Abstract
Lactococcus garvieae DCC43 produces a bacteriocin, garvicin ML (GarML), with a molecular mass of 6,004.2 Da. Data from de novo amino acid sequencing by tandem mass spectrometry and nucleotide sequencing by reverse genetics suggested that the bacteriocin is synthesized as a 63-amino-acid precursor with a 3-amino-acid leader peptide that is removed by cleavage. Subsequently, a covalent linkage between the N and C termini forms the mature version of this novel 60-amino-acid circular bacteriocin.
Ribosomally synthesized antimicrobial peptides (AP) are produced by many organisms, including mammals, birds, insects, plants, and microorganisms. In bacteria, such peptides are termed bacteriocins (10, 33), and those of lactic acid bacteria attract considerable interest as food preservatives (5, 7, 14). Many AP are more active than conventional antibiotics against pathogenic and drug-resistant Gram-positive bacteria yet display no toxicity toward eukaryotic cells (35). AP may have applications in human and veterinary medicine in the treatment of local and systemic bacterial infections (24, 40, 42).
Bacteriocins have been classified into two major groups: class I lantibiotics with posttranslationally modified amino acids and class II nonlantibiotics with nonmodified amino acids (7, 34). Circular bacteriocins may constitute a new class (18, 23, 28, 29). The circular structure appears to enhance the thermodynamic stability and structural integrity of the peptide to improve its biological stability and activity (17). To date, a few circular bacteriocins are known: enterocin AS-48 (12), reutericin 6 (43), acidocin B (27), butyrivibriocin AR10 (19), gassericin A (20), circularin A (23), subtilosin A (22), uberolysin (46), carnocyclin A (30), and lactocyclicin Q (41). These bacteriocins can be further classified according to their primary structures, biochemical characteristics, and genetic arrangements (21, 29). This study reports a novel circular bacteriocin, garvicin ML (GarML), produced by Lactococcus garvieae DCC43, isolated from Mallard ducks (Anas platyrhynchos) (39).
Strains and genetic techniques.
Antimicrobial activity was evaluated by agar diffusion tests (ADT) and microtiter plate assays (MPA) as previously described (39) (Table 1). Plasmids were isolated using a midi kit (Qiagen) with added lysozyme (40 mg/ml) and mutanolysin (500 U/ml). Plasmid-Safe ATP-dependent DNase (Epicentre) eliminated residual genomic DNA. Genomic DNA, isolated as previously described (38), was digested with blunt-end-generating restriction enzymes, and fragments were ligated to an EcoRV-digested pCR-Blunt II-TOPO vector (Invitrogen). PCR was done in 50-μl mixtures, using 100 pmol of each primer and 1 U of Phusion high-fidelity DNA polymerase (Finnzymes). PCR fragments were isolated with QIAquick kits for purification or gel extraction (Qiagen). DNA was sequenced with a PRISM BigDye terminator cycle sequencing kit and an automatic DNA sequencer, model 377 (Applied Biosystems). Homology searches, using the BLAST algorithm (2), were done from the website of the National Center for Biotechnology Information (NCBI).
TABLE 1.
Antimicrobial activities and inhibitory spectrum of fractions generated from the purification of garvicin ML produced by L. garvieae DCC43a
| Indicator strain | Sourceb | Halo of inhibition (mm2) using indicated garvicin ML fractionc |
|||||
|---|---|---|---|---|---|---|---|
| SN | AS | GF | SE | OE | RP | ||
| Lactobacillus reuteri 20016 | DSM | —d | — | — | 51 | 369 | 378 |
| Lactobacillus helveticus 15009 | ATCC | — | — | 22 | 26 | 129 | 492 |
| Lactobacillus curvatus 2739 | NCFB | — | — | — | — | 221 | 177 |
| Lactobacillus casei 334 | ATCC | 83 | 215 | 153 | 191 | 1,048 | 1,174 |
| Lactobacillus acidophilus 4356 | ATCC | — | — | — | 25 | 149 | 236 |
| Lactobacillus sakei 2714 | NCFB | 94 | 204 | 163 | 249 | 1,445 | 1,445 |
| Lactococcus lactis BB24 | FVM | 97 | 186 | 150 | 183 | 941 | 928 |
| Lactococcuslactis NZ9000 | NIZO | — | 69 | 37 | 77 | 163 | 163 |
| Lactococcuslactis DPC5598 | DPC | 163 | 98 | 113 | 141 | 1,075 | 1,541 |
| Lactococcusgarvieae 5274 | CECT | — | 67 | 71 | 94 | 684 | 617 |
| Lactococcusgarvieae 5806 | CECT | 334 | 482 | 413 | 561 | 2,807 | 2,807 |
| Lactococcusgarvieae 5807 | CECT | 291 | 438 | 441 | 529 | 2,603 | 2,705 |
| Lactococcusraffinolactis 988 | CECT | — | — | — | 10 | 228 | 228 |
| Pediococcus acidilactici 347 | FVM | 94 | 193 | 153 | 235 | 1,398 | 1,398 |
| Pediococcus pentosaceus FBB61 | TNO | 21 | 144 | 108 | 193 | 1,131 | 1,131 |
| Enterococcus faecium P13 | FVM | 65 | 201 | 160 | 232 | 1,217 | 1,291 |
| Enterococcus faecium L50 | FVM | — | 63 | 47 | 83 | 574 | 719 |
| Enterococcus faecalis DBH18 | FVM | — | — | — | — | 25 | 25 |
| Enterococcus faecalis P4 | IFR | — | — | — | — | 86 | 123 |
| Propionibacterium sp. P6 | NCDO | 62 | 119 | 111 | 130 | 684 | 190 |
| Propionibacterium acidipropionici 563 | NCDO | 75 | 153 | 105 | 132 | 662 | 864 |
| Clostridium tyrobutyricum 3,5 CT | TNO | 55 | 124 | 83 | 124 | 719 | 651 |
| Clostridium tyrobutyricum 1754 | NCDO | 58 | 129 | 105 | 126 | 802 | 839 |
| Clostridium perfringens 376 | CECT | — | 45 | 29 | 31 | 351 | 352 |
| Clostridium botulinum 551 | CECT | — | 59 | 174 | 227 | 1,232 | 574 |
| Listeria monocytogenes 4032 | CECT | — | 22 | 215 | 289 | 1,574 | 662 |
| Listeria ivanovii 913 | CECT | — | 139 | 273 | 353 | 1,978 | 1,020 |
| Listeria seeligeri 917 | CECT | 34 | 87 | 212 | 292 | 1,509 | 684 |
| Listeria grayi 931 | CECT | — | 127 | 135 | 191 | 1,075 | 742 |
| Listeria welshimeri 919 | CECT | — | — | 158 | 258 | 1,291 | 452 |
| Brochothrix thermosphacta 847 | CECT | — | — | — | 31 | 255 | 283 |
| Pseudomonas fluorescens 378 | CECT | — | — | — | — | — | — |
| Escherichia coli JM109 | Invitrogen | — | — | — | — | — | — |
| Escherichia coli MC1000 | NIZO | — | — | — | — | — | — |
| Salmonella paratyphi 554 | CECT | — | — | — | — | — | — |
| Salmonella Typhimurium 443 | CECT | — | — | — | — | — | — |
| Salmonella enteritidis 4396 | CECT | — | — | — | — | — | — |
| Streptococcus pneumoniae FQ26 | HRC | 69 | 156 | 100 | 110 | 599 | 590 |
| Streptococcus pneumoniae 67620 | HRC | 65 | 135 | 94 | 128 | 707 | 596 |
| Streptococcus pneumoniae 15 M-1047 | HRC | 58 | 128 | 96 | 139 | 790 | 790 |
Purification of the bacteriocin was described by Sánchez et al. (39).
Source abbreviations: ATCC, American Type Culture Collection (Rockville, MD); CECT, Colección Española de Cultivos Tipo (Valencia, Spain); DPC, Teagasc Dairy Products Research Centre, Moorepark, Fermoy (County Cork, Ireland); DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany); FVM, Facultad de Veterinaria de Madrid (Madrid, Spain); HRC, Departamento de Microbiología, Hospital Universitario Ramón y Cajal (Madrid, Spain); IFR, Institute of Food Research (Norwich, United Kingdom); NCDO, National Collection of Dairy Organisms (Reading, United Kingdom); NCFB, National Collection of Food Bacteria (Reading, United Kingdom); NIZO, Department of Biophysical Chemistry, NIZO Food Research (Ede, Netherlands); TNO, Nutrition and Food Research (Zeist, Netherlands).
Antimicrobial activity was determined by the agar diffusion test (ADT), and the area of the halo of inhibition (mm2) is shown. Fraction abbreviations: SN, supernatant; AS, ammonium sulfate precipitation; GF, gel filtration; SE, Sepharose fast flow eluate; OE, octyl Sepharose eluate; RP, reversed-phase eluate diluted 5-fold (vol/vol) with 30% 2-propanol containing 0.1% trifluoroacetic acid.
—, no halo of inhibition.
Purification of the bacteriocin produced by L. garvieae DCC43 and mass spectrometry analysis.
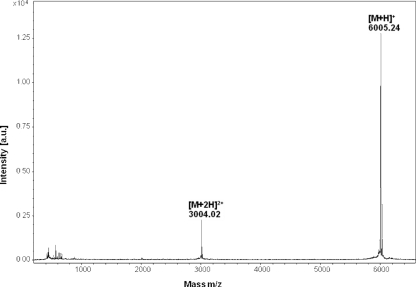
The supernatant of an overnight culture of L. garvieae DCC43 was subjected to peptide purification by ion exchange chromatography on a HiPrep 16/10 SP-XL column (GE Healthcare Biosciences) and two cycles of reversed-phase chromatography on a reversed-phase Resource RPC column (GE Healthcare Biosciences) and a Sephasil peptide C8 5-μm ST 4.6/100 column (Amersham Biosciences) integrated onto an Äkta purifier fast protein liquid chromatography system (FPLC). The molecular weight of the bacteriocin was determined by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF-MS) as described previously (9). Analysis of the purified entity, garvicin ML (GarML), showed that only the [M + H]+ and [M + 2H]2+ peaks of the bacteriocin were present, suggesting that the monoisotopic molecular mass of GarML is 6,004.2 Da (Fig. 1).
FIG. 1.
Mass spectrometry analysis of the purified garvicin ML produced by L. garvieae DCC43. a.u., absorbance units.
Proteolytic digestion of purified garvicin ML and de novo MS-MS peptide mapping.
Initial efforts to determine the N-terminal amino acid sequence of GarML by Edman degradation failed, suggesting that the peptide was either cyclic or N-terminally blocked. However, although various peptide fragmentation procedures are available (4, 23, 41), GarML was digested by trypsin, either by a standard overnight protocol or in a micropipette tip (16, 37). To facilitate de novo tandem mass spectrometry (MS-MS) peptide mapping, the peptides were derivatized with a Lys tag and/or 4-sulfophenyl isothiocyanate (SPITC; Sigma-Aldrich) (26, 36). Digestion of GarML with trypsin produced two major peptide fragments of 1,652 Da and 3,581 Da, and their amino acid sequences are shown in Fig. 2A.
FIG. 2.
Determination of the amino acid and nucleotide sequences of garvicin ML produced by L. garvieae DC443. (A) Amino acid sequences obtained by de novo MS-MS peptide mapping of the major peptide fragments obtained after trypsin digestion of garvicin ML, and the degenerate primers designed based on the sequences. (B) The 119-bp nucleotide sequence obtained after amplification of genomic DNA from L. garvieae DCC43 with primers DP7/DP10, and its deduced amino acid sequence. The nucleotide sequences corresponding to primers DP7 and DP10 are underlined. (C) From the above-cited nucleotide sequence, using reverse genetics, a sequence of 264 bp and its deduced amino acid sequence were obtained. A putative ribosome binding site (RBS) is underlined. An asterisk identifies the translation stop codon. The predicted cleavage site of the leader peptide is indicated by a vertical arrow.
Identification of the structural gene and DNA and protein sequence analysis of garvicin ML.
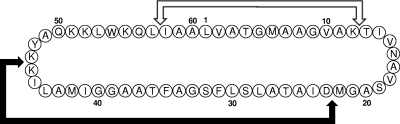
Based on the known amino acid sequence of the two major peptide fragments, four degenerate primers (DP7 to DP10) were designed for PCR amplification and DNA sequencing of the gene encoding mature GarML (Fig. 2A). Only the primer pair DP7/DP10 produced a PCR fragment (119 bp) that matched the amino acid sequence of the trypsin digests of GarML (Fig. 2B). New primers were designed by primer walking, and specific PCR fragments were sequenced and assembled into a 264-bp contig. As a result, the DNA sequence of the structural gene encoding GarML, termed garML, was obtained. The garML gene consisted of a 189-bp open reading frame (ORF) encoding a primary translation product of 63 amino acid residues, preceded by a putative ribosomal binding site (GGAGG) upstream of the methionine translation initiation codon (Fig. 2C). The deduced amino acid sequence of the minor trypsin digest from GarML (IAALVATGMAAGVAK) permitted the determination of the exact point of circularization, suggesting that GarML is synthesized as a 63-amino-acid precursor peptide which is processed between Asp3 and Leu4 to produce the 60-amino-acid mature peptide (Fig. 2). We postulate that the putative leader peptide (tripeptide) of the GarML precursor is cleaved off and cyclization takes place between the N-terminal Leu4 and the C-terminal Ala63 by a peptide bond (Fig. 3). garML is also carried on a plasmid (results not shown). To date, only one other L. garvieae bacteriocin, garviecin L1-5, produced by L. garvieae L1-5, isolated from raw cow's milk, has been reported (45).
FIG. 3.
Circular structure of garvicin ML. Double-ended arrows indicate locations of the major peptide fragments obtained after trypsin digestion of garvicin ML. The white arrow defines the 1,652-Da fragment, and the black arrow the 3,581-Da fragment.
GarML shares limited amino acid similarity (30% identity) with carnocyclin A (CclA), a circular bacteriocin from Carnobacterium maltaromaticum UAL307 (30), and a lower similarity (28% identity) with enterocin AS-48, produced by Enterococcus faecalis S-48 (12). The predicted secondary structure of GarML, obtained with both the Jpred3 (6) and the PSIPRED (32) protein structure prediction server, as well as by modeling the three-dimensional (3-D) structure of GarML with DeepView and SWISS-MODEL (http://spdbv.vital-it.ch) and ESyPred3D (25), suggests that GarML folds into a compact globular bundle comprised of four conserved α-helices enclosing a compact hydrophobic core. The structures of CclA, enterocin AS-48, circularin A, uberolysin, and lactocyclicin Q, as well as the predicted model for GarML, show that a cluster of basic amino acid residues, such as the Lys46, Lys47, Lys52, Lys53, and Lys56 residues for GarML (Fig. 3), impart a highly localized positive charge on the surface of the peptide (31). These conserved residues are likely responsible for attracting the peptides to the surface of the negatively charged membrane. Differences in antimicrobial activities among the circular bacteriocins may result from variations in the surface features of the conserved framework (17, 31). The 3-amino-acid-long leader peptide (MFD) of the GarML precursor is one of the shortest described for circular bacteriocins (23, 30, 41). However, the function of the leader peptides in the targeting and translocation of circular bacteriocins and how cyclization from the linear precursors occurs are still not understood (8, 29, 30).
Sensitivity of the bacteriocin to heat, pH, and proteolytic enzymes and antimicrobial spectrum of garvicin ML.
GarML showed resistance to temperature (80 and 100°C) and alkaline and acid pH (2 to 10), and to digestion by trypsin, pepsin, papain, and proteinase K (results not shown). The resistance of GarML to proteolytic enzymes is not due to the absence of digestion sites but to the inaccessibility of the recognition sites, probably due to a tightly folded three-dimensional structure. This could make the bacteriocin less susceptible to digestion by endoproteinases and increase its spectrum of activity (23). GarML shows a higher antibacterial activity and a broader antimicrobial spectrum as it is increasingly purified (Table 1), probably due to removal of antimicrobial inhibitors, disaggregation of the bacteriocin, or changes in conformation of the bacteriocin in the hydrophobic solvent. However, different from other circular bacteriocins (13, 41), no activity against any Gram-negative bacteria was recorded, suggesting that its mode of action may be different. Nevertheless, GarML inhibits other L. garvieae strains, and this is an interesting observation. L. garvieae is the etiological agent of lactococcosis, an emergent hyperacute, hemorrhagic septicemia that affects a range of fish and crustacea worldwide and has a considerable sanitary and economic impact in the freshwater and marine fish-farming industry (1, 11, 44). Several strategies, mostly based on the use of vaccines, bacteriocins, and probiotics, are being developed to combat L. garvieae in fish farming (3, 15). Further efforts are needed to determine the role of L. garvieae DCC43 as producer of a highly active circular bacteriocin against spoilage and pathogenic bacteria or as a potential probiotic against infections caused by other L. garvieae strains.
Nucleotide sequence accession number.
The nucleotide sequence of the structural gene encoding garvicin ML has been deposited in the GenBank database under accession number GU205098.
Acknowledgments
This work was partially supported by grants AGL2006-01042 from the Ministerio de Educación y Ciencia (MEC) and AGL2009-08348 from the Ministerio de Ciencia e Innovación (MICINN) and by grants S-0505/AGR/0265 and S2009/AGR-1489 from the Comunidad de Madrid (CAM), Spain. J. Borrero holds a research contract from the CAM.
We express our gratitude to Leah A. Martin-Visscher and John C. Vederas from the Department of Chemistry, University of Alberta, Edmonton, Alberta, Canada, for their help with the secondary structure and modeling of the 3-D structure of GarML.
Footnotes
Published ahead of print on 5 November 2010.
REFERENCES
- 1.Algöet, M., A. E. Bayley, E. G. Roberts, S. W. Feist, R. W. Wheeler, and D. W. Verner-Jeffreys. 2009. Susceptibility of selected freshwater fish species to a UK Lactococcus garvieae isolate. J. Fish Dis. 32:825-834. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunt, J., A. Newaj-Fyzul, and B. Austin. 2007. The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 30:573-579. [DOI] [PubMed] [Google Scholar]
- 4.Cintas, L. M., P. Casaus, H. Holo, P. E. Hernández, I. F. Nes, and L. S. Håvarstein. 1998. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J. Bacteriol. 180:1988-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cintas, L. M., P. Casaus, C. Herranz, I. F. Nes, and P. E. Hernández. 2001. Bacteriocins of lactic acid bacteria. Food Sci. Technol. Int. 7:281-305. [Google Scholar]
- 6.Cole, C., J. D. Barber, and G. J. Barton. 2008. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 36:W197-W201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 8.Craik, D. J. 2006. Seamless proteins tie up their loose ends. Science 311:1563-1564. [DOI] [PubMed] [Google Scholar]
- 9.Diep, D. B., L. Godager, D. Brede, and I. F. Nes. 2006. Data mining and characterization of a novel pediocin-like bacteriocin system from the genome of Pediococcus pentosaceus ATCC 25745. Microbiology 152:1649-1659. [DOI] [PubMed] [Google Scholar]
- 10.Eijsink, V. G. H., L. Axelsson, D. B. Diep, L. S. Håvarstein, H. Holo, and I. F. Nes. 2002. Production of class II bacteriocins by lactic acid bacteria: an example of biological warfare and communication. Antonie Van Leeuwenhoek 81:639-654. [DOI] [PubMed] [Google Scholar]
- 11.Fortina, M. G., G. Ricci, R. Foschino, C. Picozzi, P. Dolci, G. Zeppa, L. Coccolin, and P. L. Manachini. 2007. Phenotypic typing, technological properties and safety aspects of Lactococcus garvieae strains from dairy environments. J. Appl. Microbiol. 103:445-453. [DOI] [PubMed] [Google Scholar]
- 12.Gálvez, A., M. Maqueda, E. Valdivia, A. Quesada, and E. Montoya. 1986. Characterization and partial purification of a broad spectrum antibiotic AS-48 produced by Streptococcus faecalis. Can. J. Microbiol. 32:765-771. [DOI] [PubMed] [Google Scholar]
- 13.Gálvez, A., M. Maqueda, M. Martínez-Bueno, and E. Valdivia. 1989. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against Gram-positive and Gram-negative bacteria and other organisms. Res. Microbiol. 140:57-68. [DOI] [PubMed] [Google Scholar]
- 14.Gálvez, A., H. Abriouel, R. Lucas-López, and N. B. Omar. 2007. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 120:51-70. [DOI] [PubMed] [Google Scholar]
- 15.Gatesoupe, F. J. 2008. Updating the importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J. Mol. Microbiol. Biotechnol. 14:107-114. [DOI] [PubMed] [Google Scholar]
- 16.Gobom, J., E. Nordhoff, R. Ekman, and P. Roepstorff. 1997. Rapid micro-scale proteolysis of proteins for MALDI-MS peptide mapping using immobilized trypsin. Int. J. Mass Spectrom. Ion Processes 169-170:153-163. [Google Scholar]
- 17.Gong, X., L. A. Martin-Visscher, D. Nahirney, J. C. Vederas, and M. Duszyk. 2009. The circular bacteriocin, carnocyclin A, forms anion-selective channels in lipid bilayers. Biochim. Biophys. Acta 1788:1797-1803. [DOI] [PubMed] [Google Scholar]
- 18.Heng, N. C. K., and J. R. Tagg. 2006. What's in a name? Class distinction for bacteriocins. Nat. Rev. Microbiol. doi: 10.1038/nrmicro1273-c1. [DOI]
- 19.Kalmokoff, M. L., and R. M. Teather. 1997. Isolation and characterization of a bacteriocin (butyrivibriocin AR10) from the ruminal anaerobe Butyrivibrio fibrisolvens AR10: evidence in support of the widespread occurrence of bacteriocin-like activity among ruminal isolates of B. fibrisolvens. Appl. Environ. Microbiol. 63:394-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai, Y., T. Saito, H. Kitazawa, and T. Itoh. 1998. Gassericin A: an uncommon cyclic bacteriocin produced by Lactobacillus gasseri LA39 linked at N- and C-terminal ends. Biosci. Biotechnol. Biochem. 62:2438-2440. [DOI] [PubMed] [Google Scholar]
- 21.Kawai, Y., J. Kusnadi, R. Kemperman, J. Kok, Y. Ito, M. Endo, K. Arakawa, H. Uchida, J. Nishimura, H. Kitazawa, and T. Saito. 2009. DNA sequencing and homologous expression of a small peptide conferring immunity to gassericin A, a circular bacteriocin produced by Lactobacillus gasseri LA39. Appl. Environ. Microbiol. 75:1324-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawulka, K., T. Sprules, C. M. Diaper, R. M. Whittal, R. T. McKay, P. Mercier, P. Zuber, and J. C. Vederas. 2004. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to R-carbon cross-links: formation and reduction of R-thio-R-amino acid derivatives. Biochemistry 43:3385-3395. [DOI] [PubMed] [Google Scholar]
- 23.Kemperman, R., A. Kuipers, H. Karsens, A. Nauta, O. Kuipers, and J. Kok. 2003. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 69:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klostermann, K., F. Crispie, J. Flynn, R. P. Ross, C. Hill, and W. Meaney. 2008. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: comparison with antibiotic treatment in field trials. J. Dairy Res. 75:365-373. [DOI] [PubMed] [Google Scholar]
- 25.Lambert, C., N. Leonard, X. De Bolle, and E. Depiereux. 2002. ESyPred3D: prediction of proteins' 3D structures. Bioinformatics 18:1250-1256. [DOI] [PubMed] [Google Scholar]
- 26.Lee, Y. H., M. Kim, W. Choie, H. Min, and S. Lee. 2004. Highly informative proteome analysis by combining improved N-terminal sulfonation for de novo peptide sequencing and online capillary reverse-phase liquid chromatography/tandem mass spectrometry. Proteomics 4:1684-1694. [DOI] [PubMed] [Google Scholar]
- 27.Leer, R. J., J. M. van der Vossen, M. van Giezen, J. M. van Noort, and P. H. Pouwels. 1995. Genetic analysis of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus. Microbiology 141:1629-1635. [DOI] [PubMed] [Google Scholar]
- 28.Maqueda, M., A. Galvez, M. J. Sánchez-Barrena, C. González, A. Albert, M. Rico, and E. Valdivia. 2004. Peptide AS-48: prototype of a new class of cyclic bacteriocins. Curr. Protein Pept. Sci. 5:399-416. [DOI] [PubMed] [Google Scholar]
- 29.Maqueda, M., M. Sánchez-Hidalgo, M. Fernández, M. Montalbán-López, E. Valdivia, and M. Martínez-Bueno. 2008. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol. Rev. 32:2-22. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Visscher, L. A., M. J. van Belkum, S. Garneau-Tsodikova, R. M. Whittal, J. Zheng, L. M. McMullen, and J. C. Vederas. 2008. Isolation and characterization of carnocyclin A, a novel circular bacteriocin produced by Carnobacterium maltaromaticum UAL307. Appl. Environ. Microbiol. 74:4756-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin-Visscher, L. A., X. Gong, M. Duszyk, and J. C. Vederas. 2009. The three-dimensional structure of carnocyclin A reveals that many circular bacteriocins share a common structural motif. J. Biol. Chem. 284:28674-28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 33.Nes, I. F., D. B. Diep, L. S. Håvarstein, M. B. Brurberg, V. Eijsink, and H. Holo. 1996. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70:113-128. [DOI] [PubMed] [Google Scholar]
- 34.Nes, I. F., D. B. Diep, and H. Holo. 2007. Bacteriocin diversity in Streptococcus and Enterococcus. J. Bacteriol. 189:1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen-Meyer, J., and I. F. Nes. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 167:67-77. [PubMed] [Google Scholar]
- 36.Peters, E. C., D. M. Horn, D. C. Tully, and A. Brock. 2001. A novel multifunctional labeling reagent for enhanced protein characterization with mass spectrometry. Rapid Commun. Mass. Spectrom. 15:2387-2392. [DOI] [PubMed] [Google Scholar]
- 37.Rappsilber, J., Y. Ishihama, and M. Mann. 2003. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75:663-670. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 39.Sánchez, J., A. Basanta, B. Gómez-Sala, C. Herranz, L. M. Cintas, and P. E. Hernández. 2007. Antimicrobial and safety aspects, and biotechnological potential of bacteriocinogenic enterococci isolated from mallard ducks (Anas platyrhynchos). Int. J. Food Microbiol. 117:295-305. [DOI] [PubMed] [Google Scholar]
- 40.Sang, Y., and F. Blecha. 2008. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim. Health Res. Rev. 9:227-235. [DOI] [PubMed] [Google Scholar]
- 41.Sawa, N., T. Zendo, J. Kiyofuji, K. Himeno, J. Nakayama, and K. Sonomoto. 2009. Identification and characterization of lactocyclicin Q, a novel cyclic bacteriocin produced by Lactococcus sp. strain QU12. Appl. Environ. Microbiol. 75:1552-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sit, C. S., and J. C. Vederas. 2008. Approaches to the discovery of new antibacterial agents based on bacteriocins. Biochem. Cell Biol. 86:116-123. [DOI] [PubMed] [Google Scholar]
- 43.Toba, T., S. K. Samant, E. Yoshioka, and T. Itoh. 1991. Reutericin 6, a new bacteriocin produced by Lactobacillus reuteri LA6. Lett. Appl. Microbiol. 13:281-286. [Google Scholar]
- 44.Vela, A. I., J. Vázquez, A. Gibello, M. M. Blanco, M. A. Moreno, P. Liébana, C. Albendea, B. Alcalá, A. Méndez, L. Domínguez, and J. F. Fernández-Garayzábal. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates from other countries and sources. J. Clin. Microbiol. 38:3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villani, F., M. Aponte, G. Blaiotta, G. Mauriello, O. Pepe, and G. Moschetti. 2001. Detection and characterization of a bacteriocin, garviecin L1-5, produced by Lactococcus garvieae isolated from raw cow's milk. J. Appl. Microbiol. 90:430-439. [DOI] [PubMed] [Google Scholar]
- 46.Wirawan, R. U., K. M. Swanson, T. Kleffmann, R. W. Jack, and J. R. Tagg. 2007. Uberolysin: a novel cyclic bacteriocin produced by Streptococcus uberis. Microbiology 153:1619-1630. [DOI] [PubMed] [Google Scholar]