.4) were then transferred to the BBMV-coated plates. Parallel plates were run under identical conditions except in the presence of 10 μM unlabeled Cry11Ba. After 2 h, the plates were washed with 100 μl PBST three times. Bound biotinylated Cry11Ba protein was detected by incubation with streptavidin-horseradish peroxidase (HRP) conjugate (1:1,500) for 1 h. After washing three times with PBST, HRP activity was revealed with a freshly prepared luminol substrate (Supersignal enzyme-linked immunosorbent assay [ELISA] pico; Thermo Scientific). An X-ray film was place over the microplate in a darkroom for 1 to 5 min, and the data were quantified with NIH Image J software and analyzed using Origin (Origin Lab). Specific binding was calculated from total minus nonspecific binding. The concentration corresponding to half the saturation response of specific binding was considered the dissociation constant (Kd) and was obtained from two independent experiments using different BBMV preparations.
For competition assays, biotinylated Cry11Ba toxin (10 nM) was equilibrated with increasing amounts of unlabeled Cry11Ba or Cry4Ba protein (0.01 to 1,500 nM) in PBST (100 μl) for 1 h at room temperature. The mixtures were then transferred to plates previously coated with BBMV for 2 h. The plates were washed with PBST (100 μl). Bound biotinylated Cry11Ba protein was detected as described above. The concentration corresponding to half the maximal response was considered the 50% inhibitory concentration (IC50) and was obtained from two independent experiments using different BBMV preparations.
Competition binding assays with cadherin, ALP, and APN.
Assays of toxin binding to BBMV were done in binding buffer (100 μl, 0.1% BSA, 0.1% Tween 20, 1× PBS, pH 7.4). BBMV (10 μg protein) were incubated with biotinylated Cry11Ba toxin (10 nM) in the presence or absence of unlabeled Cry11Ba toxin, cadherin fragments, cadherin repeats (CR), Ae. aegypti ALP1 (AaeALP1) to -3, AaeAPN1, and Cry4Ba loop peptides for 1 h at room temperature. The mixtures were centrifuged at 10,000 × g for 10 min to remove unbound toxins, and the pellet then was washed three times with binding buffer. BBMV were resuspended in 20 μl of PBS and 4 μl of 6× Laemmli sample loading buffer (60% glycerol, 300 mM Tris-Cl [pH 6.8], 12 mM EDTA, 12% SDS, 864 mM 2-mercaptoethnol, 0.05% bromophenol blue). The samples were then boiled for 5 min, separated by SDS-PAGE, and electrotransferred to polyvinylidene difluoride (PVDF) membranes (Immobilon; Amersham Biosciences). The membranes were incubated with streptavidin-peroxidase conjugate (1:1,500 dilution; Amersham Biosciences) for 1 h and then visualized using luminal (ECL; Amersham Biosciences).
Expression of different proteins in E. coli.
Constructs in pQE30 encoding the Cry11Ba wild-type and the mutant toxins (α8-V256A/G257A/E258A, L1-R303A/E304A/N305, L3-N454A/K455A/L456A, and L1-H307A) (28) as well as Escherichia coli clones of cadherin fragments G10, G7, and C13 (11), AaeALP1 to -3 (10), and AaeAPN1 (12) were transformed into E. coli M15 competent cells for protein expression. Bacterial clones harboring the different plasmids were grown at 37°C in Luria-Bertani medium containing ampicillin (l00 μg/ml) and kanamycin (50 μg/ml) until the optical density at 600 nm (OD600) of the culture reached 0.3 to 0.5. Protein expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM for 4 h. Cells containing cytoplasmic inclusions were harvested by centrifugation and resuspended in B-PER solution (Amersham). Cells were then disrupted by sonication for 10 min (Sonifier 450). After centrifugation at 10,000 × g and 4°C for 10 min, the pellets were washed three times in 1% Triton X-l00 and suspended by sonication. Protein concentrations of the partially purified inclusions were determined by using a BCA kit (Pierce). Inclusions (1 to 2 mg/ml) were solubilized by incubation at 37°C for 2 to 3 h in 50 mM NaOH and then dialyzed in 50 mM Na2CO3 (pH 10.0) at 4°C overnight. The quality of these proteins was analyzed by 10% SDS-PAGE.
Competitive enzyme-linked immunosorbent assay-based binding assays.
In brief, 10 nM G10 was equilibrated with increasing concentrations (0.01 to 1,000 nM) of mutants of Cry11Ba (α8-V256A/G257A/E258A, L1-R303A/E304A/N305A, and L3-N454A/K455A/L456A) in PBST (100 μl) for 1 h at room temperature. The mixtures were then transferred to ELISA plate wells previously coated with wild-type Cry11Ba toxin and treated with blocking buffer (PBS, 0.1% Tween 20, 0.5% gelatin). After washing, the bound G10 protein was detected by polyclonal anticadherin antibody (1:2,500 dilution), followed by incubation with a goat anti-rabbit alkaline phosphatase (ALP) conjugate antibody (1:1,500 dilution). The ALP enzymatic activity was revealed with a freshly prepared substrate (3 mM nitrophenyl phosphate), and the absorbance was read at 405 nm (Molecular Devices, Sunnyvale, CA).
Mass spectrometry.
Protein bands of activated Cry11Ba toxin were excised from the SDS-polyacrylamide gel, digested with trypsin, and analyzed by nano-ultra-performance liquid chromatography/tandem mass spectrometry (nano-UPLC/MS/MS) for de novo sequencing of both peptides (Institute of Integrative Genome Biology [IIGB], University of California, Riverside). The sequences were compared to the full-length Cry11Ba sequence to identify putative cleavage sites.
Larval bioassays.
Larvicidal assays were performed using fourth-instar larvae. The assays were done at room temperature in water (200 ml), each with 25 larvae. The 50% lethal concentration (LC50) and the 95% fiducial limits were obtained by using a probit analysis program, version 1.5 (U.S. Environmental Protection Agency). Any overlap of the fiducial limits indicates a lack of statistical difference at the 95% level of confidence.
RESULTS
Dissociation constant and competitive assays of Cry11Ba toxin binding to Ae. aegypti BBMV.
A number of studies showed the interaction of Cry11Aa or Cry4Ba toxins with mosquito BBMV (5, 17, 20). Toxin affinity to BBMV has been measured with Cry11Aa, Cry4Aa and Cry4Ba, Cry1C, and the binary toxin from B. sphaericus (2, 3, 14, 33, 34). Here we determined the binding affinity of Cry11Ba toxin to Ae. aegypti BBMV using biotinylated Cry11Ba. We first demonstrated that biotin-labeled Cry11Ba remains toxic to mosquito larvae. The calculated 50% lethal concentration (LC50) of soluble Cry11Ba was 8,050 ng/ml, whereas the LC50 of biotin-labeled Cry11Ba was in the same range (10,000 ng/ml). The trypsin-activated forms of Cry11Ba or biotinylated Cry11Ba showed lower toxicity, with LC50s of 40,000 and 49,000 ng/ml, respectively. Overall, no significant difference in toxicity was observed after biotinylation of the toxin (Table 1).
TABLE 1.
Toxicity of Cry11Ba toxins with or without biotinylation against Ae. aegypti mosquito larvae
| Cry11Ba toxin | LC50, ng/ml (95% fiducial limit) |
||
|---|---|---|---|
| Inclusion body | Soluble toxin | Trypsin-activated toxin | |
| Unlabeled | 15.81 (10.2-29.37) | 8,053 (5,260-10,546) | 39,770 (31,193-50,637) |
| Biotin labeled | NDa | 9,997 (5,487-10,906) | 49,064 (35,796-57,412) |
ND, not determined.
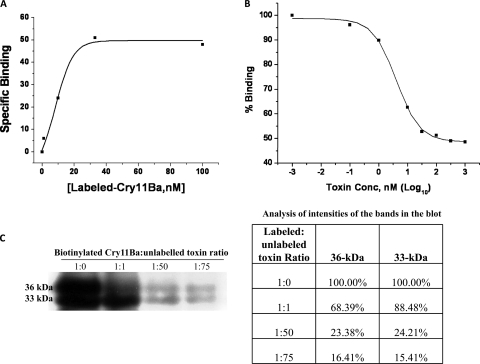
The affinity of Cry11Ba binding to BBMV was then determined using increasing concentrations of biotinylated Cry11Ba. Binding of Cry11Ba toxin to proteins on BBMV isolated from Ae. aegypti was specific and dose dependent showing a dissociation constant (Kd) of 8.2 nM (Fig. 1 A). In an alternative assay, binding of biotinylated Cry11Ba to BBMV was measured in the presence of increasing concentrations of unlabeled Cry11Ba toxin (0.001 to 1,000 nM). A half-maximal inhibitory concentration (IC50) of 3.6 nM was obtained (Fig. 1B. Furthermore, it is important to note that freshly prepared BBMV were required, presumably due to rapid degradation of BBMV proteins that bound Cry11Ba.
FIG. 1.
Binding of Cry11Ba toxin to Ae. aegypti BBMV. (A) Specific binding of biotinylated Cry11Ba toxin to BBMV was determined in the presence of increasing concentrations of labeled Cry11Ba toxin. Specific binding was obtained from total binding minus nonspecific binding. The dissociation constant (Kd) (8.2 nM) for toxin binding affinity was determined by Origin plot analysis from two different BBMV preparations. The curve shown is from a single experiment. (B) Binding of biotinylated Cry11Ba toxin to BBMV was determined in the presence of increasing concentrations of unlabeled Cry11Ba toxin. The IC50 (3.6 nM) for toxin binding affinity was determined by Origin plot analysis from three different BBMV preparations. The curve shown is from a single experiment. (C) Homologous competition in the binding of biotinylated Cry11Ba toxin to BBMV was also analyzed in solution; bound toxins to the BBMV were recovered by centrifugation, subjected to SDS-PAGE, and transferred to PVDF membranes. Biotinylated toxins were visualized with streptavidin-HRP. Different ratios of labeled to unlabeled toxins were used. At a ratio of 1:50 or 1:75, binding of biotinylated Cry11Ba was competed.
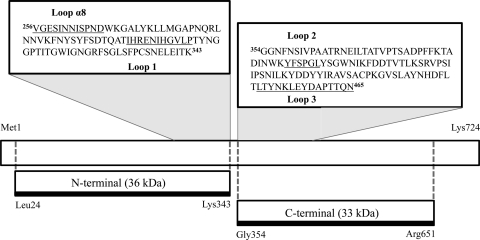
Cry11Ba inclusions were purified from a recombinant B. thuringiensis strain by use of density gradients. Upon activation, the 80-kDa Cry11Ba protein generated 33- and 36-kDa fragments. Both fragments were analyzed using tandem mass spectrometry, which showed that proteolytic cleavage occurs at the N and C termini, resulting in 24Leu and 651Arg as the corresponding termini. Intramolecular processing occurs between 343Lys and 354Gly (Fig. 2), but these two fragments are kept together by salt bridges. The N-terminal 36-kDa fragment consists of all of domain I and loops α8 and 1 of domain II, while the 33-kDa fragment represents the C-terminal end containing the rest of domain II, including loops 2 and 3, and the complete domain III of Cry11Ba toxin (Fig. 2).
FIG. 2.
Schematic analysis of Cry11Ba toxin proteolysis. The 80-kDa Cry11Ba protoxin was processed in vitro by trypsin. The Cry11Ba toxin is cleaved between 343Lys and 354Gly, generating a 36-kDa N-terminal fragment and a 33-kDa C-terminal fragment. Loop region sequences in the Cry11Ba toxin are underlined in insets. Loop α8 and loop 1 were identified in the N-terminal region of Cry11Ba, whereas loop 2 and loop 3 were in the C-terminal fragment.
The activated 33- and 36-kDa toxin fragments were labeled with biotin, and their binding to Ae. aegypti BBMV was analyzed. Homologous competition assays show that at a molar ratio of 1:1 of labeled to unlabeled Cry11Ba toxin, the unlabeled toxin competed with binding of the 36-kDa fragment, followed by competition with the 33-kDa fragment (Fig. 1C). At ratios of 1:50 and higher, binding to both fragments of the biotinylated Cry11Ba was nearly totally competed (Fig. 1C).
Aedes cadherin fragments compete with Cry11Ba binding to BBMV.
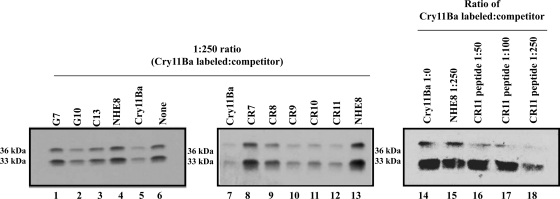
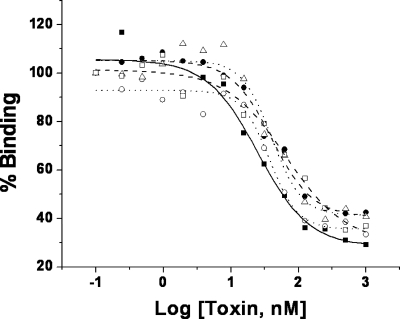
The Ae. aegypti cadherin fragments G7 (includes cadherin repeats 1 to 5), C13 (containing CR3 to -7), and G10 (containing CR7 to -11) were examined for their ability to compete Cry11Ba binding to Ae. aegypti BBMV. As shown in Fig. 3 and Table 2, the G10 fragment (Fig. 3, lane 2) significantly competed with the binding of Cry11Ba to BBMV. The G7 and C13 fragments showed lower competition, particularly to the 33-kDa fragment. In contrast, the negative-control peptide NHE8, derived from Ae. aegypti NHE ion exchanger protein, does not compete (Table 2; Fig. 3). Since the G10 fragment showed the highest competition, we further analyzed the binding competition of individual CR regions that are present in G10 (CR7 to CR11). The cadherin repeats were expressed in E. coli individually and used in toxin overlay assays. Our results indicated that CR7 to CR11 bound Cry11Ba toxin. However, CR11 appeared to be show the highest binding to Cry11Ba (data not shown). In addition, to demonstrate the role of these cadherin repeats in the binding interaction with Cry11Ba toxin, heterologous competition assays using individual CR peptides were performed. Repeats CR9 to CR11 were the most critical regions for interaction with Cry11Ba toxin, whereas CR8 competed off only half of Cry11Ba binding and CR7 did not compete at all (Fig. 3, lanes 8 to 12, and Table 2). A synthetic peptide of CR11 also showed high competition with Cry11Ba binding to BBMV (Fig. 3, lanes 16 to 18).
FIG. 3.
Cadherin is involved in binding of Cry11Ba to Ae. aegypti midgut membranes. Homologous and heterologous competition assays of binding of biotinylated Cry11Ba to Ae. aegypti BBMV were performed in the presence of different competitors, including cadherin fragments, cadherin repeats, and a cadherin synthetic peptide. Cadherin fragments G7, G10, and C13 (lanes 1 to 3, respectively) and unlabeled Cry11Ba (lane 5) were used to compete the binding of biotinylated Cry11Ba to BBMV at a 250-fold molar excess. Lane 6 shows uncompeted toxin binding. Cadherin repeats CR7 to CR11, also at 250-fold-higher concentrations, were used as competitors in lanes 8 to 12, respectively. Finally, a synthetic peptide corresponding to CR11 sequence (lanes 16 to 18) was used at molar excesses of 1:50, 1:100, and 1:250, respectively (Cry11Ba biotinylated toxin to CR11 peptide). NHE8 (lanes 4, 13, and 15), an unrelated peptide, was used as a negative control in competition experiments.
TABLE 2.
Cadherin fragments, cadherin repeats, AaeALPs, and AaeAPN1 compete with Cry11Ba binding to BBMV
| Competitorb | % of binding of biotinylated-Cry11Ba to Ae. aegypti BBMVa |
|
|---|---|---|
| Domains I-II, 36-kDa fragment | Domains II-III, 33-kDa fragment | |
| Cry11Ba toxin | 26.9 | 33.2 |
| Cadherin fragment G7 | 73.3 | 77.3 |
| Cadherin fragment G10 | 30.9 | 32.7 |
| Cadherin fragment C13 | 37.7 | 62.1 |
| Cadherin repeat 7 | 86.4 | 91.3 |
| Cadherin repeat 8 | 68.8 | 67.6 |
| Cadherin repeat 9 | 44.2 | 51.1 |
| Cadherin repeat 10 | 46.2 | 52.5 |
| Cadherin repeat 11 | 49.6 | 53.2 |
| AaeALP1 | 35.6 | 49.4 |
| AaeALP2 | 71.2 | 83.5 |
| AaeALP3 | 75.8 | 97.5 |
| AaeAPN1 | 45.7 | 49.0 |
| Peptide NHE8 | 92.9 | 100.8 |
Experiments were repeated at least two times to obtain mean data.
Competitor peptides were used at a 1:250 molar ratio excess of Cry11Ba.
Role of ALPs and APN in binding interaction with Cry11Ba toxin.
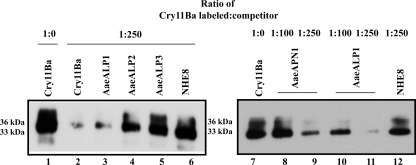
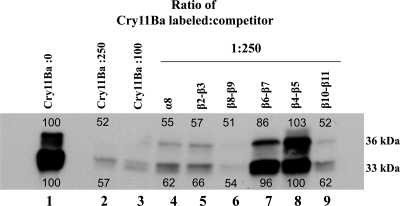
We also determined if AaeALPs (AaeALP1, AaeALP2, and AaeALP3) and AaeAPN1 are able to compete in Cry11Ba binding to BBMV. The binding of biotinylated Cry11Ba on Ae. aegypti BBMV can be competed principally with AaeALP1, while AaeALP2 and AaeALP3 barely competed Cry11Ba binding to BBMV (Fig. 4, lanes 3 to 5). For the 36-kDa Cry11Ba fragment, 64% was competed off with AaeALP1, whereas competitors such as AaeALP2 and AaeALP3 competed only 29% and 24% of toxin binding, respectively. Similarly, the 33-kDa Cry11Ba fragment was competed with AaeALP1 at 50%, whereas AaeALP2 and AaeALP3 compete in smaller amounts, at 17% and 3%, respectively. Recently, GPI-anchored APNs from An. quadrimaculatus and An. gambiae were determined to bind Cry11Ba and considered potential toxin receptors (1, 45). Here we also observed competitive binding between biotinylated Cry11Ba and AaeAPN1 (Fig. 4, lanes 8 and 9), suggesting a possible role of APN as a toxin receptor for Cry11Ba in Ae. aegypti mosquito larvae.
FIG. 4.
APN and ALP compete with Cry11Ba binding to Ae. aegypti membranes. Heterologous competition assays of binding of biotinylated Cry11Ba to Ae. aegypti BBMV were performed in the presence of different competitors, including AaeAPN1 and AaeALP1 to -3. Purified AaeAPN1 and AaeALP1 to -3 at a 100- or 250-fold molar excess were incubated with biotinylated Cry11Ba overnight before binding to Ae. aegypti BBMV. NHE8 (lane 6 and 12), an unrelated peptide, was used as a negative control.
Analyses of binding of different Cry11Ba mutant toxins to the G10 cadherin fragment.
Four Cry11Ba mutants with mutations located in different loop regions of domain II were expressed in E. coli and used to assess binding to the G10 cadherin fragment. Mutants with multiple mutations in three loop regions (loop α8-V256A/G257A/E258A, loop 1-R303A/E304A/N305A, and loop 3-N454A/K455A/L456A), all of which lost toxicity to mosquito larvae, and a single mutant (loop 1-H307A), which had slightly lower toxicity than the wild-type Cry11Ba toxin, were analyzed for their ability to bind the G10 cadherin fragment. There are differences, though minor, in the 50% effective concentrations (EC50s) of binding between the wild-type and mutant Cry11Ba toxins (Fig. 5). Thus, binding to just the cadherin fragment does not appear to be correlated with larval toxicity.
FIG. 5.
Loop mutants of Cry11Ba toxin retain their ability to bind the G10 cadherin fragment. Binding of wild-type Cry11Ba (▪) and the four loop mutants α8-V256A/G257A/E258A (○), L1-R303A/E304A/N305A (▵), L3-N454A/K455A/L456A (•), and L1-H307A (□) is shown. The G10 cadherin fragment EC50 for binding to Cry11Ba is 25 nM, and those for the four mutants were 38, 41, 40, and 66 nM, respectively. The EC50s were determined using Origin.
Assays of binding competition between Cry11Ba and Cry4Ba toxins.
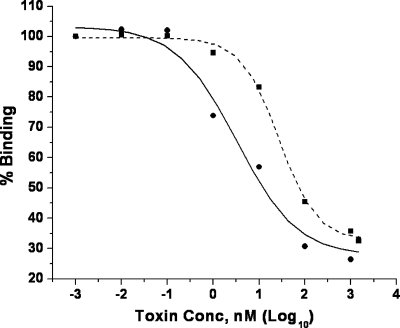
Analyses of Cry11Ba and Cry4Ba binding competition with Ae. aegypti BBMV were conducted through heterologous competitions. Competitions were conducted with labeled Cry11Ba and unlabeled Cry11Ba or Cry4Ba to determine if the two toxins share a binding site present in BBMV. The binding of biotinylated Cry11Ba to BBMV was competed in the presence of increasing concentrations of unlabeled Cry11Ba toxin as well as unlabeled Cry4Ba toxin (Fig. 6). As expected, homologous competition with Cry11Ba was more effective than heterologous competition with the Cry4Ba toxin.
FIG. 6.
Competition binding of biotinylated Cry11Ba toxin to Ae. aegypti BBMV in the presence of unlabeled Cry11Ba or Cry4Ba toxin. Results of competitive assays of binding of biotinylated Cry11Ba toxin to BBMV in the presence of Cry11Ba (•) or Cry4Ba (▪) as a competitor were plotted by using Origin. The experiment was repeated once with similar results. The concentration of Cry4Ba required to compete 50% of the binding of biotinylated Cry11Ba to BBMV was 42 nM.
To identify the critical loop regions of Cry4Ba involved in this binding, we used heterologous binding competition assays to determine common binding sites of the two toxins. Six synthetic peptides corresponding to the loop regions of domain II of Cry4Ba (loop α8, loop β2-β3, loop β4-β5, loop β6-β7, loop β8-β9, and loop β10-β11) were synthesized and used to compete with binding of biotinylated Cry11Ba to larval midgut BBMV. Four peptides corresponding to loops α8, β2-β3, β8-β9, and β10-β11 significantly competed with Cry11Ba binding (Fig. 7). However, peptides corresponding to loops β4-β5 and β6-β7 showed negligible competition.
FIG. 7.
Identification of loop regions of Cry4Ba that compete the binding of biotinylated Cry11Ba toxin to Ae. aegypti BBMV. Competition binding of biotinylated Cry11Ba to BBMV with 100- and 250-fold molar excesses of unlabeled Cry11Ba is shown in lanes 2 and 3, respectively. Synthetic loop peptides corresponding to Cry4Ba toxin loop regions α8, β2-3, β8-9, β6-7, β4-5, and β10-11 were used in lanes 4 to 9, respectively. Four peptides, corresponding to α8, β2-β3, β8-β9, and β10-β11 (lanes 4 to 6 and 9, respectively) were able to compete the binding of biotinylated Cry11Ba to BBMV. Numbers on the gel refer to percent Cry11Ba binding to the 33- and 36-kDa bands in comparison to uncompeted Cry11Ba (lane 1). These values are the averages from two independent experiments.
DISCUSSION
Of the Cry toxins, Cry11Ba has one of the highest mosquitocidal activities against Ae. aegypti larvae, but its mode of action in this species has been barely analyzed. Here we measured the binding affinity between Cry11Ba and Ae. aegypti BBMV and found that the toxin has a high binding affinity to BBMV. This interaction was saturable and dose dependent, with a dissociation constant (Kd) of 8.2 nM. The Cry11Ba binding affinity to Ae. aegypti BBMV is higher than that of Cry11Aa to Culex pipiens BBMV, which is known to be around 20 to 30 nM (S. M. Dai and S. S. Gill, unpublished data). Previous work by de Barros Moreira Beltrao et al. (14) using whole Ae. aegypti larval membranes showed that Cry11Aa had an IC50 of 88 nM while Cry4Aa and Cry4Ba had IC50s of 99 and 521 nM, respectively. Here we show Cry11Ba has an IC50 of 3.6 nM with midgut BBMV. According to previous LC50 bioassay data, Cry11Ba is more toxic to Ae. aegypti (18.8 ng/ml) (Table 1) than Cry11Aa toxin is to C. pipiens (372.4 ng/ml) (15). Thus, there appears to be a correlation between affinity of binding to BBMV and toxicity of Cry11Aa and Cry11Ba, at least in these two mosquito species.
Upon activation, the 80-kDa Cry11Ba toxin generated 36- and 33-kDa fragments on SDS-PAGE. Cleavage occurs at protease-sensitive sites between loop 1 and loop 2. We show that at a molar ratio of 1:1 of labeled to unlabeled Cry11Ba toxins, the unlabeled toxin competed principally the binding of the 36-kDa fragment (32%), followed by competition with the 33-kDa fragment binding (12%). At higher ratios (1:50 or 1:100), binding to both fragments was nearly totally competed. The 36-kDa fragment was shown to be the N-terminal fragment, which consists of domain I and critical loops of domain II of Cry11Ba toxin (31). Hence, it is not surprising that the 36-kDa fragment was more readily competed off than the 33-kDa fragment with most of the competitors.
Since all binding experiments were conducted with biotinylated toxin, the toxicity upon labeling of Cry11Ba toxin was tested in a mosquito larva bioassay. The biotinylated toxins retained similar toxicity, comparable to that of the unlabeled toxins. The toxicities of soluble and activated Cry11Ba toxins were 500 and 2,500 times lower than that of inclusion bodies of Cry11Ba. Previous results showed solubilized crystals of B. thuringiensis subsp. israelensis were 7,000 times less toxic to Ae. aegypti larvae than intact crystals (38). Presumably, this is because mosquito larvae are filter feeders, which selectively concentrate particles while excluding water and soluble molecules.
Based on information gathered from prior studies, cadherin is a likely candidate binding site for Cry4Ba and Cry11Aa toxins in different mosquito species (11, 24). We previously showed that the anticadherin antibody inhibits the binding of Cry11Ba toxin to Ae. aegypti BBMV (31). To determine the specificity of cadherin interaction with Cry11Ba toxin, the roles of three cadherin fragments (G7, C13, and G10) covering the cadherin repeats that have been shown to be involved in Cry11Aa binding to Ae. aegypti BBMV (11) were analyzed. Our results suggest that fragment G10 (which contains cadherin repeats CR7 to CR11) was able to significantly compete with Cry11Ba binding to BBMV. Cadherin fragments G7 and C13 also competed, albeit at lower levels. We then analyzed the individual cadherin repeats that are present on G10. Binding competition assays using BBMV revealed that CR9 to CR11 are the binding regions of this receptor that interact with the Cry11Ba toxin. Moreover, a CR11 peptide can compete with Cry11Ba binding to Ae. aegypti BBMV. These results indicate that CR11 of cadherin, which is the cadherin repeat most proximal to the cell membrane, is critical for binding and plays an important role in Cry11Ba binding. Previous work performed with Cry11Aa toxin showed that CR11 from Ae. aegypti cadherin bound Cry11Aa toxin and that this region binds loop 3 of Cry11Aa toxin (11). A CR11 peptide from An. gambiae cadherin (CR11-MPED) was reported to synergize the toxicity of Cry4Ba to mosquito larvae (35), suggesting that this peptide fragment also contains a Cry4Ba toxin binding site and plays a fundamental role in toxin interaction. Importantly, Cry4Ba binds with high affinity (23 nM) to the An. gambiae cadherin fragment, CR11-MPED (35), further suggesting that binding to cadherin is important for toxicity.
ALP and APN were identified as functional receptors in many insects. A 65-kDa ALP (AaeALP1) was identified as a Cry11Aa receptor in Ae. aegypti larvae (18-20). Isoforms of ALP were also shown to be Cry4Ba binding proteins in Ae. aegypti (18-20). Recently, AgALP1 was recognized as a functional receptor for Cry11Ba in An. gambiae (25). However, there are no data on whether Ae. aegypti ALPs or APNs are involved in Cry11Ba binding. Since the brush border membranes of Ae. aegypti larvae have multiple forms of ALPs, we analyzed three ALPs (AaeALP1 to -3) and AaeAPN1 from Ae. aegypti for their ability to compete with Cry11Ba binding. Binding to Aedes BBMV could be competed with both AaeALP1 and AaeAPN1. We also demonstrated that AaeALP1 more readily competes off the binding of biotinylated Cry11Ba toxin to BBMV than AaeALP2 or AaeALP3, suggesting that in Ae. aegypti, ALP1 could be more important in the interaction with Cry11Ba than AaeALP2 or AaeALP3. In the case of An. gambiae, an AgAPN had a greater binding affinity to Cry11Ba than AgALP1 (6.4 nM versus 23.9 nM) (25, 45).
To determine what domain II loop regions are involved in receptor binding and toxicity, we analyzed the effects of different domain II loop mutations of Cry11Ba in binding and toxicity. The L1-R303A/E304A/N305A mutant, which lost almost all its toxicity against mosquito larvae in the bioassay, was found to retain an ability to bind Ae. aegypti BBMV that was similar to that of the wild-type toxin (31). In the pore-forming model, Cry monomeric toxins first bind to the cadherin receptor, resulting in toxin oligomerization (8). The oligomeric Cry toxins then bind to GPI-anchored receptors, which leads to toxin insertion into the membrane (40). Our BBMV binding assays likely exemplify the binding of monomeric toxins to receptor molecules on BBMV. It might be possible that all wild-type and mutant toxins can bind cadherin in their monomeric form. In this report, we demonstrate that there are small differences in the binding of the loop mutants analyzed and Cry11Ba toxin to the G10 fragment. One interesting possibility is that such mutants are unable to bind GPI-anchored receptors, such as ALP or APN, in their oligomeric structure. Further investigations on binding of oligomeric structures of these mutants are required in order to understand the function of these critical residues.
It is known that receptors such as cadherin, ALP, and APN (or, as determined more recently, α-amylase) are recognized by different Cry toxins such as Cry4Ba and Cry11Aa (11, 12, 18, 21, 24, 25, 40). Hence, we analyzed the competitive binding interactions between Cry11Ba and Cry4Ba toxins in Ae. aegypti BBMV. The binding of biotinylated Cry11Ba to BBMV was competed more effectively with Cry11Ba than with Cry4Ba. Since it has been reported that both toxins are active against Ae. aegypti larvae, with LC50s of 470 ng/ml for Cry4Ba (13) and 19 ng/ml for Cry11Ba (15), it might be possible that the abilities of these Cry toxins to bind BBMVs are directly correlated with their levels of toxicity. Indeed Cry4Ba has a lower affinity to Aedes BBMV than Cry11Ba (S. Likitvivatanavong and S. S. Gill, unpublished data).
We previously developed a structural model of the Cry11Ba toxin and identified exposed loop regions in domain II, and we showed that loop α8, loop 1 (or β2-β3), and loop 3 (or β10-β11) are involved in toxicity and receptor binding to BBMV of Ae. aegypti (31). For Cry4Ba, it has been revealed that loops β6-β7, β8-β9, and β10-β11 play a role in Cry4Ba toxicity, and combinations of two-loop mutants (loops β6-β7/β8-β9, loops β6-β7/β10-β11, and loops β8-β9/β10-β11) exhibited reduced binding to apical microvilli of the Ae. aegypti larval midgut (28, 41). However, there are no data on whether other loops of the Cry4Ba toxin are involved in toxicity and binding activity. Since it is known that various combinations of Cry toxins may help reduce selection of resistance in insects (9, 43), the identification of common binding sites of different Cry toxins may facilitate the development of insect control strategies that will reduce resistance selection in important insect pests. Thus, we conducted heterologous competition binding assays to determine common binding regions for both toxins. Six synthetic peptides regions (α8, β2-β3, β4-β5, β6-β7, β8-β9, and β10-β11) corresponding to the loop regions of Cry4Ba (7) were used to compete individually with the binding of biotinylated Cry11Ba to larval midgut BBMV. Four peptides corresponding to loops α8, β2-β3, β8-β9, and β10-β11 could significantly compete with the binding of biotinylated Cry11Ba to BBMV, whereas peptides corresponding to loops β4-β5 and β6-β7 showed negligible competition. These data suggest that Cry11Ba and Cry4Ba share binding regions during their interaction with Ae. aegypti BBMV.
Acknowledgments
We appreciate the technical assistance of Amy Evans and Subum Lee.
This research was funded in part through a grant from the National Institutes of Health (1R01 AI066014), grants from DGAPA/UNAM (IN218608 and IN210208-N) and CONACyT (U48631-Q), and the University of California Agricultural Experiment Station.
Footnotes
Published ahead of print on 29 October 2010.
REFERENCES
- 1.Abdullah, M. A., A. P. Valaitis, and D. H. Dean. 2006. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Rauf, M., and D. J. Ellar. 1999. Mutations of loop 2 and loop 3 residues in domain II of Bacillus thuringiensis Cry1C delta-endotoxin affect insecticidal specificity and initial binding to Spodoptera littoralis and Aedes aegypti midgut membranes. Curr. Microbiol. 39:94-98. [DOI] [PubMed] [Google Scholar]
- 3.Abdul-Rauf, M., and D. J. Ellar. 1999. Toxicity and receptor binding properties of a Bacillus thuringiensis CryIC toxin active against both lepidoptera and diptera. J. Invertebr. Pathol. 73:52-58. [DOI] [PubMed] [Google Scholar]
- 4.Arenas, I., A. Bravo, M. Soberon, and I. Gomez. 2010. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 285:12497-12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayyareddy, K., T. M. Andacht, M. A. Abdullah, and M. J. Adang. 2009. Proteomic identification of Bacillus thuringiensis subsp. israelensis toxin Cry4Ba binding proteins in midgut membranes from Aedes (Stegomyia) aegypti Linnaeus (Diptera, Culicidae) larvae. Insect Biochem. Mol. Biol. 39:279-286. [DOI] [PubMed] [Google Scholar]
- 6.Berry, C., S. O'Neil, E. Ben-Dov, A. F. Jones, L. Murphy, M. A. Quail, M. T. Holden, D. Harris, A. Zaritsky, and J. Parkhill. 2002. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl. Environ. Microbiol. 68:5082-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boonserm, P., P. Davis, D. J. Ellar, and J. Li. 2005. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 348:363-382. [DOI] [PubMed] [Google Scholar]
- 8.Bravo, A., I. Gomez, J. Conde, C. Munoz-Garay, J. Sanchez, R. Miranda, M. Zhuang, S. S. Gill, and M. Soberon. 2004. Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim. Biophys. Acta 1667:38-46. [DOI] [PubMed] [Google Scholar]
- 9.Bravo, A., and M. Soberon. 2008. How to cope with insect resistance to Bt toxins? Trends Biotechnol. 26:573-579. [DOI] [PubMed] [Google Scholar]
- 10.Chang, C., Y. M. Yu, S. M. Dai, S. K. Law, and S. S. Gill. 1993. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein, and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl. Environ. Microbiol. 59:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., K. G. Aimanova, L. E. Fernandez, A. Bravo, M. Soberon, and S. S. Gill. 2009. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Biochem. J. 424:191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J., K. G. Aimanova, S. Pan, and S. S. Gill. 2009. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem. Mol. Biol. 39:688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crickmore, N., E. J. Bone, J. A. Williams, and D. J. Ellar. 1995. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol. Lett. 131:249-254. [Google Scholar]
- 14.de Barros Moreira Beltrao, H., and M. H. Silva-Filha. 2007. Interaction of Bacillus thuringiensis svar. israelensis Cry toxins with binding sites from Aedes aegypti (Diptera: Culicidae) larvae midgut. FEMS Microbiol. Lett. 266:163-169. [DOI] [PubMed] [Google Scholar]
- 15.Delecluse, A., M. L. Rosso, and A. Ragni. 1995. Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl. Environ. Microbiol. 61:4230-4235. [DOI] [PMC free article] [PubMed]
- 16.de Maagd, R. A., A. Bravo, and N. Crickmore. 2001. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 17:193-199. [DOI] [PubMed] [Google Scholar]
- 17.Dronina, M. A., L. P. Revina, L. I. Kostina, L. A. Ganushkina, I. A. Zalunin, and G. G. Chestukhina. 2006. Toxin-binding proteins from midgut epithelium membranes of Anopheles stephensi larvae. Biochemistry (Mosc.) 71:133-139. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez, L. E., K. G. Aimanova, S. S. Gill, A. Bravo, and M. Soberon. 2006. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem. J. 394:77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez, L. E., C. Martinez-Anaya, E. Lira, J. Chen, A. Evans, S. Hernandez-Martinez, H. Lanz-Mendoza, A. Bravo, S. S. Gill, and M. Soberon. 2009. Cloning and epitope mapping of Cry11Aa-binding sites in the Cry11Aa-receptor alkaline phosphatase from Aedes aegypti. Biochemistry 48:8899-8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez, L. E., C. Perez, L. Segovia, M. H. Rodriguez, S. S. Gill, A. Bravo, and M. Soberon. 2005. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop alpha-8 of domain II. FEBS Lett. 579:3508-3514. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Luna, M. T., H. Lanz-Mendoza, S. S. Gill, A. Bravo, M. Soberon, and J. Miranda-Rios. 2010. An alpha-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae). Environ. Microbiol. 12:746-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gahan, L. J., F. Gould, and D. G. Heckle. 2001. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 293:857-860. [DOI] [PubMed] [Google Scholar]
- 23.Gill, S. S., E. A. Cowles, and V. Francis. 1995. Identification, isolation, and cloning of a Bacillus thuringiensis CryIAc toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J. Biol. Chem. 270:27277-27282. [DOI] [PubMed] [Google Scholar]
- 24.Hua, G., R. Zhang, M. A. Abdullah, and M. J. Adang. 2008. Anopheles gambiae cadherin AgCad1 binds the Cry4Ba toxin of Bacillus thuringiensis israelensis and a fragment of AgCad1 synergizes toxicity. Biochemistry 47:5101-5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua, G., R. Zhang, K. Bayyareddy, and M. J. Adang. 2009. Anopheles gambiae alkaline phosphatase is a functional receptor of Bacillus thuringiensis jegathesan Cry11Ba toxin. Biochemistry 48:9785-9793. [DOI] [PubMed] [Google Scholar]
- 26.Jurat-Fuentes, J. L., and M. J. Adang. 2004. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur. J. Biochem. 271:3127-3135. [DOI] [PubMed] [Google Scholar]
- 27.Kawalek, M. D., S. Benjamin, H. L. Lee, and S. S. Gill. 1995. Isolation and identification of novel toxins from a new mosquitocidal isolate from Malaysia, Bacillus thuringiensis subsp. jegathesan. Appl. Environ. Microbiol. 61:2965-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaokhiew, T., C. Angsuthanasombat, and C. Promptmas. 2009. Correlative effect on the toxicity of three surface-exposed loops in the receptor-binding domain of the Bacillus thuringiensis Cry4Ba toxin. FEMS Microbiol. Lett. 300:139-145. [DOI] [PubMed] [Google Scholar]
- 29.Knight, P. J., B. H. Knowles, and D. J. Ellar. 1995. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis CryIA(c) toxin. J. Biol. Chem. 270:17765-17770. [DOI] [PubMed] [Google Scholar]
- 30.Lereclus, D., H. Agaisse, M. Gominet, and J. Chaufaux. 1995. Overproduction of encapsulated insecticidal crystal proteins in a Bacillus thuringiensis spo0A mutant. Biotechnology (NY) 13:67-71. [DOI] [PubMed] [Google Scholar]
- 31.Likitvivatanavong, S., K. Aimanova, and S. S. Gill. 2009. Loop residues of the receptor binding domain of Bacillus thuringiensis Cry11Ba toxin are important for mosquitocidal activity. FEBS Lett. 583:2021-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin, S., R. W. Biggs, M. S. Sisterson, L. Shriver, C. Ellers-Kirk, D. Higginson, D. Holley, L. J. Gahan, D. G. Heckel, Y. Carriere, T. J. Dennehy, J. K. Brown, and B. E. Tabashnik. 2003. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. U. S. A. 100:5004-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen-Leroux, C., and J. F. Charles. 1992. Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur. J. Biochem. 210:585-590. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen-Leroux, C., J. F. Charles, I. Thiery, and G. P. Georghiou. 1995. Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur. J. Biochem. 228:206-210. [DOI] [PubMed] [Google Scholar]
- 35.Park, Y., G. Hua, M. A. Abdullah, K. Rahman, and M. J. Adang. 2009. Cadherin fragments from Anopheles gambiae synergize Bacillus thuringiensis Cry4Ba's toxicity against Aedes aegypti larvae. Appl. Environ. Microbiol. 75:7280-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopal, R., S. Sivakumar, N. Agrawal, P. Malhotra, and R. K. Bhatnagar. 2002. Silencing of midgut aminopeptidase N of Spodoptera litura by dsRNA establishes its role as BT toxin receptor. J. Biol. Chem. 107:1. [DOI] [PubMed] [Google Scholar]
- 37.Sangadala, S., F. S. Walters, L. H. English, and M. J. Adang. 1994. A mixture of Manduca sexta aminopeptidase and phosphatase enhances Bacillus thuringiensis insecticidal CryIA(c) toxin binding and 86Rb(+)-K+ efflux in vitro. J. Biol. Chem. 269:10088-10092. [PubMed] [Google Scholar]
- 38.Schnell, D. J., M. A. Pfannenstiel, and K. W. Nickerson. 1984. Bioassay of solubilized Bacillus thuringiensis var. israelensis crystals by attachment to latex beads. Science 223:1191-1193. [DOI] [PubMed] [Google Scholar]
- 39.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soberon, M., S. S. Gill, and A. Bravo. 2009. Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol. Life Sci. 66:1337-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuntitippawan, T., P. Boonserm, G. Katzenmeier, and C. Angsuthanasombat. 2005. Targeted mutagenesis of loop residues in the receptor-binding domain of the Bacillus thuringiensis Cry4Ba toxin affects larvicidal activity. FEMS Microbiol. Lett. 242:325-332. [DOI] [PubMed] [Google Scholar]
- 42.Vadlamudi, R. K., E. Weber, I. Ji, T. H. Ji, and L. A. Bulla, Jr. 1995. Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J. Biol. Chem. 270:5490-5494. [DOI] [PubMed] [Google Scholar]
- 43.Wirth, M. C., A. Zaritsky, E. Ben-Dov, R. Manasherob, V. Khasdan, S. Boussiba, and W. E. Walton. 2007. Cross-resistance spectra of Culex quinquefasciatus resistant to mosquitocidal toxins of Bacillus thuringiensis towards recombinant Escherichia coli expressing genes from B. thuringiensis ssp. israelensis. Environ. Microbiol. 9:1393-1401. [DOI] [PubMed] [Google Scholar]
- 44.Xu, X., L. Yu, and Y. Wu. 2005. Disruption of a cadherin gene associated with resistance to Cry1Ac δ-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl. Environ. Microbiol. 71:948-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, R., G. Hua, T. M. Andacht, and M. J. Adang. 2008. A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry 47:11263-11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, R., G. Hua, J. L. Urbauer, and M. J. Adang. 2010. Synergistic and inhibitory effects of aminopeptidase peptides on Bacillus thuringiensis Cry11Ba toxicity in the mosquito Anopheles gambiae. Biochemistry 49:8512-8919. [DOI] [PubMed] [Google Scholar]