Abstract
A targeted metagenomic approach was applied to investigate magnetotactic bacteria (MTB) within the phylum Nitrospirae in Lake Miyun near Beijing, China. Five fosmids containing rRNA operons were identified. Comparative sequence analysis of a total of 172 kb provided new insights into their genome organization and revealed unexpected subgenomic diversity of uncultivated MTB in the phylum Nitrospirae. In addition, affiliation of two novel MTB with the phylum Nitrospirae was verified by fluorescence in situ hybridization. One of them was morphologically similar to “Candidatus Magnetobacterium bavaricum,” but the other differed substantially in cell shape and magnetosome organization from all previously described “Ca. Magnetobacterium bavaricum”-like bacteria.
Magnetotactic bacteria (MTB) are a phylogenetically and morphologically diverse group of microorganisms which can synthesize iron minerals of magnetite (Fe3O4) or greigite (Fe3S4) within complex subcellular structures, the magnetosomes (3, 4). These biologically mineralized crystals are nanometer sized and have species-specific shapes, implying that their formation is under strict genetic control (10, 18). Magnetosomes allow MTB to swim along the Earth's magnetic field lines and thereby orient themselves in chemically stratified aquatic environments (4). Currently, most reported MTB belong to Proteobacteria, while three uncultivated species are affiliated with the phylum Nitrospirae (2, 12).
MTB within the phylum Nitrospirae were first identified through a 16S rRNA gene-based survey in Lake Chiemsee near Munich, Germany (24). The giant rod-shaped bacterium named “Candidatus Magnetobacterium bavaricum” can synthesize hundreds of bullet-shaped magnetosomes arranged into several bundles of chains within individual cells. Up to 7 × 105 cells of “Ca. Magnetobacterium bavaricum” per cm3 in the first few millimeters of sediment were found, and based on their large biovolume they are likely to play an important ecological role in biogeochemical cycling of iron and sulfur and sedimentary magnetism (9, 19, 24). Recently, MTB which are phylogenetically affiliated within the phylum Nitrospirae (known as “Ca. Magnetobacterium bavaricum”-like bacteria) have also been detected in Lake Waller See near Bremen, Germany (5), Lake Miyun near Beijing, China (13-15), and even hot springs in Nevada (12). Since these MTB have proven recalcitrant to cultivation, little is known about their genomic content or physiological potential.
Recently, we have developed an efficient strategy for targeted metagenomic analysis of uncultivated MTB, including “Ca. Magnetobacterium bavaricum” (8, 9). Our results have shown the first insight into the genome of “Ca. Magnetobacterium bavaricum,” which contains a gene encoding a putative RubisCO-like protein (RLP), potentially involved in sulfur metabolism (9). In the present study, we used a metagenomic approach to characterize the diversity and partial genomic sequences of related “Ca. Magnetobacterium bavaricum”-like bacteria in Lake Miyun, China.
Sediments from the topmost 5 to 10 cm from Lake Miyun (40°29′19.45″N, 117°0′25.71″E) were sampled and subsequently aliquoted into 600-ml plastic bottles (microcosms) in the laboratory. Each microcosm was checked periodically by light microscopy using the hanging-drop method (6). Nineteen of more than 100 microcosms were found to be rich in “Ca. Magnetobacterium bavaricum”-like bacteria (>103 cells/cm3). Thus, they were selected for subsequent metagenomic analysis. A two-step magnetic enrichment strategy was applied to collect and purify MTB from sediments. Briefly, the south pole of a bar magnet was placed on the north side of each microcosm near the water-sediment interface to enrich the MTB. After 1 h of collection, MTB were removed from the bottle and further purified by using the “MTB trap” (8). The purified MTB cells were then used for genomic DNA extraction and fosmid library construction, the detailed procedures of which have been described previously (8).
In order to identify fosmids containing 16S rRNA genes of “Ca. Magnetobacterium bavaricum”-like bacteria, high-throughput screens of fosmid library clone pools were performed by PCR using the bacterial universal primer 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) (14) as the forward primer and a “Ca. Magnetobacterium bavaricum”-specific probe (5′-GCCATCCCCTCGCTTACT-3′; named BaP in this study) (24) as the reverse primer. Individual clones revealing positive PCR results were subjected to subsequent similar PCR analysis. PCR products of positive clones were sequenced for verification and phylogenetic identification. Finally, five fosmids were identified, completely shotgun sequenced, and characterized by bioinformatic gene prediction, representing a total of 172 kb of genomic information.
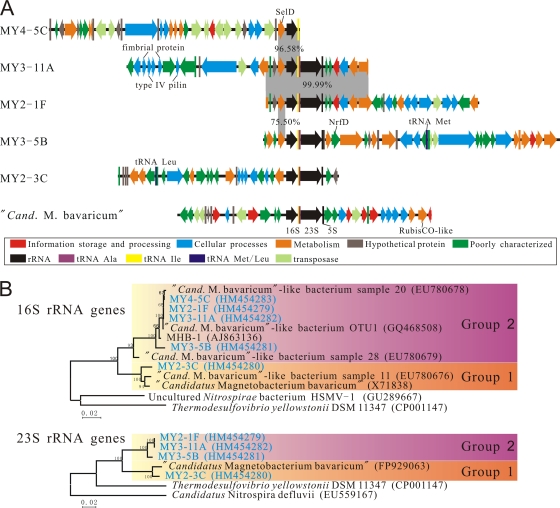
Like those of “Ca. Magnetobacterium bavaricum” (9), all fosmids except MY4-5C contained a typical bacterium-like rRNA operon organization that consisted of a 16S rRNA gene, tRNA Ile, tRNA Ala, 23S rRNA gene, and 5S rRNA gene (Fig. 1 A). The sizes of the fosmid inserts were between 30,297 bp and 41,797 bp, while the average G+C content varied from 45.1% to 50.2% (Table 1). Between 23 and 35 predicted open reading frames (ORFs) were identified in each fosmid (Fig. 1A). The majority of ORFs in all fosmids had most-similar orthologs among the Deltaproteobacteria and Nitrospirae (Table 1). The number of ORFs with no significant similarities to protein-encoding genes in the NCBI database (represented by hypothetical proteins in Table 1) ranged from 2 to 9 per clone. Between 0 and 9 genes per clone were identified as potentially encoding transposases. Analyses, using BLAST and clusters of orthologous groups (COGs), of other ORFs revealed that the predicted gene products are mostly involved in cellular processes and metabolism (Fig. 1A; see Table S1 in the supplemental material). For example, one predicted protein encoded by fosmid MY3-5B was found to be polysulfide reductase NrfD (Fig. 1A), an integral transmembrane protein that was thought to interact with menaquinone during electron transfer, suggesting a potential anaerobic respiratory system of this bacterium (11, 23). Notably, another gene encoding a highly conserved selenophosphate synthetase (SelD) was found adjacent to the 16S rRNA gene on fosmid fragments MY4-5C, MY3-11A, MY2-1F, and MY3-5B (Fig. 1A); SelD generates the selenium donor for selenocysteine biosynthesis in bacteria, suggesting the trait of selenium utilization (22). Furthermore, we found four genes encoding putative fimbrial proteins and type IV pilins on fosmid MY3-11A (Fig. 1A), which might be involved in pilus biosynthesis (7).
FIG. 1.
(A) Organizations of predicted genes located on fosmids MY4-5C, MY3-11A, MY2-1F, MY3-5B, and MY2-3C of “Ca. Magnetobacterium bavaricum”-like bacteria and the fosmid fragment isolated from “Ca. Magnetobacterium bavaricum” (9). For putative gene functions, see Table S1 in the supplemental material. (B) Neighbor-joining phylogenetic trees of 16S and 23S rRNA genes carried by the fosmids compared to those for other related bacteria. Sequences determined in this study are in blue. GenBank accession numbers are given in parentheses. Numbers at nodes represent bootstrap values (100-fold resampling analyses).
TABLE 1.
General genomic features of the fosmids MY2-1F, MY3-11A, MY4-5C, MY2-3C, and MY3-5B
| Feature | Value for: |
||||
|---|---|---|---|---|---|
| MY4-5C | MY3-11A | MY2-1F | MY3-5B | MY2-3C | |
| Size (bp) | 35,154 | 34,185 | 30,297 | 41,797 | 30,357 |
| G+C content (%) | 45.1 | 47.5 | 47.3 | 50.0 | 50.2 |
| No. of predicted ORFs | 35 | 23 | 23 | 32 | 28 |
| No. of tRNA genes | 1 | 2 | 2 | 3 | 3 |
| No. of hypothetical proteins | 9 | 2 | 2 | 4 | 5 |
| No. of transposases | 9 | 1 | 1 | 2 | 0 |
| No. of proteins with most similar ortholog in Nitrospirae | 5 | 3 | 4 | 7 | 5 |
| No. of proteins with most similar ortholog in Deltaproteobacteria | 8 | 8 | 7 | 8 | 5 |
Phylogenetic analysis of the complete 16S and 23S rRNA genes from the fosmids consistently showed a clear relationship with “Ca. Magnetobacterium bavaricum” and known “Ca. Magnetobacterium bavaricum”-like bacteria of the phylum Nitrospirae (Fig. 1B). Phylogenetic trees of 16S rRNA genes were calculated by using maximum-parsimony and maximum-likelihood methods (data not shown); the trees are identical in topology to the neighbor-joining tree (Fig. 1B). MTB of the phylum Nitrospirae within this study formed two distinguishable groups (groups 1 and 2), defined by nearly 5% sequence divergence and high bootstrap values (Fig. 1B). This result is further corroborated by phylogenetic analysis of 23S rRNA genes, which revealed a congruent branching pattern (Fig. 1B).
Clones MY2-1F and MY3-11A contained an overlapping region of 14,507 bp with only two nucleotide mismatches and identical 16S/23S/5S rRNA operons (Fig. 1A), indicating that these two fosmids were from the same species and assembled into a contig of about 50 kb, which so far represents the largest continuous genomic stretch determined from a Nitrospirae-like MTB. However, the genomic organizations appeared very divergent on other clones, including MY4-5C, whose 16S rRNA gene was 99.98% identical to those of MY2-1F and MY3-11A. This poor conservation may suggest that either multiple rearrangements occurred in these regions (20, 21) or the genomes of “Ca. Magnetobacterium bavaricum”-like bacteria contained more than one 16S/23S/5S rRNA operon.
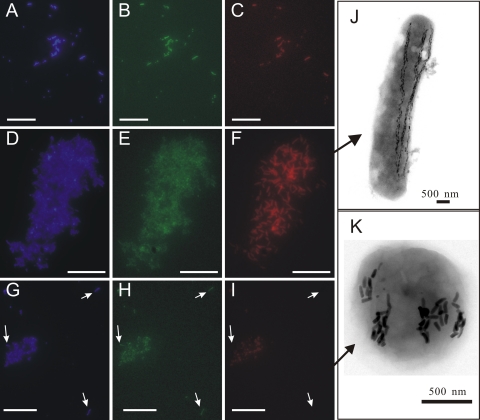
To identify the “Ca. Magnetobacterium bavaricum”-like cells from which sequences originated, two probes (Mba1000 [5′-TTATCCTTTCGGACTCCTACCACT-3′] and Mba1019 [5′-CTCCCTTACGGGAAAGTCGCTCA-3′]) deduced from 16S rRNA gene sequences of each group were developed for fluorescence in situ hybridization (FISH) experiments. Most enriched MTB cells detectable with bacterial universal probe EUB338 (1), including giant rod-shaped cells and small coccoid-to-ovoid bacteria, could simultaneously be visualized with probe BaP, which is specific for “Ca. Magnetobacterium bavaricum”-like bacteria (Fig. 2 A to C). Probe Mba1000, which was designed to specifically detect group 1, exclusively recognized the giant rod-shaped bacteria that had morphology similar to that of “Ca. Magnetobacterium bavaricum” (Fig. 2D to F and J). Thus, these bacteria belong to group 1. Probe Mba1019 was specific for group 2 except for a single mismatch with sequence MY3-5B. Unexpectedly, this probe recognized coccoid-to-ovoid cells with lengths of 1 to 2 μm and diameters of 0.5 to 1 μm, indicating that these morphotypes also belong to the phylum Nitrospirae (Fig. 2G to I). These cells contained 4 or 5 braid-like chain bundles consisting of bullet-shaped magnetosomes (Fig. 2K). However, several coccoid bacteria almost similar in size and shape present in the magnetic collections could not be hybridized by probe BaP, Mba1000, or Mba1019. These cocci might be Alphaproteobacteria MTB that often coexist with “Ca. Magnetobacterium bavaricum”-like bacteria in Lake Miyun as described previously (14-17). Transmission electron microscopic analysis demonstrated that these cells had different magnetosome morphologies and arrangements (see Fig. S1 in the supplemental material).
FIG. 2.
Fluorescence in situ hybridization (A to I) and transmission electron micrographs (J and K) of “Ca. Magnetobacterium bavaricum”-like bacteria in the magnetic enrichment from the Lake Miyun sediments. (A to C) The same microscopic field after staining with DAPI (A), after hybridization with 5′-6-carboxyfluorescein (FAM)-labeled bacterial universal probe EUB338 (B), and after hybridization with 5′-Cy3-labeled probe BaP, specific for all “Ca. Magnetobacterium bavaricum”-like bacteria (C). (D to F) The same microscopic field after staining with DAPI (D), after hybridization with 5′-FAM-labeled bacterial universal probe EUB338 (E), and after hybridization with 5′-Cy3-labeled probe Mba1000, specific for group 1 of “Ca. M. bavaricum”-like bacteria (F). Only the giant rod-shaped bacteria (J) hybridized with the specific probe Mba1000, whereas the accompanying cells were not stained. (G to I) The same microscopic field after staining with DAPI (G), after hybridization with 5′-FAM-labeled bacterial universal probe EUB338 (H), and after hybridization with 5′-Cy3-labeled probe Mba1019, specific for group 2 of “Ca. Magnetobacterium bavaricum”-like bacteria (I). Only the coccoid-to-ovoid bacteria containing bullet-shaped magnetosomes (K) were stained with the specific probe Mba1019. Arrows indicate the giant rod-shaped cells as a negative control. Bars (A to I), 50 μm.
Overall, the metagenomic analysis of “Ca. Magnetobacterium bavaricum”-like bacteria significantly expanded the variety of MTB known to be affiliated within the phylum Nitrospirae. Our data provided new insights into the genomic structure and gene content of “Ca. Magnetobacterium bavaricum”-like bacteria and revealed several putative metabolic functions. Although the 16S rRNA genes were closely related, gene content and synteny of colocalized genes were poorly conserved, indicating a higher diversity of these bacteria within the phylum Nitrospirae than previously expected. In total, 172 kb of genomic information and 141 predicted genes were identified. The COG category assignment showed that the majority of genes in the five fosmids encode proteins that are involved in cellular processes, metabolism, and signal transduction. Our approach for targeted genome analysis presented here, with the addition of future work, will help to elucidate the putative physiologies and ecological roles of these intriguing, deep-branching, and yet-uncultured bacteria.
Nucleotide sequence accession numbers.
GenBank accession numbers for the five fosmids sequenced in this study are HM454279 to HM454283.
Supplementary Material
Acknowledgments
We thank Jinhua Li for the electron microscopy observations and Bi Li and Changqian Cao for help with field sampling. We are grateful to three anonymous reviewers for their valuable comments.
This work was supported by the CAS/SAFEA International Partnership Program for Creative Research Teams (KZCX2-YW-T10), the CAS project, and NSFC grant 40821091.
Footnotes
Published ahead of print on 5 November 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amann, R., L. Krumholz, and D. A. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R., J. Peplies, and D. Schüler. 2006. Diversity and taxonomy of magnetotactic bacteria, p. 25-36. In D. Schüler (ed.), Magnetoreception and magnetosomes in bacteria. Springer, Berlin, Germany.
- 3.Bazylinski, D. A., and R. B. Frankel. 2004. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2:217-230. [DOI] [PubMed] [Google Scholar]
- 4.Faivre, D., and D. Schüler. 2008. Magnetotactic bacteria and magnetosomes. Chem. Rev. 108:4875-4898. [DOI] [PubMed] [Google Scholar]
- 5.Flies, C. B., J. Peplies, and D. Schüler. 2005. Combined approach for characterization of uncultivated magnetotactic bacteria from various aquatic environments. Appl. Environ. Microbiol. 71:2723-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg, M., K. Canter, I. Mahler, and A. Tornheim. 2005. Observation of magnetoreceptive behavior in a multicellular magnetotactic prokaryote in higher than geomagnetic fields. Biophys. J. 88:1496-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs, M., and J. S. Mattick. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10:233-243. [DOI] [PubMed] [Google Scholar]
- 8.Jogler, C., W. Lin, A. Meyerdierks, M. Kube, E. Katzmann, C. Flies, Y. Pan, R. Amann, R. Reinhardt, and D. Schüler. 2009. Towards cloning the magnetotactic metagenome: identification of magnetosome island gene clusters in uncultivated magnetotactic bacteria from different aquatic sediments. Appl. Environ. Microbiol. 75:3972-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jogler, C., M. Niebler, W. Lin, M. Kube, G. Wanner, S. Kolinko, P. Stief, A. Beck, D. DeBeer, N. Petersen, Y. Pan, R. Amann, R. Reinhardt, and D. Schüler. 19 April 2010. Cultivation-independent characterization of “Candidatus Magnetobacterium bavaricum” via ultrastructural, geochemical, ecological and metagenomic methods. Environ. Microbiol. doi: 10.1111/j.1462-2920.2010.02220.x. [DOI] [PubMed]
- 10.Jogler, C., and D. Schüler. 2009. Genomics, genetics, and cell biology of magnetosome formation. Annu. Rev. Microbiol. 63:501-521. [DOI] [PubMed] [Google Scholar]
- 11.Jormakka, M., K. Yokoyama, T. Yano, M. Tamakoshi, S. Akimoto, T. Shimamura, P. Curmi, and S. Iwata. 2008. Molecular mechanism of energy conservation in polysulfide respiration. Nat. Struct. Mol. Biol. 15:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefèvre, C. T., F. Abreu, M. L. Schmidt, U. Lins, R. B. Frankel, B. P. Hedlund, and D. A. Bazylinski. 2010. Moderately thermophilic magnetotactic bacteria from hot springs in Nevada. Appl. Environ. Microbiol. 76:3740-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, J., Y. Pan, Q. Liu, K. Yu-Zhang, N. Menguy, R. Che, H. Qin, W. Lin, W. Wu, and N. Petersen. 2010. Biomineralization, crystallography and magnetic properties of bullet-shaped magnetite magnetosomes in giant rod magnetotactic bacteria. Earth Planet. Sci. Lett. 293:368-376. [Google Scholar]
- 14.Lin, W., J. Li, D. Schüler, C. Jogler, and Y. Pan. 2009. Diversity analysis of magnetotactic bacteria in Lake Miyun, northern China, by restriction fragment length polymorphism. Syst. Appl. Microbiol. 32:342-350. [DOI] [PubMed] [Google Scholar]
- 15.Lin, W., and Y. Pan. 2010. Temporal variation of magnetotactic bacterial communities in two freshwater sediment microcosms. FEMS Microbiol. Lett. 302:85-92. [DOI] [PubMed] [Google Scholar]
- 16.Lin, W., and Y. X. Pan. 2009. Specific primers for the detection of freshwater alphaproteobacterial magnetotactic cocci. Int. Microbiol. 12:237-242. [PubMed] [Google Scholar]
- 17.Lin, W., L. Tian, J. Li, and Y. Pan. 2008. Does capillary racetrack-based enrichment reflect the diversity of uncultivated magnetotactic cocci in environmental samples? FEMS Microbiol. Lett. 279:202-206. [DOI] [PubMed] [Google Scholar]
- 18.Murat, D., A. Quinlan, H. Vali, and A. Komeili. 2010. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc. Natl. Acad. Sci. U. S. A. 107:5593-5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan, Y., N. Petersen, M. Winklhofer, A. Davila, Q. Liu, T. Frederichs, M. Hanzlik, and R. Zhu. 2005. Rock magnetic properties of uncultured magnetotactic bacteria. Earth Planet. Sci. Lett. 237:311-325. [Google Scholar]
- 20.Quaiser, A., T. Ochsenreiter, C. Lanz, S. C. Schuster, A. H. Treusch, J. Eck, and C. Schleper. 2003. Acidobacteria form a coherent but highly diverse group within the bacterial domain: evidence from environmental genomics. Mol. Microbiol. 50:563-575. [DOI] [PubMed] [Google Scholar]
- 21.Rocap, G., D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 2002. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl. Environ. Microbiol. 68:1180-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero, H., Y. Zhang, V. N. Gladyshev, and G. Salinas. 2005. Evolution of selenium utilization traits. Genome Biol. 6:R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon, J., and M. Kern. 2008. Quinone-reactive proteins devoid of haem b form widespread membrane-bound electron transport modules in bacterial respiration. Biochem. Soc. Trans. 36:1011-1016. [DOI] [PubMed] [Google Scholar]
- 24.Spring, S., R. Amann, W. Ludwig, K.-H. Schleifer, H. van Gemerden, and N. Petersen. 1993. Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl. Environ. Microbiol. 59:2397-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.