Abstract
Objectives:
An unsuccessful attempt to reach the apical area or to place the retrograde material is a major difficulty in periradicular surgery. The aim of this study was to compare the histological evaluation of the healing process following an orthograde versus a retrograde application of mineral trioxide aggregate (MTA) as a root-end filling material during apical surgery on cats’ teeth in order to find out whether orthograde placement of MTA before surgery can be used instead of retrograde placement during surgery.
Methods:
In this experimental study, 24 canine teeth in 12 mature and healthy cats were filled with either MTA or gutta-percha in an orthograde manner. Two weeks later, the teeth with MTA were surgically exposed and resected to the set-MTA within the canals. The teeth previously filled by gutta-percha were also surgically exposed, and retrograde cavities were prepared at the root ends and filled with fresh-MTA. After 8 weeks, the animals were euthanized by vital perfusion. Six-micron histological slices were prepared from samples, stained by Hematoxylin & Eosin, and histologically studied by means of a light microscope. The collected data was analyzed by the Chi-square and the T-test.
Results:
One of the samples in the fresh-MTA group was omitted during processing because of inappropriate sectioning. In the set-MTA group, 5 out of 12 showed chronic abscess, while in the fresh-MTA group, 2 out of 11 were discovered to have chronic abscess; however, no significant difference was observed (P>.05). Hard tissue healing (cementum, bone, cementum + bone formation) in the set-MTA and fresh-MTA groups were 7 out of 12 and 9 out of 11, respectively. While healing seemed more likely to occur in the fresh-MTA group, the difference was statistically insignificant (P>.05). The magnitude of bone, cementum, or bone and cementum formation showed slight differences between the two groups; however, the figures failed to show any marked differences (P>.05).
Conclusions:
Orthograde placement of MTA could be used as an obturation material before surgery. In this way, after root-end resection, there would be no need for root-end preparation and filling procedures.
Keywords: Healing, Mineral Trioxide Aggregate, Root end filling
INTRODUCTION
Different types of materials have been used to fill the root-end cavity, including amalgam, gutta-percha, composite resins, glass ionomers, and zinc-oxide eugenol cements, like IRM and Super EBA. Recently, mineral trioxide aggregate (MTA) has been introduced to the market as the most favorable and biocompatible retrofilling material.1,2
Torabinejad et al3 found that dentoalveolar healing adjacent to the MTA root-end fillings results in regeneration of the periapical tissues, including apical cementogenesis. Moreover, Mitchell et al4 showed successful growth of osteoblast cells adjacent to MTA. Furthermore, Torabinejad et al5 investigated the tissue reaction to two types of implanted root-end filling materials, and MTA proved to be the more tissue friendly. The characteristics of apical cementogenesis adjacent to MTA have been studied in cats,6 dogs,3 and monkeys.7
Andelin et al8 compared the microleakage in resected fresh and set MTA in an in vitro study. The results showed no significant difference in dye leakage between the two groups. Based on these results, it seems that the resection of set MTA does not affect its sealing ability. Apaydin et al9 compared hard-tissue healing after the application of fresh and set MTA as a root-end filling material. The results indicated that although freshly placed MTA resulted in a higher incidence of cementum formation, there is no significant difference in the amount of cementum or osseous healing associated with freshly placed or set MTA.
The purpose of this study was to histologically compare the healing process after the orthograde application of MTA as set MTA to its retrograde application as fresh MTA in cats’ canine teeth.
MATERIALS AND METHODS
In this animal study, 24 fully developed mandibular and maxillary canines were randomly selected in 12 healthy cats with an average weight of 2–3 kg. These animals were kept under the supervision of a veterinarian and the Animal Protection Unit at the Dental Research Center of the Mashhad University of Medical Sciences. The proposal of this research was approved by the Ethical Committee of the Mashhad University of Medical Sciences.
All dental procedures were performed under general anesthesia, which was provided by an intra-muscular injection of 10 mg/kg of Ketamin HCl (woerden-Holland) and 1 mg/kg of Xylozine (Woerden-Holland).
The experimental teeth (n=24) were randomly divided into 2 groups of 12 teeth each (set MTA group and fresh MTA group). In the first session, occlusal access was gained to the pulp chambers of each tooth; then the pulp was extirpated and the root canal prepared with FlexoFiles (Dentsply Maillefer, Tulsa, USA). A uniform flare was achieved throughout the canals with the subsequent use of Gates-Glidden burs numbers 1, 2, and 3 for the coronal part and with a step-back technique for the apical part.
For the teeth in the first group (the set MTA group), the roots were entirely obturated with MTA (ProRoot, Dentsply Tulsa Dental, Tulsa, USA) by using a plugger. For the teeth in the second group (fresh MTA group), the roots were entirely filled with a lateral condensation of gutta-percha (Ariadent Co., Iran). The coronal portions of all teeth in both groups were sealed with amalgam (Sinalux Co., Iran).
In the second session, after two weeks a full thickness mucoperiosteal buccal flap with one releasing incision was reflected. This allowed access to the periradicular tissue in the mandibular and maxillary canine region. The cortical bone over the root ends was removed using a #6 round bur in a high-speed handpiece, using copious saline irrigation. The root ends in both groups were resected with fissure burs approximately 3 mm from the apex at an angle approximately 45 degrees to the long axis of the root. For the teeth in the set MTA group, nothing more was done, whereas for the teeth in the fresh MTA group, root-end preparations were also made at cut roots to the depth of 3 mm. The root-end cavities prepared in the aforementioned way were filled with MTA according to the manufacturer’s recommendation. After this stage, all mucoperiosteal flaps were replaced and sutured with 4-0 silk (Supa Co., Iran) sutures.
All animals were then injected with 24 ml of 10% Dextrose serum (Daroo-pakhsh Co., Iran) subcutaneously. This was done to prevent side effects from general anesthesia during recovery, such as mortality/morbidity due to lack of appetite and glucosuria. Furthermore, 20,000 Iu/kg of penicillin (6.3.3, Sobhan Co., Iran) was intramuscularly injected to prevent infection. Intramuscular injection of vitamin B-complex (Daroo-pakhsh Co., Iran) was also done, according to the vet’s recommendation in order to increase appetite of the cats.
The animals were euthanized 8 weeks after the second surgical procedure by perfusion with 10% buffered formalin. Mandibular and maxillary block sections containing the canine teeth and surrounding tissue were obtained. One of the fresh-MTA samples was destroyed during processing due to inappropriate sectioning and was omitted from the data.
These specimens were demineralized in 10% formic acid and then dehydrated in 70%, 80%, and 100% alcohol, subsequently. Once the specimens were embedded in paraffin, serial buccolingual sections of 6-μm thickness were cut through the center of the apical formation along the long axis of the teeth. Selected sections were stained with Hematoxylin & Eosin and were evaluated under a light microscope with 40x magnification (Olympus Co., Japan) by a pathologist without prior knowledge of the study.
The healing was assessed by the presence or absence of newly formed bone and/or cementum adjacent to the root-end filling. Digital photomicrographs were used to measure the cementum area on a scale of micrometers for a more accurate measurement of healing. The presence of inflammation was also considered as evidence of failure to heal. The data were analyzed with Chi-square and T-tests.
RESULTS
While inflammation was noted in 5 set-MTA (n=12) and in 2 fresh-MTA (n=11) samples, healing (formation of cementum and/or bone) was found in 7 set-MTA (n=12) and in 9 fresh-MTA (n=11) samples. However, the differences proved to be statistically insignificant (P>.05).
Among the samples that showed evidence of healing, cementum formation happened adjacent to the MTA in 4 set-MTA (n=12) and in 6 fresh-MTA (n=11) samples. The mean area of cementum formation was 158.7 μm2 and 308.3 μm2 in the set-MTA (n=12) and fresh-MTA (n=11) groups, respectively (Table 1). Bone formation was also detected in one sample in each group. Furthermore, 2 cases in the set-MTA and 2 in the fresh-MTA group showed evidence of both bone and cementum formation. Interestingly, all of the aforementioned differences between the two groups were found not to be statistically significant (P>.05)
Table 1.
Mean area of cementum formation in two study groups.
| N | Mean | SD | P-value | |
|---|---|---|---|---|
| Fresh MTA | 11 | 308.3 | 294.3 | 0.19 |
| Set MTA | 12 | 158.7 | 234.9 | 0.19 |
Figures 1 and 2 demonstrate the osseous and cementum formation adjacent to the set and fresh MTA.
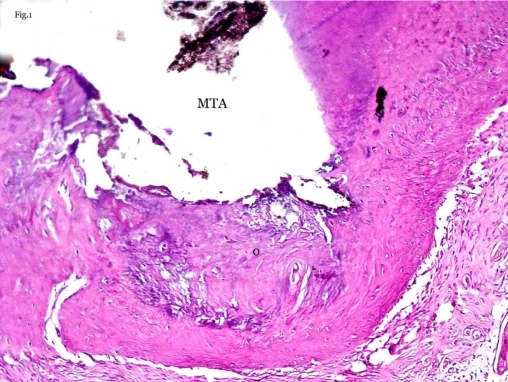
Figure 1.
Dentoalveolar healing in fresh-MTA group. Bone formation adjacent to MTA that contains lacunae with osteocytes (O) (40X magnification-Hematoxylin & Eosin staining).
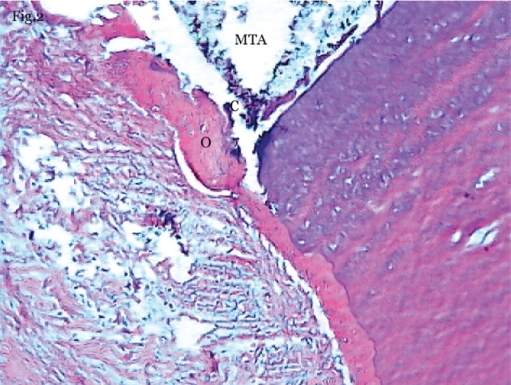
Figure 2.
Dentoalveolar healing in set-MTA group. Bone formation contains lacunae with osteocytes (O) and cellular cementum (C) formation adjacent to MTA (100X magnification-Hematoxylin & Eosin staining).
DISCUSSION
Periradicular surgery may be required if endodontic treatment fails. During this procedure, a part of the root which has not been completely cleared from debris and microorganisms is resected, followed by sealing of the resected section with a suitable root-end filling material. This kind of surgery is often performed in necrotic canals with persistent apical periodontitis where bacteria inside the rarefied apical area need to be cleaned by retropreparation procedures.
One of the best materials proposed for this purpose is MTA.10 The results of Torabinejad et al’s study showed that resecting the root-end section containing the set MTA does not significantly alter the sealing ability and the biocompatibility of MTA. Torabinejad et al11 showed that no difference exists in the zone of lysis between set and freshly mixed material. Researchers in another study showed that periradicular inflammation adjacent to root ends filled with MTA is minimal, whereas inflammation was observed at all root ends filled with amalgam. They concluded that MTA can be used as a suitable root-end filling material.12
Our results are in agreement with some other studies that have been carried out on set materials. Osorio et al,13 in 1998, tested set MTA on human gingival fibroblasts and found that it does not have cytotoxic effects. This finding supports the fact that a resected surface of set MTA does not seem to hinder hard tissue formation. Zhu et al14 also found in 1999 that the osteoblasts spread and made intimate contact with the set material. In another 1999 study, Mitchell et al15 found that set MTA was biocompatible when tested with a culture of human osteosarcoma cells.
The setting of MTA is one of the main factors that is vital for the healing process.16 Gancedo et al16 showed that the effect of curing time depends on humidity and that, in the absence of humidity, the push-out strength did not increase after 3 days. Buding et al17 also illustrated that moisture around the apical foramen where the MTA is placed is insufficient for optimum setting. Another study carried out by Walker et al18 showed that leaving a moistened cotton pallet in place for 24 hours can result in optimized flexural strength.
In a study on dogs’ premolar teeth, Apaydin et al9 discovered that hard tissue healing is similar in both set and fresh MTA. In contrast, Hatchmeister et al19 recently found that MTA apical plugs placed by the orthograde method in teeth with open apices exhibit somewhat more bacterial leakage than those placed with the retrograde method. This may be related to the packing technique used and the resultant density of MTA. Apaydin et al9 believe that this difference is due mainly to the extent of the compaction of materials in various studies.
The results of our study and Apaydin et al’s study9 showed that although no significant difference exists between the healing process for set and fresh MTA, fresh MTA may display better results. The data is depicted in Table 2. These findings suggest that further in vitro and in vivo studies are warranted to elucidate whether set MTA can perform precisely the same as fresh MTA. It is presumed that the setting of excessive MTA in root canals can be problematic, so an excessive amount of MTA in root canals should be avoided; also sufficient moisture is necessary in order to help the MTA set better.9
Table 2.
The comparison between the present study and the Apaydin et al’s9 study.
| Study | Type of animal | Sample size | Type of tooth | Mean area of cementum formation | |
|---|---|---|---|---|---|
| Set | Fresh | ||||
| Apaydin et al’s 9 study | Beagle dog | 24 | Mandibular premolar | 32.83 μm2 | 49.54 μm2 |
| Present study | Persian cat | 23 | Maxilla and mandibular canine | 158.7 μm2 | 308.3 μm2 |
One sample of fresh-MTA group was omitted during sample processing.
Taking into consideration the favorable healing process adjacent to set MTA, it can be stated that this material can potentially replace fresh MTA in apical surgery in the future. The advantages of this method would include less need for vasoconstrictor local anesthetics (which stop bleeding during surgery to make it easier to place retrofilling material), no root-end preparation, and no resultant microcracks, The indication might include patients with medical contraindication for vasoconstrictors and those cases in which surgical access for retropreparation and root-end filling is anticipated to be difficult and time consuming. However, a limitation is the root canals with intracanal posts, in which it is impossible to place the MTA in an orthograde manner before the surgical procedure.10
CONCLUSIONS
Orthograde placement of MTA could be used as an obturation material before surgery. In this way, after root-end resection, there would be no need for root-end preparation and filling procedures.
Acknowledgments
This study was supported in part by a grant from the Research Council of Mashhad University of the Medical Sciences. The authors would like to express sincere gratitude to the director of the Mashhad Dental Research Center. The grant number is 83119/03.09.2004, which was funded by the Office of Vice Chancellor for Research of the Mashhad University of Medical Sciences.
REFERENCES
- 1.Torabinejad M, Smith PW, Kettering JD, Pitt Ford TR. Bacterial leakage of mineral trioxide aggregate as a root-end filling material. J Endod. 1995;21:109–112. doi: 10.1016/s0099-2399(06)80433-4. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Pitt Ford TR, McKendry D, Abedi HR, Miller DA, Kariyawasam SP. Histological assessment of mineral trioxide aggregate as a root-end filling material in monkeys. J Endod. 1997;23:225–228. doi: 10.1016/S0099-2399(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root end filling in dogs. J Endod. 1995;21:603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate biomaterials. J Endod. 1999;20:167–173. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 5.Torabinejad M, Hong CU, Pitt Ford TR, Kaiyawasam SP. Tissue reaction to implanted super-EBA and mineral trioxide aggregate in the mandible of guinea pigs. J Endod. 1995;21:569–571. doi: 10.1016/s0099-2399(06)80987-8. [DOI] [PubMed] [Google Scholar]
- 6.Maguire H, Torabinejad M, McKendry D, McMillan P, Simon JH. Effects of resorbable membrane placement and human osteogenic protein-1 on hard tissue healing after periradicular surgery in cats. J Endod. 1998;24:720–725. doi: 10.1016/S0099-2399(98)80161-1. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995;21:489–492. doi: 10.1016/s0099-2399(06)80518-2. [DOI] [PubMed] [Google Scholar]
- 8.Andelin WE, Browning DF, Hsu GHR, Rolond DD, Torabinejad M. Microleakage of resected MTA. J Endod. 2002;28:573–574. doi: 10.1097/00004770-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Apaydin ES, Shabahang S, Torabinejad M. Hard tissue healing following fresh or set MTA as root - end filling material. J Endod. 2004;30:21–24. doi: 10.1097/00004770-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Torabinejad M, Chivian N, Rubinstein R. Practical lessons in endodontic surgery. Quintessence Co; Chicago: 1998. [Google Scholar]
- 11.Torabinejad M, Hong CU, Pitt Ford TR, Kettering JD. Cytotoxicity of four root end filling materials. J Endod. 1995;21:489–492. doi: 10.1016/s0099-2399(06)80518-2. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Pitt Ford TR, McKendry DJ, Abedi HR, Miller DA, Kariyawasam SP. Histologic assessment of Mineral Trioxide Aggregate as a root-end filling in monkeys. Int Endod J. 2009;42:408–411. doi: 10.1111/j.1365-2591.2009.01556.x. [DOI] [PubMed] [Google Scholar]
- 13.Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod. 1998;24:91–96. doi: 10.1016/S0099-2399(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Q, Haglund R, Safavi KE, Spångberg LSW. Adhesion of human osteoblasts on root end filling materials. J Endod. 2000;26:404–406. doi: 10.1097/00004770-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell PJ, Pitt Ford TR, Torabinejad M, McDonald F. Osteoblast biocompatibility of mineral trioxide aggregate. Biomaterials. 1999;20:167–173. doi: 10.1016/s0142-9612(98)00157-4. [DOI] [PubMed] [Google Scholar]
- 16.Gancedo-Caravia L, Garcia-Barbero E. Influence of humidity and setting time on the push-out strength of mineral trioxide aggregate obturations. J Endod. 2006;32:894–896. doi: 10.1016/j.joen.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Budig CG, Eleazer PD. In vitro comparison of the setting of dry ProRoot MTA by moisture absorbed through the root. J Endod. 2008;34:712–714. doi: 10.1016/j.joen.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Walker MP, Diliberto A, Lee C. Effect of setting conditions on mineral trioxide aggregate flexural strength. J Endod. 2006;32:334–336. doi: 10.1016/j.joen.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Hachmeister DR, Schindler WG, Walker WA, 3rd, Thomas DD. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28:386–390. doi: 10.1097/00004770-200205000-00010. [DOI] [PubMed] [Google Scholar]