Abstract
Spores of Bacillus subtilis strains with (wild type) or without (α−β−) most DNA-binding α/β-type small, acid-soluble proteins (SASP) were prepared in medium with additional MnCl2 concentrations of 0.3 μM to 1 mM. These haploid spores had Mn levels that varied up to 180-fold and Mn/Fe ratios that varied up to 300-fold. However, the resistance of these spores to desiccation, wet heat, dry heat, and in particular ionizing radiation was unaffected by their level of Mn or their Mn/Fe ratio; this was also the case for wild-type spore resistance to hydrogen peroxide (H2O2). However, α−β− spores were more sensitive to H2O2 when they had high Mn levels and a high Mn/Fe ratio. These results suggest that Mn levels alone are not essential for wild-type bacterial spores' extreme resistance properties, in particular ionizing radiation, although high Mn levels sensitize α−β− spores to H2O2, probably by repressing expression of the auxiliary DNA-protective protein MrgA. Notably, Mn2+ complexed with the abundant spore molecule dipicolinic acid (DPA) with or without inorganic phosphate was very effective at protecting a restriction enzyme against ionizing radiation in vitro, and Ca2+ complexed with DPA and phosphate was also very effective in this regard. These latter data suggest that protein protection in spores against treatments such as ionizing radiation that generate reactive oxygen species may be due in part to the spores' high levels of DPA conjugated to divalent metal ions, predominantly Ca2+, much like high levels of Mn2+ complexed with small molecules protect the bacterium Deinococcus radiodurans against ionizing radiation.
Spores of Bacillus species are extremely resistant to a variety of harsh treatments, including wet and dry heat, desiccation, toxic chemicals such as peroxides, and UV and gamma irradiation (57, 58). Since spores of some species are vectors of food spoilage and food-borne disease, as well as the disease anthrax, there is continued interest in the causes of the spores' extreme resistance and mechanisms to modulate this resistance. A number of factors have been identified that cause spore resistance, including the following: (i) the thick spore coats; (ii) a low core water content; (iii) the high level of mineral ions in the core complexed with the abundant core small molecule pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) (∼20% of core dry weight); (iv) the saturation of spore DNA with a novel group of DNA-binding proteins, the α/β-type small, acid-soluble spore proteins (SASP) (35, 51, 56-58). These latter proteins protect DNA in the spore core so well that spore killing by agents such as wet heat, hydrogen peroxide (H2O2), and other peroxides is not through DNA damage but is likely via damage to one or more spore proteins (12, 13, 47, 58, 64).
There is recent evidence from studies of several bacterial systems indicating that a number of killing treatments, in particular desiccation, gamma irradiation, and UV radiation, exert their effects to a significant degree through the generation of reactive oxygen species (ROS), which cause cell killing through damage to proteins, perhaps enzymes that repair oxidative DNA damage (1, 7, 15-18, 23, 28, 32, 39, 59). Strikingly, the resistance of at least some bacteria to these agents is sensitive to cellular levels of Mn, with resistance rising markedly as cytosolic Mn concentrations increase (15-18, 23, 29, 32). It has been suggested that Mn2+ complexes scavenge ROS in vivo, thus in effect increasing cell resistance to agents that kill cells through ROS generation. Although Mn2+ is most commonly associated with its role as a catalytic and/or structural protein cofactor (40, 60, 65), the majority of cellular Mn in radiation-resistant bacteria (e.g., Deinococcus radiodurans and Lactobacillus plantarum) appear to exist as low-molecular-weight Mn2+ complexes (2, 16, 18). Intracellular Mn2+ speciation within Saccharomyces cerevisiae has also recently been probed through measurements of 1H and 31P electron-nuclear double resonance signal intensities, which support an important role for the orthophosphate (Pi) complex of Mn2+ in cellular resistance to oxidative stress in this eukaryote (40). Collectively, those studies indicate that Mn2+-Pi complexes, but not Mn2+-polyphosphate or Mn2+-pyrophosphate, serve as global cellular antioxidants which can functionally compensate for the loss of antioxidant enzymes (2-4,15). The benefits of Mn accumulation in cells are also likely to extend to protecting active sites of enzymes from oxidative damage. Replacement of Fe2+ and other divalent cations (e.g., Mg2+ and Cu2+) with Mn2+ as the mononuclear cofactor in enzymes would thus protect active sites from oxidative damage (1). Thus, the proximal protective effects afforded to enzymes by Mn2+ and its complexes are attributed to the removal of ROS generated by the Fenton reaction and other physico-chemical redox processes (1, 15, 18, 28). Generally, vegetative bacteria that cannot increase their Mn concentrations are more sensitive to agents that generate ROS, including H2O2, desiccation, UV radiation, and ionizing radiation (1).
As noted above, spores of Bacillus species are extremely resistant to agents that can generate ROS. Spores also contain high levels of divalent cations, including Mn2+ and Ca2+, most of which are complexed with DPA, and the levels of particular cations can markedly influence spore resistance (5, 27, 36, 62). It is thus possible that the normally high levels of Mn in spores play a significant role in their resistance, in particular to agents that generate ROS. However, there has been no study of the effects of Mn and other divalent cation levels on spore resistance to ROS generation, especially by gamma radiation. Indeed, the effects of various spore constituents other than α/β-type SASP on spore gamma radiation resistance have not been studied at all (41, 50, 58). Consequently, in this work we prepared Bacillus subtilis spores (+/− α/β-type SASP) with very different Mn levels and Mn/Fe ratios, and we measured the resistance of the spores to a variety of oxidizing agents. We also examined the abilities of DPA plus various divalent cations and Pi to protect a restriction enzyme against ionizing radiation in vitro.
MATERIALS AND METHODS
B. subtilis strains used and spore purification.
The B. subtilis strains used are isogenic with and derived from strain PS832, a prototrophic laboratory derivative of strain 168. These strains are PS533 carrying plasmid pUB110 encoding resistance to kanamycin (10 μg/ml) (54), PS578 (termed α−β−) lacking the sspA and sspB genes that encode the spore's two major α/β-type SASP and also carrying plasmid pUB110 (54), and PS2507, which is resistant to chloramphenicol (3 μg/ml) and lacks plasmid pUB110 and the sspA and sspB genes as well as the mrgA gene, whose product is involved in some aspects of B. subtilis cell resistance to H2O2 (8, 9). Spores of these strains were prepared at 37°C in liquid 2× SG medium, but with various amounts of MnCl2 added, and spores were harvested, purified, and stored as described previously (43). As an additional purification step to remove loosely bound manganese ions, purified spores at an optical density at 600 nm (OD600) of 50 to 300 were incubated for 1 to 5 h at 4°C in 10 mM EDTA, washed four times with an equal volume of water, and stored in water. All spores used in this work were free (98%) from growing or sporulating cells, germinated spores, and cell debris as observed by phase-contrast microscopy.
Analytical methods.
For analyses of Mn and Fe levels, ∼5 mg (dry weight) of spores or 16 mg of Difco nutrient broth was digested with concentrated nitric acid, and any remaining organic material was oxidized by the addition of 30% hydrogen peroxide. The Mn and Fe levels in these digests were determined by inductively coupled plasma-mass spectrometry at the Dartmouth College Trace Elements Analysis Core in Hanover, NH.
Analyses of spore resistance to wet heat, dry heat, desiccation, H2O2, and gamma radiation were carried out as follows, generally using established protocols (21, 45, 52, 53). In all cases aliquots of untreated and treated spores were diluted serially in water, aliquots were spotted on Luria broth medium plates (46) containing kanamycin (10 μg/ml), plates were incubated 24 to 36 h at 37°C until no further colonies appeared, and colonies were counted. For wet heat resistance, spores were incubated at an OD600 of 1 at either 85°C (PS533) or 80°C (PS578), at various times aliquots were diluted in water at 23°C, and survivors were determined as described above. For dry heat resistance, 1 ml of spores at an OD600 of 1 were freeze-dried, samples were heated at either 120°C (PS533) or 90°C (PS578) in an oil bath for various times, samples were rehydrated with 1 ml water, the tubes were briefly immersed in a bath sonicator to disperse the spores, survivors were determined as described above, and the OD600 of the suspended spores was measured to assess spore recovery. For desiccation resistance, 1-ml aliquots of spores at an OD600 of 3 were centrifuged, and the pellets were frozen and lyophilized. After 18 to 72 h of desiccation pellets were rehydrated in 1 ml of water for 1 to 3 h on ice with spore dispersion assisted by brief sonication, small (10-μl) aliquots were taken for determination of survivors, the remaining suspension was centrifuged, and the pellets were relyophilized. Following determination of the spore viability values as described above after 3 to 12 freeze-drying and rehydration cycles, the OD600 of the final spore suspension was determined in order to calculate total spore recovery. For H2O2 resistance, spores at an OD600 of 1 were incubated at 23 or 20°C in 50 mM KPO4 buffer (pH 7.4) plus 5% H2O2. At various times, aliquots were diluted 1/100 in 50 mM KPO4 buffer (pH 7.4), with 1 μg of beef liver catalase added, and survival was determined as described above after incubation for at least 15 min. For determination of gamma radiation resistance, spores at an OD600 of 10 in 4°C water were exposed to a 60Co source to give exposures of 1 to 20 kGy, and survivors were quantitated as described above. All measurements of spore survival during various treatments were carried out at least in duplicate and with at least two independent spore preparations made with different concentrations of MnCl2 added to the sporulation medium.
DPA was extracted from spores and quantitated as described previously (45, 49). Determinations of spore wet densities were by equilibrium isopycnic centrifugation in gradients of 45 to 75% Nycodenz (Sigma Chemical Co., St. Louis, MO) as described previously (12). Small amounts of beads with a density of 1.139 g/ml (Sigma Chemical Co., St. Louis, MO) were added to each gradient to allow correction for any differences between gradients.
BamHI irradiation and activity assays.
Irradiation of the restriction enzyme BamHI was on ice in air with 60Co at 4.2 kGy/h. Dilutions of reagents were with Nanopure H2O (resistivity, 18.2 megohms-cm; Barnstead nanopure water purification system; Thermo Scientific, Waltham, MA). Postirradiation activity of BamHI was analyzed as described previously (17, 18). Briefly, BamHI (5,000 units/μl; a special-order reagent prepared without bovine serum albumin; New England Biolabs, Ipswich, MA) was diluted in the indicated reagent mixtures to 3.8 units/μl; the reagent mixtures were boiled and then cooled on ice before adding BamHI. Typically, 50-μl aliquots of the BamHI mixtures were irradiated in 0.5-ml tubes. Following irradiation, 5 μl of each BamHI sample was assayed for residual endonuclease activity in a separate reaction mixture (final volume, 50 μl) containing 200 ng λ phage DNA, 50 mM NaCl, 10 mM Tris-HCl (pH 7.9), 10 mM MgCl2, and 1 mM dithiothreitol. BamHI-λ DNA mixtures were incubated for 1.25 h at 37°C, followed by 0.8% agarose gel electrophoresis as described previously (17, 18). All experiments were repeated at least three times. All chemicals used in these analyses were the highest-quality reagents and were obtained from Sigma Chemical Co., St. Louis, MO.
RESULTS
Mn levels in B. subtilis spores.
While B. subtilis sporulates in several defined media, complex media generally give higher yields of spores and are more convenient to prepare. Consequently, we chose to use 2× SG medium, a complex medium that gives good sporulation (43). This medium as routinely used has 10 μM MnCl2 added, in addition to the low concentration of Mn (∼0.5 μM) determined to be present in the complex medium without MnCl2 supplementation (data not shown), although the oxidation state of this Mn is not known. Additional Mn2+ is routinely added to this complex sporulation medium, since sporulation in Mn-deficient medium is very poor, at least in part because of the absolute Mn2+ requirement of the enzyme phosphoglycerate mutase (33, 44, 61). In preliminary experiments we found that liquid 2× SG medium without added MnCl2 gave only poor sporulation (data not shown). However, supplementation with 0.3 μM MnCl2 gave good sporulation, equivalent to that in complete 2× SG medium, and addition of MnCl2 up to 1 mM also gave similar sporulation (data not shown).
Because we were interested in the effects of Mn levels in the spore core on resistance of key spore molecules such as DNA and enzymes, most of which are in the spore core, it was important to show that the Mn in spores made in medium with different Mn concentrations was largely in the core and not adsorbed in the spore outer layers. The latter was of special concern for spores prepared in medium with high Mn concentrations, since adsorption of Mn-containing precipitates to spore outer layers has been reported (22, 48). Consequently, Mn levels were determined in wild-type spores following spore purification but before and after treatment with the chelator EDTA either once or twice (Table 1). Such an EDTA treatment has recently been shown to remove at least the great majority of Mn adsorbed in spore outer layers (63). In contrast, removal of Mn2+ and other cations chelated to DPA in the spore core, while still retaining spore viability, requires titration of spores to pH 4 with HCl and incubation at ∼60°C (36). One EDTA treatment at 4°C of spores made in medium with 10 μM additional MnCl2 led to removal of only a small amount of Mn, but when spores were made with 1 mM additional MnCl2, the first EDTA treatment removed ∼70% of the spore-associated Mn (Table 1). However, in both cases a second EDTA treatment at 4°C did not reduce spore Mn levels further (Table 1).
TABLE 1.
Mn levels in spores with and without EDTA treatmenta
| Treatment of purified spores | Mn level (μg/g [dry wt]) in spores prepared with: |
|
|---|---|---|
| 10 μM Mn | 1 mΜ Mn | |
| None | 1,775 | 13,078 |
| One EDTA treatment | 1,526 | 3,847 |
| Two EDTA treatments | 1,515 | 4,106 |
Spores of B. subtilis PS533 (wild type) were prepared with various concentrations of MnCl2 added to 2× SG medium, the spores were purified, and Mn levels were determined before or after one or two EDTA treatments as described in Materials and Methods.
Using purified wild-type spores that had been treated once with EDTA, we found that their Mn content increased markedly when we used medium with 0.3 to 100 μM additional MnCl2, although there was little further increase in medium with 1 mM additional MnCl2 (Table 2). In going from medium with 0.3 μM additional MnCl2 to medium with 1 mM additional MnCl2, Mn levels in wild-type spores increased ∼180-fold, Fe levels decreased ∼2-fold, and the Mn/Fe ratio increased ∼300-fold (Table 2). In contrast to the large changes in Mn levels in spores made in medium with different MnCl2 concentrations, spores with these different Mn levels had identical levels of DPA (all within 15%) (data not shown). As a consequence, in spores prepared with 0.3 μM additional MnCl2, the Mn/DPA molar ratio was ∼0.001, and this value increased to ∼0.18 in spores made with 1 mM MnCl2 (data not shown). Spores made with different Mn levels also had identical (within experimental error) core water contents, as determined by measuring the spore core wet densities (data not shown).
TABLE 2.
Mn and Fe levels in spores prepared with different Mn2+ concentrations added to sporulation mediuma
| Mn2+ concn (μM) | Metal level (μg/g [dry wt]) (range) and Mn/Fe ratio in cell type |
|||||
|---|---|---|---|---|---|---|
| Wild type |
α−β− |
|||||
| Mn | Fe | Mn/Fe | Mn | Fe | Mn/Fe | |
| 0.3 | 24 (21-27) | 87 (72-101) | 0.3 | 49 (39-59) | 79 (66-93) | 0.6 |
| 1 | 65 (61-69) | 83 (79-87) | 0.8 | 125 (107-142) | 79 (77-81) | 1.6 |
| 10 | 1,025 (989-1,061) | 75 (74-76) | 15 | 1,592 (1,300-1,884) | 69 (60-77) | 23 |
| 100 | 3,621 (3,272-3,896) | 44 (32-60) | 82 | 3,405 (3,355-3,455) | 63 (58-68) | 54 |
| 1,000 | 4,140 (3,863-4,416) | 42 (30-54) | 99 | 3,829 (3,592-4,066) | 50 (37-63) | 77 |
Spores of various strains were prepared with various additional MnCl2 concentrations, the spores were purified and EDTA treated, and analysis of Mn and Fe levels in EDTA-treated spore samples was carried out as described in Materials and Methods. All values reported are averages for two independent spore preparations. Values in parentheses (ranges) are the results for individual spore preparations.
Resistance of wild-type spores prepared with different Mn levels.
With spores prepared in different Mn levels in hand, it was then possible to examine the effects of Mn levels on spore resistance properties. Wild-type spores made in complete 2× SG medium are resistant to at least 25 freeze-dryings (21, 58). Analysis of PS533 spores with an ∼180-fold difference in their Mn levels showed that these spores exhibited no (≤30%) killing after 12 freeze-dryings (Table 3). In contrast to desiccation, wild-type B. subtilis spores are killed by wet heat, dry heat, H2O2, and gamma radiation (57). However, wild-type spores with levels of Mn that differed ∼180-fold exhibited essentially identical killing by wet heat, dry heat, H2O2, and gamma radiation, with the most severe treatments giving 99 to 99.9% spore killing (Fig. 1 A to D).
TABLE 3.
Desiccation killing of spores in medium with different Mn levelsa
| Mn2+ concn (μM) | % viability in spore type after indicated no. of freeze-drying cycles |
||
|---|---|---|---|
| Wild type |
α−β− |
||
| 12 cycles | 3 cycles | 6 cycles | |
| 0.3 | ≥70 | 11 | 0.7 |
| 1 | ≥70 | 7 | 0.6 |
| 10 | ≥70 | 10 | 1 |
| 100 | ≥70 | 15 | 0.6 |
| 1,000 | ≥70 | 9 | 0.3 |
Spores of various strains were prepared in medium containing various additional MnCl2 concentrations, the spores were purified and EDTA treated, and resistance to desiccation was determined as described in Materials and Methods.
FIG. 1.
Resistance properties of wild-type B. subtilis spores made in different Mn levels. Spores of B. subtilis PS53 (wild type) were prepared in media with different amounts of added MnCl2, the spores were purified and EDTA treated, and their resistance properties were measured as described in Materials and Methods. All values reported are the results of at least duplicate measurements on one set of spores prepared together, and essentially identical results were obtained with two other independent sets of spore preparations. The resistance properties measured were to wet heat (A), dry heat (B), H2O2 (measured at 23°C) (C), and gamma radiation (D). The symbols used represent the different amounts of Mn2+ added to sporulation medium to make spores: ○, 0.3 μM; •, 1 μM; ▵, 10 μM; ▴, 100 μM; □, 1 mM.
Resistance of α−β− spores with different Mn levels.
As noted above, the α/β-type SASP are important factors in the resistance properties of wild-type spores, in particular to desiccation, wet heat, dry heat, H2O2, and gamma radiation (41, 58). As a consequence, α−β− B. subtilis spores that lack the two major α/β-type SASP are more sensitive to these agents, although α−β− spores have DPA levels and core water contents very similar to those of wild-type spores (37, 55) (data not shown). Thus, it was of interest to examine the resistance properties of α−β− spores with different Mn levels. PS578 (α−β−) spores made with different additional MnCl2 concentrations added to 2× SG medium had Mn levels similar to those in wild-type spores prepared with the same Mn2+ concentrations, although α−β− spores prepared with lower additional MnCl2 in the sporulation medium had higher amounts of Mn than wild-type spores prepared comparably and slightly higher Fe levels than wild-type spores prepared with high additional MnCl2 concentrations (Table 2). Because of these latter differences, the Mn/Fe ratio in α−β− spores rose only ∼130-fold on comparison of spores prepared with 0.3 μM to those with 1 mM MnCl2 (Table 2). As found with spores of the wild-type strain, α−β− spores with different Mn levels also had similar levels of DPA (within 15%) (data not shown).
In contrast to wild-type spores, α−β− spores made in normal 2× SG medium are sensitive to desiccation, losing approximately 1 log of viability for every three freeze-drying cycles (21). This was also found in the current work (Table 3). However, α−β− spores with up to an ∼80-fold difference in their Mn content exhibited no significant differences in their killing by freeze-drying, up to a level of ≥99% killing (Table 3). Analysis of the killing of α−β− spores by wet heat, dry heat, and H2O2 again showed that these spores were more sensitive to these agents than were wild-type spores, as expected (56, 57) (Fig. 2 A to C; compare with Fig. 1A to C); the gamma radiation resistance of α−β− spores was also lower than that of wild-type spores (compare Fig. 2D with Fig. 1D). However, up to an ∼80-fold difference in α−β− spore Mn content had no significant effect on the spores' killing by wet heat, dry heat, or gamma radiation (Fig. 2A, B, and D). In contrast, α−β− spores with lower Mn levels were significantly more resistant to H2O2 than were spores with the highest Mn levels (Fig. 2C).
FIG. 2.
Resistance properties of α−β− B. subtilis spores prepared with different Mn levels. Spores of B. subtilis PS578 (α−β−) were prepared in media with different amounts of added MnCl2, the spores were purified and EDTA treated, and their resistance properties were measured as described in Materials and Methods. All values reported are the results of at least duplicate measurements on one set of spores prepared together, and essentially identical results were obtained with another independent set of spores prepared together. The resistance properties measured were to wet heat (A), dry heat (B), H2O2 (measured at 23°C) (C), and gamma radiation (D). The symbols used represent the different amounts of Mn2+ added to sporulation medium to make spores: ○, 0.3 μM; •, 1 μM; ▵, 10 μM; ▴, 100 μM; □, 1 mM.
The elevated H2O2 resistance of α−β− spores with low Mn levels was unexpected, but a possible explanation for this phenomenon is that low Mn levels during sporulation may greatly increase the expression of the DNA-protective protein MrgA due to the derepression of the PerR regulon, since Mn is a corepressor of this regulon (9). While α−β− mrgA spores have been reported to exhibit identical H2O2 resistance to α−β− spores (8), the spores in the latter study were prepared in complete 2× SG medium, in which mrgA may be largely be repressed (8, 9). Consequently, spores of an α−β− mrgA strain were prepared with various Mn levels, and their H2O2 resistance was measured (Fig. 3; note that the resistance of these spores was tested at a slightly lower temperature than the α−β− spores in Fig. 2C). Strikingly, α−β− mrgA spores prepared with 0.3 μM Mn had much lower H2O2 resistance than α−β− spores prepared at this Mn concentration, although preparation of α−β− mrgA spores with 1 μM Mn resulted in higher resistance (Fig. 2C and 3). However, when α−β− mrgA spores were prepared with 1 to 100 μM Mn, the spores' H2O2 resistance decreased significantly, as was seen with α−β− spores (Fig. 2C and 3). Note that in contrast to the α−β− spores of strain PS578, the α−β− mrgA spores lacked plasmid pUB110. However, the absence of plasmid pUB110 has no noticeable effect on α−β− spore resistance to H2O2 (B. Setlow and P. Setlow, unpublished data).
FIG. 3.
Hydrogen peroxide resistance of α−β− mrgA spores prepared with different Mn levels. Spores of strain PS2507 (α−β− mrgA) were prepared with different Mn concentrations in the sporulation medium, the spores were purified, and spore resistance to H2O2 at 20°C was determined as described in Materials and Methods. The symbols represent the added Mn2+ concentrations under which spores were made: ○, 0.3 μM; •, 1 μM; ▵, 10 μM; ▴, 100 μM.
Effects of DPA, divalent cations, and orthophosphate on protein radioprotection in vitro.
Recent studies have indicated that a major defense against extreme gamma radiation and desiccation damage in polyploid prokaryotic cells, accounting for the distinctive shoulders in their dose-response relationships, is a greatly enhanced capacity for scavenging ROS (15). In the extremely radiation- and desiccation-resistant bacterium D. radiodurans, the accumulation of low-molecular-weight complexes of Mn2+ with Pi, peptides, and certain nucleosides has been implicated in protecting the proteome from oxidation (18). Proteins thereby protected include homologous recombination enzymes needed to repair double-strand breaks (DSBs).
In standard rich sporulation medium, dormant spores of at least several Bacillus species accumulate high levels of Mn2+ (25 to 50 μmol/g [dry weight] of spores), DPA (∼450 μmol/g [dry weight]), Ca2+ (∼400 μmol/g [dry weight]), and Pi (∼15 μmol) (42, 57). While these small molecules are solely in the spore core, calculating their actual concentrations there is difficult, since the level of at least DPA undoubtedly exceeds its solubility. However, if we assumed that (i) these small molecules are soluble, (ii) the core is ∼70% of the spore dry weight, and (iii) ∼40% of the core's wet weight is water (24, 57, 58), this would give approximate concentrations of these molecules ranging from 30 mM (Pi) to 900 mM (DPA). Previous in vitro studies have shown that small organic compounds (e.g., uridine) that contain a primary, secondary, or tertiary amine group flanked by two carbonyl oxygens form stable Mn2+ complexes when deprotonated (30). Such Mn complexes are extremely radioprotective of irradiated DNA-modifying enzymes and metabolic enzymes, preventing protein carbonylation and preserving their activity (18). DPA falls within this structural rubric and is a good Mn chelator (11), and it was therefore tested for its ability to protect an enzyme from ionizing radiation when combined with phosphate buffer, Mn2+, Ca2+, and Mg2+.
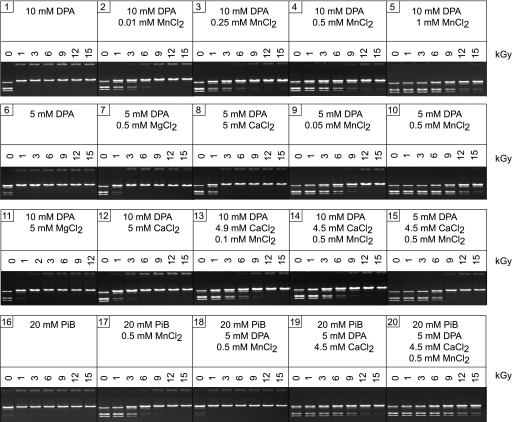
Using the activity of the restriction endonuclease BamHI as a reporter of protein function, we tested the radioprotective properties of mixtures of DPA, cations, and Pi at concentrations for most of these species that are well below those in spores—Mn2+ at 0.01 to 1 mM, DPA at 5 to 10 mM, and Pi at 20 mM—although these values for Pi and divalent cations are in the ranges for many cell types, including eukaryotes (40). BamHI is readily inactivated in aerobic aqueous solutions by ROS generated by 150 Gy (17), and Mn2+ alone is not significantly radioprotective of this enzyme (18). Individually, Pi buffer and DPA did not protect BamHI from 1 kGy under aerobic conditions (Fig. 4, gels 1 and 16), although the combination of Mn2+ and DPA was highly radioprotective, preserving the activity of BamHI to 9 kGy (Fig. 4, gels 2 to 5 and 10). However, when Pi buffer was added to an equivalent mixture of Mn2+-DPA, radioprotection was lost (Fig. 4, gel 18). Unexpectedly, we found that although mixtures of DPA and Ca2+ or Mg2+ were not significantly radioprotective (Fig. 4, gels 7, 8, 11, and 12), mixtures of DPA-Pi (Fig. 4, gel 19) or Mn2+-DPA-Pi (Fig. 4, gel 20) with Ca2+ added were extremely radioprotective, with BamHI surviving 9 to 15 kGy. Thus, the ROS-scavenging effects of DPA, Ca2+, Mn2+, and Pi were highly synergistic, particularly with Mn2+, as reported for Pi, Mn2+, nucleosides, and peptides that accumulated in D. radiodurans (18).
FIG. 4.
Radioprotection of BamHI by reconstituted mixtures of DPA, Mn2+, Ca2+, and potassium phosphate buffer (PiB; pH 7.4). Irradiation levels are shown in kGy (numbers above each gel) and were performed on ice under aerobic conditions; postirradiation activity of BamHI was determined by incubation with λ phage DNA followed by agarose gel electrophoresis as described in Materials and Methods. Each gel is representative of two or three independent trials conducted for each reaction-irradiation mixture.
DISCUSSION
The first studies implicating Mn2+ ions in the removal of ROS were in the 1950s, when chloroplasts were shown to contain plentiful Mn, both free and bound, and the oxidation of Mn2+ by illuminated chloroplasts was shown to be due to superoxide (31). The superoxide theory of oxygen toxicity emerged in the 1970s, and the enzyme superoxide dismutase (SOD), which catalytically scavenges superoxide, was considered to be the dominant, essential defense against this radical in aerobic organisms (38). In one of the early tests of this theory, a variety of microorganisms were surveyed for their contents of SOD and the H2O2-decomposing enzyme catalase. Surprisingly, a few aerobic bacteria were identified which did not contain SOD or catalase, although they did actively accumulate Mn (2). For example, superoxide scavenging in L. plantarum cell extracts was shown to be due to dialyzable forms of Mn2+ (2). Subsequent investigation showed that Mn2+ binds a variety of ligands in vitro, the complexes of which scavenge different ROS to varying degrees (3). Irwin Fridovich and colleagues showed that Mn2+ acted as a stoichiometric scavenger of superoxide in pyrophosphate buffer but as a catalytic scavenger in Pi buffer or medium containing carboxylic acids (3). Studies by Earl Stadtman and colleagues further showed that Mn2+ could form complexes with amino acids or peptides that catalytically decompose H2O2 (6). More recently, a critical role for Mn2+ accumulation was demonstrated in radiation-resistant bacteria as a mechanism for surviving extreme doses of gamma radiation that was not dependent on antioxidant enzymes (16). Evidently, the accumulation of intracellular Mn2+ complexes in bacteria can provide levels of protection from ROS which equal or even exceed the ROS-scavenging capacities of enzymes. Strong lines of evidence from different laboratories have thus converged on the conclusion that the accumulation of Mn2+ with Pi together with certain organic metabolites represents a widespread strategy for combating oxidative stress (4, 18, 40). The nature(s) of the cellular complexes, however, remains unknown, principally because it has not been possible to reliably determine Mn speciation within cells, because low-molecular-weight Mn complexes exchange their ligands rapidly in solution, and standard procedures that disrupt cells likely alter this speciation.
It has been proposed that Mn2+-dependent chemical antioxidants protect DNA repair functions in extremely radiation- and desiccation-resistant bacteria (15). However, bacteria are not expected to benefit from such protection if their genome copy number is less than two, as the most severe form of DNA damage in irradiated cells, the DSB, is difficult to repair when homologous repair templates are absent (15). In contrast, the presence of chemical antioxidants that protect proteins in polyploid bacteria is expected to substantially increase the efficiency of DSB repair (15). Wild-type B. subtilis spores are haploid and display survival curves which lack the distinctive shoulders of radiation-resistant polyploid cells (Fig. 1D). Work in this communication shows that the Mn level in wild-type B. subtilis spores is not a significant factor in their resistance to a variety of killing agents. The DSB lesion yields for all vegetative prokaryotic and eukaryotic cells examined following ionizing radiation treatments fall within a narrow range (0.002 to 0.008 DSB/Gy/Mbp) (15, 25). As the water content of the spore core as a percentage of wet weight is 2- to 3-fold lower than in the vegetative cell's protoplast and DNA in spores is tightly bound by SASP (24, 57, 58), DSB yields in B. subtilis spores exposed to ionizing radiation are expected to be significantly lower than in vegetative cells, perhaps only a few DSBs per haploid genome (4.2 Mbp) at 3,000 Gy (Fig. 1C). However, DSB yields have not been determined in such irradiated spores. Over a several-hundred-fold range of spore Mn levels and Mn/Fe ratios, our findings that B. subtilis spores were not more resistant to ionizing radiation, desiccation, or H2O2 can be explained at least in part by the fact that these spores are haploid, since haploidy will limit DSB repair irrespective of any increase in the efficiency of DNA repair mediated by Mn-dependent protection. Future analysis of the effects of Mn levels on the gamma radiation resistance of Bacillus megaterium spores, which are digenomic (34), might give insight into the importance of DSB repair in spore gamma radiation resistance. Indeed, there is a report that preparation of B. megaterium spores with high, Mn levels increases their UV resistance (20).
The results with α−β− spores having different Mn levels were largely, but not completely, similar to those with wild-type spores, even though α−β− spores were more sensitive than wild-type spores to all the agents tested, as expected. For gamma irradiation, α−β− spores were ∼3-fold more sensitive than wild-type spores, something that was not seen previously when spores were irradiated with a 60Co source (26) but was observed more recently when spores were exposed to an X-ray source or to high-energy-charged particles (41). The resistance of α−β− spores to desiccation and gamma irradiation, both of which kill spores largely by DNA damage other than base loss (19), was independent of their Mn levels.
In contrast to these results, α−β− spore resistance to H2O2, which also kills these spores by DNA damage (52, 58), was quite sensitive to the Mn levels in spores, and surprisingly, α−β− spores with low Mn levels were more resistant to H2O2 than were α−β− spores with high Mn levels. The low H2O2 resistance of α−β− mrgA spores prepared at 0.3 μM Mn suggests that MrgA expressed at low Mn concentrations in sporulation medium (9) can contribute significantly to the H2O2 resistance of α−β− spores. In addition, when α−β− mrgA spores were prepared with 1 μM Mn, their H2O2 resistance increased, suggesting that an intermediate Mn level may protect spores against H2O2 in the absence of both MrgA and α/β-type SASP. However, further increases in spore Mn levels resulted in decreased H2O2 resistance of the α−β− mrgA spores, just as was seen with α−β− spores. The reason that elevated Mn levels in sporulation sensitize spores lacking α/β-type SASP and MrgA to H2O2 is not clear, but a reasonable hypothesis is that elevated Mn levels during sporulation may lead to Mn-dependent repression, perhaps via PerR, of expression of one or more additional genes whose products are involved in spore resistance to H2O2. It is also perhaps notable that base changes generated in α−β− spores by dry heat or desiccation are identical but are considerably different from the base changes generated in α−β− spores by hydrogen peroxide (19). A similar loss in oxidative stress resistance in Mn2+-accumulating D. radiodurans cells is observed when these cells are grown in medium supplemented with high (>100 μM) concentrations of Mn2+, which leads to Mn overaccumulation, loss of radioresistance, and toxicity (10). Notably, Mn2+ solutions exposed to ionizing radiation in vitro display a concentration-related response to dose, with increasing yields of Mn3+ generated as doses of gamma radiation increase (17), and Mn3+ is a strong oxidant (2, 14). Thus, radioprotection by Mn2+ may manifest itself only within a narrow range of Mn concentrations, and then only in the presence of proton donor ligands such as Pi and carboxylic acids (2, 4, 18).
In D. radiodurans, the cytosolic accumulation of small complexes consisting of Mn2+, Pi, and small peptides (7 to 22 amino acids in length) at 0.2 to 3 mM has been strongly implicated in protecting the proteome from oxidation (18). This raises the possibility that a route to oxidative stress resistance in cells that express Mn2+ uptake systems is via metabolite accumulation. Bacillus spores contain depots not only of Mn but also of DPA, a compound absent from non-spore-forming bacteria and present in spores at concentrations above those of any other low-molecular-weight solute. We found that at or below their likely concentrations in spores, mixtures of Mn2+ (0.5 mM), DPA (5 mM), Ca (4.5 mM), and Pi (20 mM) protected the highly ROS-sensitive enzyme BamHI from gamma ray doses (15 kGy) which far exceeded the absolute limits of B. subtilis spore survival (∼6 kGy) (Fig. 1D). In mixtures of Mn2+, DPA, Ca2+, and Pi, Mn ions were the most consequential in protecting BamHI (Fig. 4). Yet, the current findings show that increasing the Mn content of B. subtilis spores does not increase their resistance to ionizing radiation, desiccation, or H2O2. As noted above, B. subtilis spores are haploid, which could have masked the benefits stemming from protein protection conferred by the accumulation of ROS-scavenging Mn complexes. However, it is also possible that just the spore core's high levels of complexes of DPA plus Pi and either Ca2+ or Mn2+ are sufficient to provide elevated ionizing radiation resistance for B. subtilis spores, with Ca2+ and Mn2+ having interchangeable roles, much as we saw in the protection of BamHI against gamma irradiation in vitro. In this regard it might be informative to analyze the gamma irradiation resistance of B. subtilis spores that lack DPA, although these spores (i) are generally quite unstable and germinate spontaneously and (ii) have a significantly elevated spore water content (35, 45, 51).
In contrast to B. subtilis spores, Bacillus megaterium spores, which survive 8 kGy, are diploid and display shoulders in their gamma radiation survival curves (34); this is a characteristic shared by other radiation-resistant polyploid organisms (15). It would thus be of interest to examine the effects of levels of Mn on the gamma radiation resistance of B. megaterium spores, as noted above. Unfortunately, Bacillus species do not sporulate when Mn is limiting, which makes it difficult to prepare spores with intracellular concentrations of Mn lower than 25 μg/g (dry weight of spores). However, a number of biochemical approaches have been developed recently to characterize the role of Mn complexes in D. radiodurans (18), and these approaches could serve as a model to study equivalent processes in B. subtilis and B. megaterium spores.
Acknowledgments
This work was supported by grants from the Army Research Office (P.S.) and by the Air Force Office of Scientific Research (M.J.D.).
We are grateful to Brian Jackson of Dartmouth College's Trace Element Analysis Core for assistance with the Mn and Fe analyses, to Barbara Setlow and Will Garner for assistance with some experiments, and to John Helmann for suggesting the possible role of MrgA in protection of α−β− spores against H2O2.
Footnotes
Published ahead of print on 5 November 2010.
REFERENCES
- 1.Anjem, A., S. Varghese, and J. A. Imlay. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72:844-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, F. S., and I. Fridovich. 1981. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 145:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archibald, F. S., and I. Fridovich. 1982. The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214:452-463. [DOI] [PubMed] [Google Scholar]
- 4.Barnese, K., E. B. Gralla, D. E. Cabelli, and J. S. Valentine. 2008. Manganous phosphate acts as a superoxide dismutase. J. Am. Chem. Soc. 130:4604-4606. [DOI] [PubMed] [Google Scholar]
- 5.Bender, G. R., and R. E. Marquis. 1985. Spore heat resistance and specific mineralization. Appl. Environ. Microbiol. 50:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlett, B. S., P. B. Chock, M. B. Yim, and E. R. Stadtman. 1990. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc. Natl. Acad. Sci. U. S. A. 87:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosshard, F., K. Riedel, T. Schneider, C. Geiser, M. Bucheli, and T. Egli. 2010. Protein oxidation and aggregation in UVA-irradiated Escherichia coli cells as signs of accelerated cellular senescence. Environ. Microbiol. 12:2931-2945. [DOI] [PubMed] [Google Scholar]
- 8.Casillas-Martinez, L., and P. Setlow. 1997. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat and oxidizing agents. J. Bacteriol. 179:7420-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, L., L. Keramati, and J. D. Helmann. 1995. Coordinate regulation of Bacillus subtilis peroxide stress genes by hydrogen peroxide and metal ions. Proc. Natl. Acad. Sci. U. S. A. 92:8190-8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou, F. I., and S. T. Tan. 1990. Manganese (II) induces cell division and increases in superoxide dismutase and catalase activities in an aging deinococcal culture. J. Bacteriol. 172:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung, L., K. S. Rajan, E. Merdinger, and N. Grecz. 1971. Coordinative binding of divalent cations with ligands related to bacterial spores. Equilibrium studies. Biophys. J. 11:469-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman, W. H., D. Chen, Y.-Q. Li, A. E. Cowan, and P. Setlow. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 189:8458-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman, W. H., and P. Setlow. 2009. Analysis of damage due to moist heat treatment of spores of Bacillus subtilis. J. Appl. Microbiol. 106:1600-1607. [DOI] [PubMed] [Google Scholar]
- 14.Curnutte, J. T., M. L. Karnovsky, and B. M. Babior. 1976. Manganese dependent NADPH oxidation by granulocyte particles. J. Clin. Invest. 57:1059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly, M. J. 2009. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat. Rev. Microbiol. 7:237-245. [DOI] [PubMed] [Google Scholar]
- 16.Daly, M. J., E. K. Gaidamakova, V. Y. Matrasova, A. Vasilenko, M. Zhai, A. Venkateswaran, M. Hess, M. V. Omelchenko, H. M. Kostandarithes, K. S. Makarova, L. P. Wackett, J. K. Fredrickson, and D. Ghosal. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025-1028. [DOI] [PubMed] [Google Scholar]
- 17.Daly, M. J., E. K. Gaidamakova, V. Y. Matrasova, A. Vasilenko, M. Zhai, R. D. Leapman, B. Lai, B. Ravel, S.-M. Li, K. M. Kemner, and J. K. Fredrickson. 2007. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 5:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly, M. J., E. K. Gaidamakova, V. Y. Matrosova, J. G. Kiang, R. Fukumoto, D.-Y. Lee, N. B. Wehr, G. Viteri, B. S. Bertlett, and R. L. Levine. 2010. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One 5:e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Carmen-Huesca-Espitia, L., C. Caley, I. Bagyan, and P. Setlow. 2002. Base-change mutations induced by various treatments of Bacillus subtilis spores with and without DNA protective small, acid-soluble proteins. Mutat. Res. 503:77-84. [DOI] [PubMed] [Google Scholar]
- 20.Donnellan, J. E., Jr., and R. S. Stafford. 1968. The ultraviolet photochemistry and photobiology of vegetative cells and spores of Bacillus megaterium. Biophys. J. 8:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairhead, H., B. Setlow, W. M. Waites, and P. Setlow. 1994. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by freeze-drying. Appl. Environ. Microbiol. 60:2647-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis, C. A., and B. M. Tebo. 1999. Marine Bacillus spores as catalysts for oxidative precipitation and sorption of metals. J. Mol. Microbiol. Biotechnol. 1:71-78. [PubMed] [Google Scholar]
- 23.Fredrickson, J. K., S.-M. W. Li, E. K. Gaidamakova, V. Y. Matrosova, M. Zhai, H. M. Sulloway, J. C. Scholten, M. G. Brown, D. L. Balkwill, and M. J. Daly. 2008. Protein oxidation: key to bacterial desiccation resistance. ISME J. 2:393-403. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-64. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development: structural and functional analysis of bacterial sporulation and germination. American Society for Microbiology, Washington, DC.
- 25.Gladyshev, E., and M. Meselson. 2008. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 105:5139-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hackett, R. H., and P. Setlow. 1988. Properties of spores of Bacillus subtilis strains which lack the major small, acid-soluble protein. J. Bacteriol. 170:1403-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igura, N., Y. Kamimura, M. S. Islam, and I. Hayakawa. 2003. Effects of minerals on resistance of Bacillus subtilis spores to heat and hydrostatic pressure. Appl. Environ. Microbiol. 69:6307-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imlay, J. A. 2008. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77:755-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inaoka, T., Y. Matsumura, and T. Tsuchido. 1999. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 181:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knobloch, B., W. Linert, and H. Sigel. 2005. Metal ion-binding properties of (N3)-deprotonated uridine, thymidine, and related pyrimidine nucleosides in aqueous solution. Proc. Natl. Acad. Sci. U. S. A. 102:7459-7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kono, Y., M.-A. Takahashi, and K. Asada. 1976. Oxidation of manganous pyrophosphate by superoxide radicals and illuminated spinach chloroplasts. Arch. Biochem. Biophys. 174:454-462. [DOI] [PubMed] [Google Scholar]
- 32.Kriško, A., and M. Radman. 2010. Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans. Proc. Natl. Acad. Aci. U. S. A. 107:14373-14377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn, N. J., B. Setlow, and P. Setlow. 1993. Manganese (II) activation of 3-phosphoglycerate mutase of Bacillus megaterium: pH-sensitive interconversion of active and inactive forms. Arch. Biochem. Biophys. 306:342-349. [DOI] [PubMed] [Google Scholar]
- 34.Levinson, H. S., and M. T. Hyatt. 1960. Some effects of heat and ionizing radiation on spores of Bacillus megaterium. J. Bacteriol. 80:441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magge, A., A. C. Granger, P. G. Wahome, B. Setlow, V. R. Vepachedu, C. A. Loshon, L. Peng, D. Chen, Y.-Q. Li, and P. Setlow. 2008. Role of dipicolinic acid in the germination, stability and viability of spores of Bacillus subtilis. J. Bacteriol. 190:4798-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquis, R. E., and G. R. Bender. 1985. Mineralization and heat resistance of spores. J. Bacteriol. 161:789-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason, J. M., and P. Setlow. 1986. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J. Bacteriol. 167:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCord, J. M., B. B. Keele, Jr., and I. Fridovich. 1971. An enzyme-based theory of obligate anaerobiosis: the physiological function of supeoxide dismutase. Proc. Natl. Acad. Sci. U. S. A. 68:1024-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwan, A. G. 2009. New insights into the protective effect of manganese against oxidative stress. Mol. Microbiol. 72:812-814. [DOI] [PubMed] [Google Scholar]
- 40.McNaughton, R. L., A. R. Reddi, M. H. S. Clement, A. Sharma, K. Barnese, L. Rosenfeld, E. B. Gralla, J. S. Valentine, V. C. Culotta, and B. M. Hoffman. 2010. Probing in vivo Mn2+ speciation and oxidative stress resistance in yeast cells with electron-nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 107:15335-15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moeller, R., P. Setlow, G. Horneck, T. Berger, G. Reitz, P. Rettberg, A. J. Doherty, R. Okayasu, and W. L. Nicholson. 2008. Roles of the major small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X-rays and high-energy charged-particle bombardment. J. Bacteriol. 190:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson, D. L., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination: phosphate metabolism during germination. J. Biol. Chem. 245:1146-1155. [PubMed] [Google Scholar]
- 43.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom.
- 44.Oh, Y. K., and E. Freese. 1976. Manganese requirement of phosphoglycerate phosphomutase and its consequences for growth and sporulation of Bacillus subtilis. J. Bacteriol. 127:739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palop, A., G. C. Rutherford, and R. E. Marquis. 1998. Inactivation of enzymes within spores of Bacillus megaterium ATC19213 by hydroperoxides. Can. J. Microbiol. 44:465-470. [PubMed] [Google Scholar]
- 48.Rosson, R. A., and K. H. Nealson. 1982. Manganese binding and oxidation by spores of a marine Bacillus. J. Bacteriol. 151:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotman, Y., and M. L. Fields. 1967. A modified reagent for dipicolinic acid analysis. Anal. Biochem. 22:168. [DOI] [PubMed] [Google Scholar]
- 50.Russell, A. D. 1982. The destruction of bacterial spores, p. 111-151. Academic Press, London, United Kingdom.
- 51.Setlow, B., S. Atluri, R. Kitchel, K. Koziol-Dube, and P. Setlow. 2006. Role of dipicolinic acid in the resistance and stability of spores of Bacillus subtilis. J. Bacteriol. 188:3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setlow, B., and P. Setlow. 1993. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl. Environ. Microbiol. 59:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Setlow, B., and P. Setlow. 1995. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl. Environ. Microbiol. 61:2787-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Setlow, B., and P. Setlow. 1998. Heat killing of Bacillus subtilis spores in water is not due to oxidative damage. Appl. Environ. Microbiol. 64:4109-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Setlow, P. 1988. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu. Rev. Microbiol. 42:19-38. [DOI] [PubMed] [Google Scholar]
- 57.Setlow, P. 1994. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 76:49S-60S. [DOI] [PubMed] [Google Scholar]
- 58.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 59.Shuryak, I., and D. J. Brenner. 2009. A model for interactions between radiation-induced oxidative stress, protein and DNA damage in Deinococcus radiodurans. J. Theor. Biol. 261:305-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sigel, A., and H. Sigel. 2000. Metal ions in biological systems. Marcel Dekker, New York, NY.
- 61.Singh, R. P., and P. Setlow. 1979. Purification and properties of phosphoglycerate phosphomutase from spores and cells of Bacillus megaterium. J. Bacteriol. 137:1024-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Slepecky, R., and J. W. Foster. 1959. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J. Bacteriol. 78:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sunde, E. P., P. Setlow, L. Hederstedt, and B. Halle. 2009. The physical state of water in bacterial spores. Proc. Natl. Acad. Sci. U. S. A. 106:19334-19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warth, A. D. 1980. Heat stability of Bacillus cereus enzymes within spores and in extracts. J. Bacteriol. 143:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wieghardt, K. 1989. The active sites in manganese-containing metalloproteins and inorganic model complexes. Angew. Chem. Int. Ed. Engl. 28:1153-1172. [Google Scholar]