Abstract
Although extracellular signal-regulated kinase (ERK) activity is essential for the acquisition of a variety of associative learning tasks, its involvement in the acquisition and extinction of ethanol (EtOH)-induced conditioned place preference (CPP) remains unknown. Therefore, in these experiments we examined the effects of the ERK-kinase (MEK)-inhibitor SL327 on acquisition and expression of EtOH-CPP as well as the dose- and time-dependent effects of SL327 on CPP extinction. The parametric findings of Experiment 1 showed that three 30-min (but not 15- or 5-min) non-reinforced trials were required to completely extinguish EtOH-CPP in male, DBA/2J mice. In Experiments 2 & 3, SL327 (30 and 50 mg/kg), administered 30 or 90 min prior to extinction trials, was unable to impair EtOH-CPP extinction. Experiment 4 showed that SL327 (50 mg/kg) had no effect on acquisition of EtOH-CPP or the development of EtOH-induced sensitization during conditioning. When administered prior to testing in Experiments 5 & 6, SL327 did not alter expression of EtOH-CPP but did reduce test activity. Importantly, SL327 significantly reduced pERK protein levels when assessed in the dorsal striatum and motor cortex (Experiment 7). Together, these data suggest that EtOH-related learning and EtOH reward in mice, as assessed with CPP, are not impaired by the systemically administered MEK-inhibitor SL327.
Keywords: extinction, ERK, SL327, conditioned place preference, ethanol, DBA/2J
1. Introduction
Reinstatement procedures such as contextual renewal and spontaneous recovery have revealed that extinction of associative learning, like acquisition, requires the formation of new memories (Bouton, 2004). Furthermore, these procedures show that extinction is unique in that although it inhibits responding, the original association remains intact. As such, the processes underlying initial acquisition and subsequent extinction involve both shared, and unique, neurobiological substrates. For example, NMDA-receptor activation is necessary for both acquisition (Miserendino et al., 1990) and extinction (Falls et al., 1992), but D-cycloserine (DCS), a partial agonist of the NMDA-receptor, only facilitates extinction of conditioned fear (Davis et al., 2006). Interestingly, the extinction-facilitating effects of DCS are dependent on intracellular signaling through the mitogen-activated protein kinase (MAPK)-pathway (Yang and Lu, 2005). Signaling via one such MAPK, the extracellular signal-regulated kinase (ERK), requires phosphorylation of ERK1/2 via MAPK-kinase (MEK), in order to successfully transmit a range of extracellular signals, including those involved in learning and memory (Adams and Sweatt, 2002). Accordingly, MEK inhibitors block acquisition learning in a number of behavioral tasks, including the water maze (Selcher et al., 1999), cocaine conditioned place preference (Valjent et al., 2000) and cue- and context-conditioned fear (Atkins et al., 1998). Similarly, the extinction of conditioned fear is blocked by direct administration of MEK inhibitors into the amygdala, medial prefrontal cortex, or hippocampus (Herry et al., 2006; Hugues et al., 2004; Szapiro et al., 2003, respectively). However, few studies have examined the involvement of ERK-signaling in extinction of appetitive associative behaviors, such as conditioned place preference.
Conditioned place preference (CPP) is an animal model of drug-seeking behavior that allows for direct manipulations of both acquisition and extinction learning as well as the rewarding properties of drugs of abuse. Understanding the differences between acquisition and extinction of CPP could help identify novel targets for pharmacotherapies that could facilitate the rehabilitation process and reduce the rate of relapse. Thus far, few studies have examined the involvement of ERK-signaling in drug-induced CPP. Inhibition of ERK-signaling with SL327, the only systemically administered MEK inhibitor, impairs acquisition of cocaine-, Δ9-tetrahydrocannabinol (THC)-, and 3,4-methylenedioxymethamphetamine (MDMA)-induced CPP in mice (Valjent et al., 2000, 2001; Salzmann et al., 2003). The effect of MEK-inhibition on extinction of CPP has received little attention. Although one study has reported that injection of SL327 before a single extinction session impaired partial extinction of cocaine-CPP (Valjent et al., 2006), it remains unclear how experimental parameters including route of administration, dose, and pre-treatment interval influence the effect of different MEK inhibitors on extinction learning and whether extinction of fear and CPP are differentially dependent on ERK activity. Furthermore, the involvement of the ERK pathway in the acquisition, expression, and extinction of alcohol-seeking behaviors in mice remains undetermined. Therefore, we performed a series of experiments to examine the dose- and time-dependent effects of SL327 on the extinction of ethanol (EtOH)-induced CPP in mice. Additionally, we examined the effects of SL327 on acquisition and expression of EtOH-induced CPP. Finally, we used western immunoblot analysis of phosphorylated ERK (pERK) levels in multiple brain regions to confirm that SL327 had crossed the blood-brain-barrier and was actively inhibiting ERK signaling. These findings further characterize both the shared and unique biochemical substrates that underlie the acquisition and extinction of drug- and EtOH-induced associative learning and provide insight to the biochemical substrates of EtOH reward in mice.
2. Materials and Methods
2.1 Subjects
Adult, male DBA/2J mice (n=432) were obtained from Jackson Laboratory (Sacramento, CA, USA) at 6 weeks of age and allowed to acclimate to the animal colony for 2 weeks before experiments commenced. Mice were housed, four to a cage, in cob bedding in a Thoren rack with water and food available ad libitum throughout each experiment. All experiments were conducted during the light phase (7:00–19:00). The Oregon Health & Science University IACUC approved all experimental procedures.
2.2 Drugs
Ethanol (20% v/v in isotonic saline) was administered at a dose of 2 g/kg (12.5 ml/kg) in Experiments 1–6 and 2.5 g/kg (16 ml/kg) in Experiment 7. The MEK inhibitor SL327 (Ascent Scientific, Princeton, NJ, USA) was first dissolved in 100% DMSO then diluted with dH2O, prepared fresh daily. For Experiment 2, SL327 was prepared in 15% DMSO to concentrations of 1.5 and 2.5 mg/ml and administered at 20 ml/kg to achieve doses of 30 and 50 mg/kg, respectively. This drug preparation was identical to that used by Faccidomo et al. (2009). In Experiments 3–7, SL327 was prepared in 50% DMSO to concentrations of 6 and 10 mg/ml and administered at 5 ml/kg to achieve the same doses. This drug preparation was identical to that used by Mouledous et al. (2007) and Matsuda et al. (2010). Matched vehicles were administered at identical injection volumes and pre-treatment intervals for each experiment. All drug and vehicle injections were administered intraperitoneally (IP).
2.3 Place Preference Apparatus
All behavioral procedures were performed in custom made, acrylic and aluminum conditioning boxes (30 × 15 × 15 cm), each of which was enclosed in a sound-attenuating chamber (Model E10–20, Colbourn Instruments, Allentown, PA, USA). A set of six infrared emitters and detectors, mounted 5 cm apart and 2.2 cm above the floor of the box, were used to obtain spatial location and locomotor activity data throughout conditioning, extinction and testing. The conditioned stimuli (CSs) consisted of two distinct tactile cues—grid and hole floors. Grid floors (2.3 mm stainless steel rods, 6.4 mm apart) and hole floors (16-gauge stainless steel perforated with 6.4-mm round holes) were interchangeable allowing for either full- or split-cue configurations during conditioning/extinction and testing, respectively. These cues are unbiased in that naïve DBA/2J mice show equal preference for the two floors during drug-free preference tests (Cunningham et al., 2003).
2.4 Place Preference Procedure
All CPP experiments consisted of unbiased designs and procedures similar to those previously described in detail by this laboratory (Cunningham et al., 2006b).
Experiment 1: Effect of trial duration on extinction of EtOH-CPP
On Day 1, mice (n=96) were given a saline injection (12.5 ml/kg) and habituated to the conditioning apparatus, equipped with white paper flooring, for 5 min. On Days 2–5, animals received daily CPP conditioning trials during which EtOH (2 g/kg) was paired with one of the tactile cues (e.g., Grid) while saline was paired with the other cue (e.g., Hole) on alternating days. The floor with which EtOH was paired (Conditioning Subgroup) and trial-type order (S-E-S-E or E-S-E-S) were fully counterbalanced. On Day 6, all animals received a drug-free, 15-min preference test during which both tactile cues were presented and place preference was assessed. Animals were matched for preference then divided into 4 groups that differed in extinction-trial duration: No Extinction, Ext-5 min, Ext-15 min, and Ext-30 min. During the 3 days of extinction (Days 7–9) the Ext-5 min, Ext-15 min, and Ext-30 min groups received three, non-reinforced exposures to each of the CS+ and CS− cues separately (each preceded by a saline injection) for their assigned durations. Trials occurred in the morning and afternoon of each day with order of cue exposure counterbalanced. The No Extinction group was weighed daily during this phase. On Day 10, all animals received a second, drug-free 15-min preference test.
Experiments 2 and 3: Effect of SL327 on extinction of EtOH-CPP
Animals (n=96 for each experiment) received EtOH-CPP conditioning and testing identical to that in Experiment 1 (see above). After Test 1, mice were matched for preference and assigned to one of four groups: No Extinction, Vehicle, SL-30, and SL-50. During the 4 days of extinction (Days 7–10), the Vehicle, SL-30, and SL-50 groups received four, 30-min, non-reinforced exposures to the CS+ and CS− cues separately. Animals received Vehicle (15% DMSO), 30 mg/kg SL327 (SL-30), or 50 mg/kg SL327 (SL-50) 30 min before each CS+ trial; saline (5 ml/kg) was injected 30 min before each CS− extinction trial. All mice received a saline injection (12.5 ml/kg) immediately before each CS+ and CS− trial. In order to eliminate any possible carry-over effects of SL327, cue exposure order was not counterbalanced during extinction (i.e., CS+ trials occurred in the afternoon). On Day 11 all animals received a second, drug-free 15-min preference test. Experiment 3 was identical to Experiment 2 except that SL327 was administered 90 min prior to the non-reinforced CS+ extinction trials and it was administered in a 50% DMSO vehicle. The pre-trial interval was increased in Experiment 3 in order to reduce the potential for any aversive effects of SL327 to enter into association with the CS+ cue during extinction (see Figure 2). The vehicle was changed in order to better reflect the administration parameters of other previously published studies (e.g. Mouledous et al., 2007).
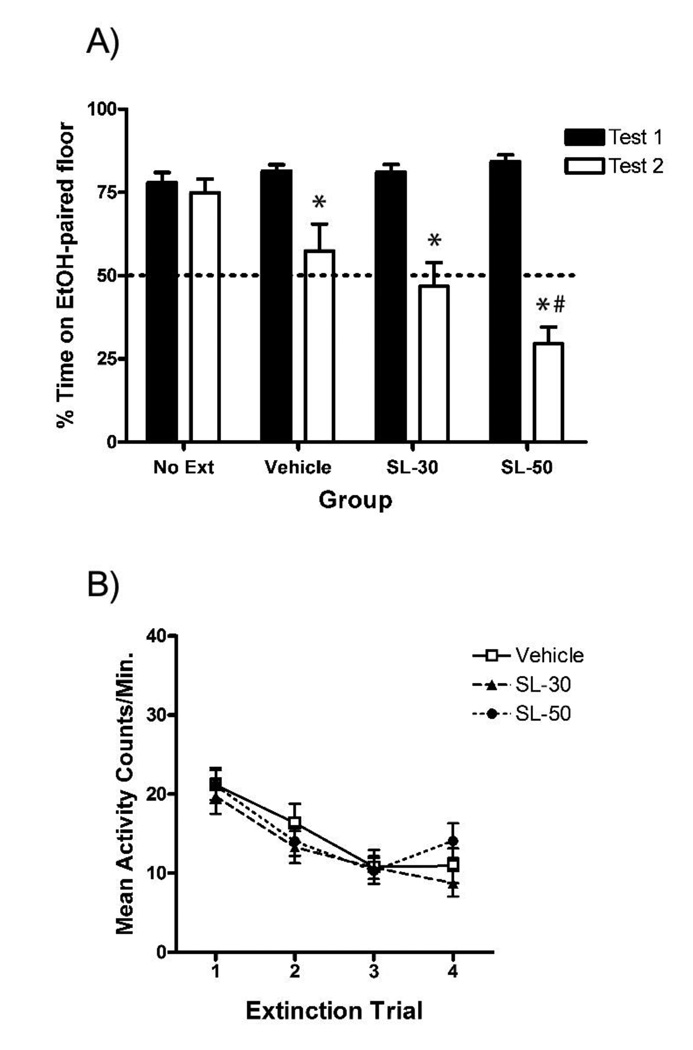
Figure 2. The systemic MEK inhibitor SL327 was unable to impair extinction of EtOH-CPP.
(A) All groups showed significant preference for the EtOH-paired floor following acquisition (Test 1). Administration of 30 mg/kg SL327 (SL-30) or 50 mg/kg SL327 (SL-50) 30-min before extinction trials did not impair normal extinction as seen in the Vehicle group (Test 2). The SL-50 group showed a significant place aversion following extinction. (B) All groups showed a decrease in activity over the course of extinction trials in Experiment 2. Pre-treatment with SL327 did not significantly reduce extinction activity as compared to the Vehicle group. Error bars indicate standard error of the mean. A total of 24 subjects (25%) were removed because of failure to express >50% preference on Test 1. An additional two subjects were removed because of procedural errors.
* denotes a significant decrease in preference on Test 2 compared to Test 1 (p < .05).
# denotes a significant difference in preference from Vehicle group on Test 2 (p < .05).
Experiment 4: Effect of SL327 on acquisition of EtOH-CPP
Mice (n=48) were randomly assigned to one of two groups: Vehicle or SL-50. All animals received EtOH-CPP conditioning identical to that in the previous experiments with the exception that each group received either Vehicle (50% DMSO) or SL327 (50 mg/kg), 90 min before each of the two EtOH (CS+) conditioning trials. On CS− trials, all animals received pre-injections of saline 90 min before another injection of saline. On Days 6–11, both groups received daily 30-min, drug-free preference tests with only a saline pre-injection.
Experiments 5 & 6: Effect of SL327 on expression of EtOH-CPP
In Experiment 5, mice (n=48) received conditioning identical to that in previous experiments in that they received 2 CS+ and 2 CS− trials in an alternating manner over the course of 4 days. Following conditioning, mice received a 5 ml/kg injection of Vehicle (50% DMSO) or SL327 (50 mg/kg), 90 min prior to a saline injection (12.5 ml/kg) followed immediately by a standard, 30-min place preference test. Experiment 6 used mice (n=48) from an unrelated experiment in which half of the mice had initially been trained using a one-compartment procedure (similar to that used in Experiments 1–5) or a two-compartment procedure (see Cunningham et al., 2006b). Consistent with previous findings (Cunningham et al., 2006c), preliminary statistical analysis showed no difference in the CPP produced by these two training procedures. Thus, this factor was omitted in the analyses reported here. Mice received a total of 4 CS+ and 4 CS− conditioning trials, with 30-min preference tests (after saline pre-injection) on the day after the 4th and 8th days of conditioning. The final expression test was given one day later, preceded by either a Vehicle or SL327 (50 mg/kg) injection 90 min before the saline pre-injection.
2.5 Western Blot Procedure
Experiment 7: Effect of SL327 on pERK levels in the dorsal striatum and motor cortex
Animals from the No Extinction group of Experiment 3 (n=24) were randomly assigned to one of four groups: Vehicle-Saline, Vehicle-EtOH, SL-Saline, SL-EtOH. Each group received a pre-injection of Vehicle (50% DMSO) or SL327 (50 mg/kg) 90 min before an injection of Saline or EtOH (2.5 g/kg). Five min later, animals were euthanized by CO2 asphyxiation, brains were extracted, and dorsal striatum and motor cortex were dissected from a 1 mm-thick brain slice corresponding to Bregma +0.86mm. Wet weights were taken and tissue was rapidly frozen in dry ice and stored at −80°C. Tissue samples were prepared by sonication in 20x w/v ice cold buffer containing 50 mM Tris HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 µl/ml phosphatase inhibitor cocktail and 10 µl/ml protease inhibitor cocktail (Calbiochem, San Diego, CA, USA). Samples were then incubated for 20 min at 4°C, followed by centrifugation at 14,000g for 15 min at 4°C. The supernatant was collected and stored at −80°C. Protein content was analyzed by BCA detection (Thermo Fisher Scientific, Rockford, IL, USA) and samples were incubated in Laemmli’s Sample Loading buffer for 45 min at 37°C. Following incubation, 20 µg of protein was loaded onto 10% SDS-PAGE gels and run for 2 hr at 100 V in a running buffer containing 25mM Tris base, 20 mM glycine and 0.1% SDS. Proteins were then transferred to PVDF membrane at 30 V overnight at 4° C in a transfer buffer containing 50 mM Tris base, 40 mM glycine, and 20% methanol. Membranes were then blocked for 1 hr at room temperature in Tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA) followed by a 1 hr incubation at room temperature with anti-pERK 44/42 or anti-ERK antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA) in TBS containing 5% BSA. Membranes were washed three times for 10 min in TBS and incubated for 45 min at room temperature with anti-rabbit IgG-AP secondary (1:2500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TBS containing 5% BSA. Finally, membranes were washed three times for 10 min in TBS and incubated for 5 min with ECF substrate (GE Healthcare, Piscataway, NJ, USA). Membranes were scanned using an Ultralum imaging system and bands were quantified using Ultraquant 6.0 software (Ultralum, Claremont, CA, USA).
2.6 Statistical Analysis
Place preference data were presented and analyzed using one-way analysis of variance (ANOVA) of the percentage of each test spent on the EtOH-paired floor (% Time on EtOH-paired floor). When extinction of EtOH-CPP was assessed, Test was included as a factor in a repeated-measures ANOVA. Additionally, Table 1 includes the raw-score means and statistical comparisons between the counterbalanced Conditioning Subgroups (G+ and G−) within each group (Cunningham et al., 2003). Post-hoc comparisons of G+ and G− subgroups were Bonferroni-corrected (overall α=.05).
Table 1.
Preference Test Data including Conditioning Subgroup (expressed as Time on Grid Floor)
| Exp. | Group | Conditioning Subgroup |
n | First Test: Time on Grid Floor (sec/min) |
Post hoc tests |
Final Test: Time on Grid Floor (sec/min) |
Post hoc tests |
|---|---|---|---|---|---|---|---|
| 1 | No Ext | G+ | 9 | 44.1 ± 2.4 | ] * | 42.7 ± 1.9 | ] * |
| G− | 9 | 17.9 ± 2.8 | 18.4 ± 2.0 | ||||
| Ext-5 min | G+ | 8 | 48.5 ± 1.9 | ] * | 47.4 ± 2.9 | ] * | |
| G− | 10 | 16.4 ± 2.3 | 16.8 ± 4.2 | ||||
| Ext-15 min | G+ | 9 | 44.1 ± 1.8 | ] * | 40.1 ± 6.1 | ] * | |
| G− | 9 | 15.5 ± 1.7 | 17.9 ± 4.4 | ||||
| Ext-30 min | G+ | 9 | 45.6 ± 1.9 | ] * | 29.8 ± 5.6 | ||
| G− | 8 | 17.1 ± 2.4 | 28.9 ± 6.9 | ||||
| 2 | No Ext | G+ | 10 | 50.3 ± 2.3 | ] * | 45.8 ± 3.3 | ] * |
| G− | 10 | 16.8 ± 2.5 | 15.9 ± 3.8 | ||||
| Vehicle | G+ | 9 | 49.0 ± 1.3 | ] * | 36.0 ± 5.7 | ||
| G− | 7 | 11.3 ± 2.1 | 27.5 ± 8.7 | ||||
| SL-30 | G+ | 9 | 49.3 ± 2.0 | ] * | 15.7 ± 5.1 | ||
| G− | 10 | 11.9 ± 1.9 | 20.8 ± 4.5 | ||||
| SL-50 | G+ | 8 | 51.7 ± 1.9 | ] * | 20.1 ± 4.2 | ] # | |
| G− | 8 | 10.6 ± 1.4 | 44.6 ± 4.4 | ||||
| 3 | No Ext | G+ | 10 | 52.0 ± 2.1 | ] * | 51.4 ± 1.6 | ] * |
| G− | 8 | 13.3 ± 3.1 | 11.7 ± 2.2 | ||||
| Vehicle | G+ | 10 | 47.9 ± 2.3 | ] * | 27.8 ± 7.5 | ||
| G− | 7 | 9.4 ± 1.7 | 12.9 ± 7.0 | ||||
| SL-30 | G+ | 10 | 48.7 ± 2.0 | ] * | 25.7 ± 7.1 | ||
| G− | 6 | 11.7 ± 2.2 | 37.8 ± 8.2 | ||||
| SL-50 | G+ | 11 | 48.9 ± 2.1 | ] * | 37.7 ± 6.2 | ||
| G− | 9 | 10.8 ± 2.3 | 29.7 ± 8.6 | ||||
| 4 | Vehicle | G+ | 12 | 46.4 ± 3.0 | ] * | 40.6 ± 2.7 | ] * |
| G− | 12 | 16.8 ± 3.8 | 18.8 ± 4.2 | ||||
| SL-50 | G+ | 12 | 48.9 ± 3.0 | ] * | 37.3 ± 3.7 | ] * | |
| G− | 12 | 23.1 ± 4.6 | 22.0 ± 4.9 | ||||
| 5 | Vehicle | G+ | 12 | 34.6 ± 3.5 | ] * | na | |
| G− | 12 | 20.3 ± 2.0 | na | ||||
| SL-50 | G+ | 12 | 45.4 ± 4.5 | ] * | na | ||
| G− | 11 | 21.2 ± 6.6 | na | ||||
| 6 | Vehicle | G+ | 12 | 41.4 ± 2.1 | ] * | na | |
| G− | 12 | 16.4 ± 1.6 | na | ||||
| SL-50 | G+ | 12 | 45.5 ± 4.1 | ] * | na | ||
| G− | 12 | 19.1 ± 4.4 | na |
Significant place preference (*) and aversion (#) as determined by Bonferroni-corrected post-hoc comparisons of G+ and G− subgroups (p < .05).
Conditioning and extinction locomotor activity was analyzed using repeated-measures ANOVAs with Trial and Group as factors. Ethanol-induced sensitization that occurred during conditioning of EtOH-CPP was analyzed using repeated-measures ANOVA with Trial, Trial-Type, and Group as factors. Post-hoc within- and between-subject comparisons were Bonferroni-corrected (overall α=.05).
Because the goal of Experiments 1–3 was to specifically manipulate extinction of an acquired EtOH-CPP, we decided, a priori, to remove animals that failed to express a place preference of greater than 50% on Test 1. These experiments revealed that approximately 25% of all subjects failed to express significant preference following the 2-trial conditioning procedure outlined above (total number of subjects removed from each experiment is reported in the figure legends). Removal of “non-learners” from analyses in order to examine the effects of extinction-specific manipulations has previously been reported (e.g. Bouton et al., 2008; Weber et al., 2007).
For western blot analysis, pERK immunoreactivity was first normalized to ERK levels and presented and analyzed after being normalized to the Vehicle-Saline group for each gel. Separate two-way ANOVAs, with Pre-injection (Vehicle or SL327) and Injection (Saline or EtOH) as factors, were initially used for dorsal striatum and motor cortex analyses. However, following initial analysis, injection groups were collapsed, normalized to the Vehicle group, and the effect of SL327 was further analyzed by independent t-tests comparing the Pre-injection groups for each brain region.
3. Results
3.1 Effect of trial duration on extinction of EtOH-CPP (Exp 1)
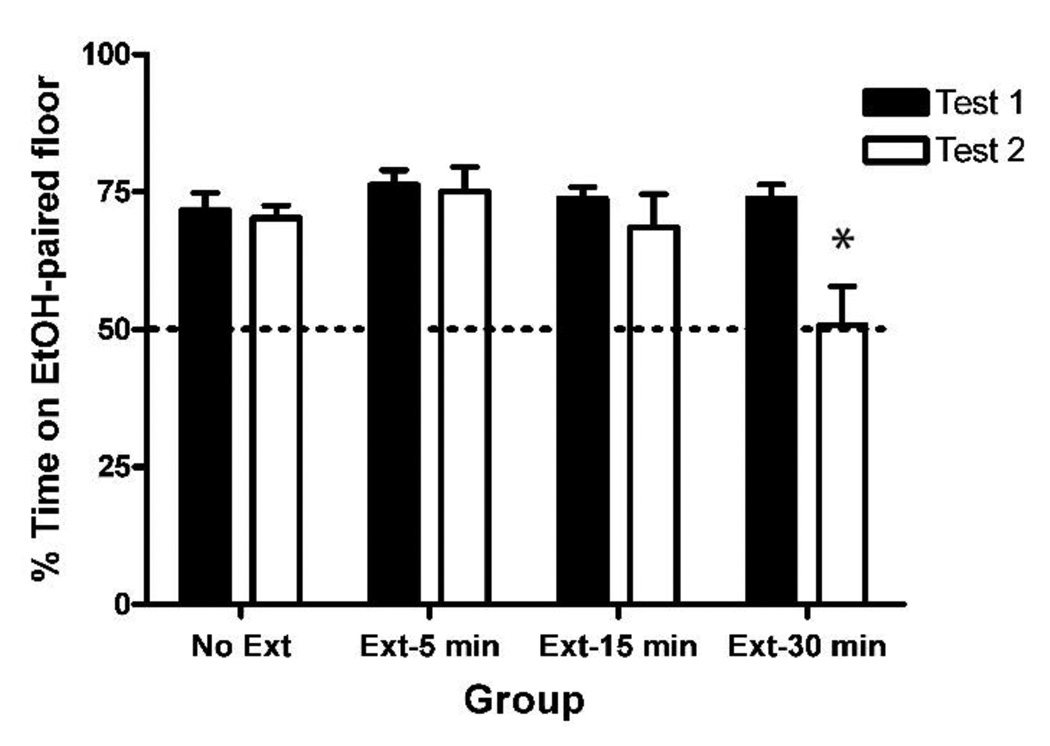
Experiment 1 was performed in order to determine the optimal extinction parameters in our procedure by varying extinction-trial durations. Figure 1 shows that on Test 1 following conditioning, all four groups showed significant preference for the EtOH-paired floor. However, after extinction (Test 2) only the group that had received the 30 min trial duration (Ext-30 min) showed significant extinction of EtOH-CPP. A two-way ANOVA revealed a significant Test × Group interaction [F(3,67) = 4.8, p < .01] and subsequent paired t-tests for each group revealed that only the Ext-30 min group showed significant extinction [t(16) = 4.0 p < .005]. As Table 1 shows, including the Conditioning Subgroups (G+ and G−) in the analysis further confirmed these findings. Therefore, Experiment 1 revealed that a trial duration of 30 min is necessary to completely extinguish EtOH-CPP. As such, all subsequent extinction experiments utilized a 30-min trial duration.
Figure 1. Extinction of EtOH-CPP was determined by trial duration.
All groups showed significant preference for the EtOH-paired floor following acquisition (Test 1). Following extinction, however, the group that received a 30-min (but not 15- or 5-min) extinction trial duration showed complete extinction of EtOH-CPP (Test 2). Error bars indicate standard error of the mean. A total of 23 subjects (24%) were removed because of failure to express >50% preference on Test 1. An additional two subjects were removed because of procedural errors.
* denotes a significant decrease in preference on Test 2 compared to Test 1 (p < .05).
3.2 Effect of systemic administration of the MEK inhibitor, SL327, on extinction of EtOH-CPP (Exps 2 & 3)
In order to determine the involvement of ERK activity in extinction of EtOH-CPP, the MEK inhibitor SL327 was administered prior to the 30-min, non-reinforced CS+ cue exposures during each extinction trial. As Figure 2a shows, SL327 did not impair extinction of EtOH-CPP—that is, all groups that underwent extinction showed a significant decrease in preference on Test 2. These findings were supported by a significant Test × Group interaction [F(3,67) = 12.4, p < .001] and significant main effects of Test [F(1,67) = 88.4, p < .001] and Group [F(3,67) = 5.5, p < .005]. Paired t-tests comparing preference on Tests 1 and 2 revealed significant extinction in all but the No Extinction group (p’s < .05). Further analysis revealed a simple main effect of Group only on Test 2 [F(3,67) = 16.5, p < .001]. Post-hoc comparisons of Test 2 preferences showed that the SL-50, but not SL-30, group differed significantly from the Vehicle group (p < .05). Therefore, the highest dose of SL327 appeared to be aversive and, when administered 30 min before cue exposure, resulted in a decrease in preference below the indifference point (50% preference) on Test 2. This finding suggested that a dose of 50 mg/kg SL327 possessed aversive properties that may have actually counter-conditioned the initial EtOH-CPP, resulting in a significant avoidance of, not just an indifference for, the previously EtOH-paired cue.
As Figure 2b shows, CS+-trial locomotor activity decreased over the course of extinction (significant main effect of Trial [F(3,144) = 34.5, p < .001]), but did not differ between drug treatment groups (no significant Group × Trial interaction, p > .05).
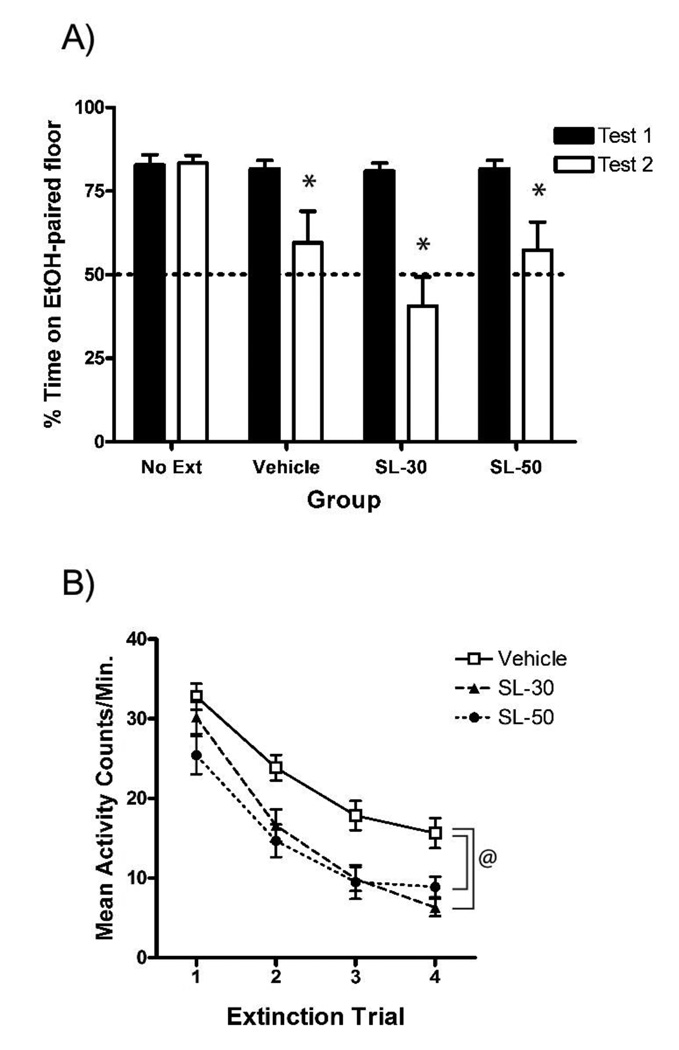
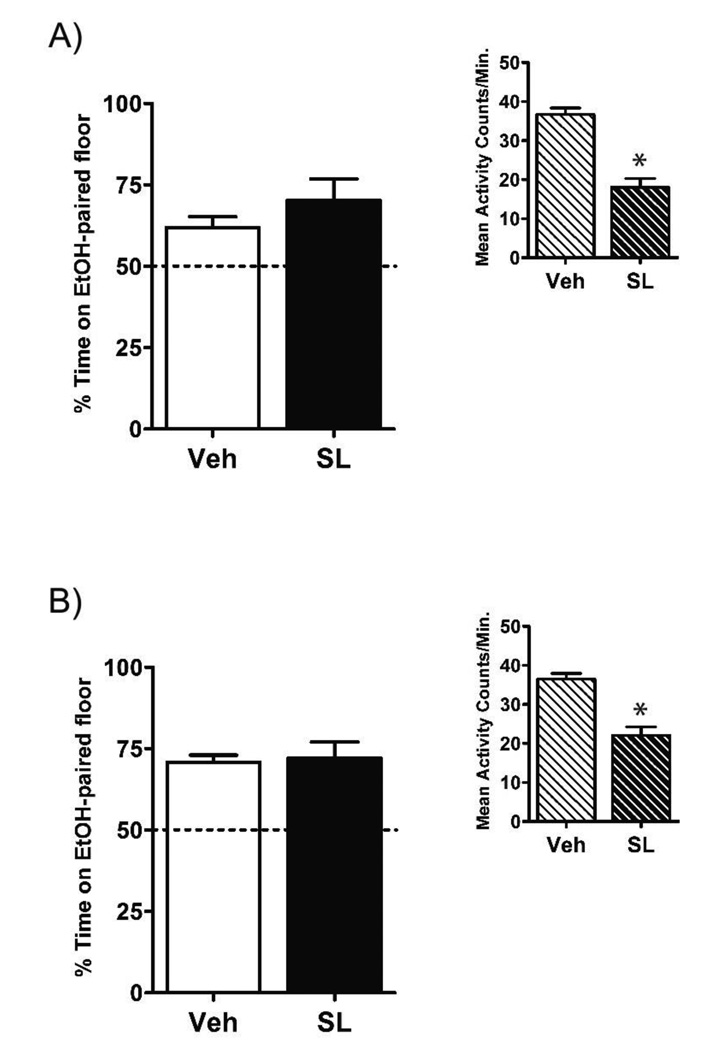
Because the SL-50 group in Experiment 2 developed a place aversion during extinction, the parameters of SL327 administration in Experiment 3 were changed in hopes of eliminating this effect. By extending the pre-trial interval to 90 min, we hoped to reduce the aversive properties of SL327 and/or weaken the ability of any aversive properties to enter into an association with the CS+ cue during extinction (i.e., prevent counter-conditioning a place aversion). The vehicle was also changed to 50% DMSO in order to better reflect the SL327 administration parameters used in previous experiments (e.g. Mouledous et al., 2007). As seen in Figure 3a, the results showed that although these manipulations eliminated the place aversion seen in Experiment 2, neither dose of SL327 interfered with normal extinction of EtOH-CPP. Analysis revealed a significant Test × Group interaction [F(3,67) = 4.6, p < .01] as well as main effects of both Test [F(1,67) = 31.0, p < .001] and Group [F(3,67) = 4.4, p < .01]. Paired t-tests comparing preference on Tests 1 and 2 revealed significant extinction in all but the No Extinction group (p’s < .05). Further analysis revealed a simple main effect of Group only on Test 2 [F(3,67) = 8.9, p < .001]. Subsequent post-hoc comparisons of Test 2 preferences revealed that neither the Ext-30 nor Ext-50 group differed from the Vehicle group (p’s > .05). Therefore, when administered at 30- or 90-min pre-trial intervals, neither dose of SL327 impaired extinction of EtOH-CPP in mice.
Figure 3. SL327, administered 90-min prior to trials, did not impair extinction of EtOH-CPP.
(A) All groups showed significant preference for the EtOH-paired floor following acquisition (Test 1). Administration of SL327 (30 or 50 mg/kg) did not impair the normal extinction apparent in the Vehicle group (Test 2). (B) Extending the pre-treatment interval to 90 min unmasked a general activity-suppressing effect of both doses of SL327 as compared to the Vehicle group. Error bars indicate standard error of the mean. A total of 22 subjects (23%) were removed because of failure to express >50% preference on Test 1. An additional three subjects were removed because of procedural errors.
* denotes a significant decrease in preference on Test 2 compared to Test 1 (p < .05).
@ denotes a significant difference in extinction activity as compared to the Vehicle group (p < .05).
In contrast to Experiment 2, however, both doses of SL327 reduced locomotor activity during extinction trials when compared to vehicle-treated animals (Figure 3b). A one-way ANOVA revealed significant main effects of Group [F(2,50) = 8.3, p < .005] and Trial [F(3,150) = 127.8, p < .001]. Subsequent post-hoc analysis showed that the Vehicle group exhibited higher locomotor activity than both the SL327 groups (p’s < .01). These data suggest that although CS+-trial locomotor activity decreased over the course of extinction, both doses of SL327 caused a general reduction in activity.
The combined results of Experiments 2 and 3 showed that inhibition of ERK-signaling with the systemic MEK inhibitor, SL327, did not impair extinction learning. This effect was evident at two doses that were administered in two vehicles, at two pre-trial intervals. However, despite failing to prevent extinction, these doses of SL327 did reduce locomotor activity when administered 90 min before the non-reinforced extinction trials.
3.3 Effect of SL327 on acquisition of EtOH-CPP (Exp 4)
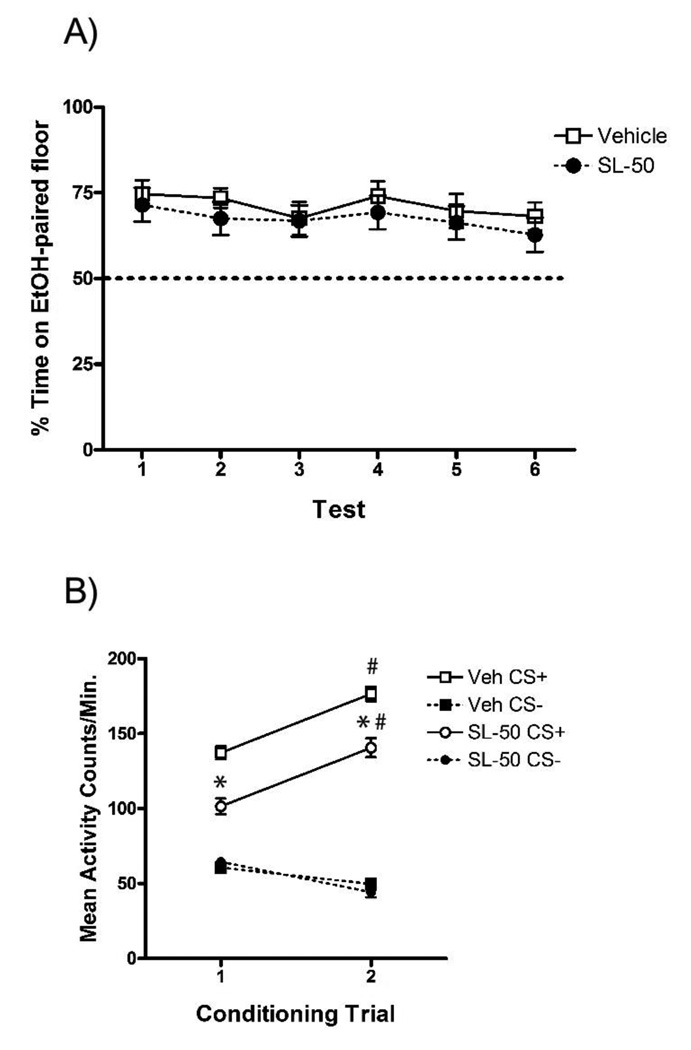
Although SL327 did not prevent extinction of EtOH-CPP, previously published experiments have reported that SL327 impairs acquisition of CPP (e.g., Valjent et al., 2000). Thus, Experiment 4 was performed to extend these previous findings by examining the effects of SL327 on acquisition of EtOH-CPP in mice. The results of Experiment 4 showed that SL327 (50 mg/kg) administered 90 min prior to CS+ conditioning trials did not impair acquisition of EtOH-CPP (Figure 4, Test 1). In order to assess the persistence of CPP, both groups received five additional drug-free preference tests. Analysis of the preference across all six tests revealed no group differences in EtOH-CPP. This was confirmed by the absence of either a Test × Group interaction or main effect of Group (p’s > .05). These data showed that SL327 was unable to impair either EtOH reward or the memory formation necessary for acquisition of EtOH-CPP in mice. Furthermore, this finding persisted across multiple, drug-free preference tests.
Figure 4. SL327 had no effect on acquisition of EtOH-CPP.
(A) SL327 (50 mg/kg) did not prevent the development of EtOH-CPP as both groups showed significant preference for the EtOH-paired floor following acquisition (Test 1). Significant EtOH-CPP of both the Vehicle and SL-50 groups persisted across five subsequent tests for both groups. (B) When administered during CPP acquisition, SL327 (50 mg/kg) caused a general reduction in EtOH-induced activity. However, SL327 did not impair the development of EtOH-induced sensitization across the two acquisition trials. Error bars indicate standard error of the mean.
* denotes a significant increase in EtOH-induced activity from CS+-trial 1 to 2 (p < .05).
# denotes a significant difference in EtOH-induced activity between the Vehicle and SL-50 group (p < .05).
Analysis of the locomotor activity during conditioning revealed that, similar to Experiment 3, SL327 reduced locomotor activity. However, SL327 did not prevent the development of EtOH-induced sensitization that normally occurs during EtOH-CPP conditioning trials (Figure 4b). These findings were supported by significant interactions of Trial × Trial Type [F(1,46) = 111.5, p < .001] and Group × Trial Type [F(1,46) = 25.0, p < .001] as well as significant main effects of Group [F(1,46) = 23.4, p < .001], Trial [F(1,46) = 34.2, p < .001], and Trial Type [F(1,46) = 578.7, p < .001], but no significant Group × Trial × Trial Type interaction (p > .05). Paired t-tests comparing EtOH-induced activity levels on Trials 1 and 2 revealed significant increases for both the Vehicle [t(23) = 6.5, p < .001] and SL-50 [t(23) = 7.6, p < .001] groups. Furthermore, the Vehicle group showed significantly greater levels of EtOH-induced activity than the SL-50 group on both Trial 1 [t(46) = 5.2, p < .001] and Trial 2 [t(46) = 4.5, p < .001]. These data showed that although SL327 reduced locomotor activity, it did not interfere with development of EtOH-induced sensitization.
3.4 Effect of SL327 on expression of EtOH-CPP following 2- and 4-trial conditioning procedures (Exps 5 & 6)
Experiments 5 and 6 were performed in order to assess the effect of SL327 on expression of EtOH-CPP following normal 2-trial, and extended 4-trial, conditioning. The results of Experiment 5 showed that both the Vehicle- and SL327-treated groups showed similar levels of preference for the EtOH-paired floor as confirmed by an independent t-test (p > .05). SL327 did, however, significantly reduce test activity [t(45) = 6.8, p < .001]. These effects were replicated when animals that had received extended CPP conditioning (4 trials) were tested in that SL327 had no effect on expression of EtOH-CPP (p > .05) while significantly reducing test activity [t(46) = 5.5, p < .001]. These results suggest that inhibiting ERK activity with SL327 does not interfere with the conditioned motivational effects of EtOH or its retrieval from memory as assessed during a drug-free CPP test.
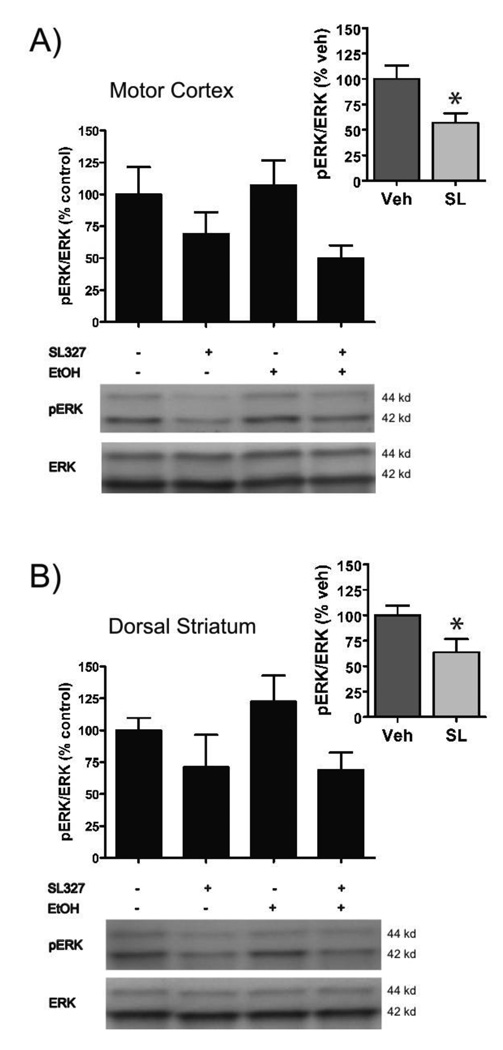
3.5 Effect of SL327 on pERK levels in the dorsal striatum and motor cortex (Exp 7)
Experiment 7 was performed in order to confirm that SL327 had crossed the blood-brain-barrier and was reducing ERK activity. Western immunoblot analysis of pERK protein levels in the dorsal striatum and motor cortex were examined as these regions have shown EtOH-induced activation (Asyyed et al., 2006) while the dorsal striatum has repeatedly been used to assess MEK-inhibition by SL327 in previous CPP experiments (Valjent et al., 2000 & 2001; Salzmann et al., 2003). As shown in Figure 6, although levels of pERK were not significantly increased 5 min after an EtOH injection, pre-treatment with SL327 caused a significant reduction in both the dorsal striatum and motor cortex. Because the initial Pre-treatment × Injection ANOVA for each region revealed only a significant main effect of Pre-treatment (Dorsal Striatum: [F(1,20) = 5.1, p < .05], Motor Cortex: [F(1,20) = 6.3, p < .05]) and no significant interaction or main effect of EtOH injection (p’s > .05), the EtOH and Saline injection groups were collapsed for further analysis of the SL327 effect. Subsequent independent t-tests comparing SL327 and Vehicle pre-treated animals revealed that SL327 significantly reduced pERK levels in both the dorsal striatum [t(22) = 2.3, p < .05] and motor cortex [t(22) = 2.7, p < .05] (Figure 6, insets). Specifically, SL327 administration resulted in a reduction of approximately 40% of the pERK levels in both brain regions. Therefore, despite having no effect on learning behavior in Experiments 2–4, or on expression of CPP in Experiments 5–6, SL327 was, in fact, crossing the blood-brain-barrier and significantly reducing pERK levels in multiple brain regions.
Figure 6. SL327 significantly reduced pERK levels in the motor cortex and dorsal striatum.
(A) Although EtOH pre-treatment did not significantly increase pERK levels in the motor cortex, SL327 (50 mg/kg) caused an approximate 40% decrease in pERK levels (inset). (B) As in the motor cortex, EtOH did not enhance pERK levels but SL327 caused an approximate 40% inhibition of pERK levels in the dorsal striatum (inset). Error bars indicate standard error of the mean.
* denotes a significant decrease in pERK/ERK levels as compared to the Vehicle-treated group (p < .05).
4. Discussion
These experiments showed that systemic administration of the MEK-inhibitor SL327 had no effect on acquisition, expression, or extinction of EtOH-induced CPP in mice. Specifically, the results of Experiment 2 and 3 showed no effect of two doses of SL327 (30 and 50 mg/kg) on extinction of EtOH-CPP. This outcome was replicated when two different vehicles and pre-trial intervals were used. Additionally, Experiment 4 showed that SL327 (50 mg/kg) did not impair acquisition of EtOH-CPP or development of EtOH-induced sensitization when administered before each of two CS+ conditioning trials. Furthermore, when administered only before the CPP test, SL327 did not alter expression of EtOH-CPP after either 2- or 4-trial conditioning procedures. Importantly, these outcomes were not simply due to a lack of ERK-activity inhibition by SL327, as Experiment 7 showed that SL327 significantly reduced pERK levels in both the dorsal striatum and motor cortex by approximately 40%. Taken together, these data suggest that the initial memory formation involved in acquisition, as well as the inhibitory learning unique to extinction, of EtOH-seeking behavior in mice did not require intact ERK signaling. Additionally, these experiments showed that neither EtOH reward nor the conditioned rewarding effects of EtOH were altered by inhibition of ERK signaling with SL327.
The lack of an effect of SL327 on these different stages of EtOH-CPP is not easily attributed to insensitivity of our procedure and parameters to pharmacological and neurobiological manipulations. Indeed, our laboratory has previously reported many studies showing significant effects of various treatments on the acquisition (Boyce-Rustay & Cunningham, 2004; Chester & Cunningham, 1999; Cunningham & Gremel, 2006a; Gremel & Cunningham, 2008), expression (Bechtholt & Cunningham, 2005; Gremel & Cunningham, 2007, 2008, 2009, 2010) and extinction (e.g., Cunningham et al., 1995, 1998) of EtOH-CPP using the same mouse strain, equipment and procedures described here. Thus, we are confident that the procedures used in the current set of experiments were appropriate for detecting potential effects of SL327 on EtOH-CPP.
4.1 ERK-signaling and extinction
Our findings are consistent with a previous study that showed that injection of SL327 (30 mg/kg IP) before 15 non-reinforced CS exposures had no impact on extinction of conditioned fear in mice (Matsuda et al., 2010). In contrast, Valjent et al. (2006) reported that injection of SL327 (50 mg/kg IP) before a single non-reinforced CS exposure impaired extinction of cocaine CPP in mice. However, because the control group in the Valjent et al. (2006) study showed only partial extinction, it is unclear whether the effects of SL327 would have persisted had the experiment included more trials that resulted in complete extinction in the control group. Nevertheless, the finding that extinction of EtOH-CPP does not depend on ERK signaling is consistent with previous studies (Groblewski et al., 2009) showing that extinction of EtOH-CPP was not altered by DCS, which has been reported to facilitate extinction of conditioned fear via the ERK pathway (Yang and Lu, 2005). Taken together, such findings suggest that extinction learning may not depend on ERK signaling.
However, several previous studies have shown that extinction of conditioned fear is impaired by direct injection of the MEK inhibitors U0126 or PD98059 into the hippocampus, medial prefrontal cortex or basolateral amygdala (BLA) (Szapiro et al., 2003; Hugues et al., 2004; Herry et al., 2006). The discrepancy between these findings and the more recent finding that systemically administered SL327 had no effect on fear extinction (Matsuda et al., 2010) might be due to unknown differences in the impact of these various MEK inhibitors on ERK-signal transduction pathways or in the level of MEK inhibition produced by systemically administered SL327 in brain areas critical for fear extinction. Unfortunately, Matsuda et al. did not measure pERK levels in brain following SL327 injection, preventing direct comparison to the changes produced by SL327 in our study or previous studies. Nevertheless, those investigators found that SL327 reversed the extinction-enhancing effect of systemically administered d-serine, an effect that was attributed to a reduction of ERK phosphorylation in brain.
It is important to note that subjects received only a single exposure to the MEK inhibitor during extinction in all of the aforementioned studies. In contrast, Experiments 2 and 3 of the current manuscript included an SL327 injection before each of four consecutive extinction trials. This procedural difference raises the possibility that repeatedly administering SL327 could have diminished its extinction-impairing effects (i.e., SL327 might have impaired extinction on the first or second trial, but did not prevent extinction on later trials). It would be difficult to evaluate this hypothesis in the EtOH-CPP procedure because a single extinction trial yields little to no extinction in control mice (unpublished findings), reducing the ability to detect impairment by SL327 exposure. Nevertheless, the finding that a single exposure to SL327 had no effect on fear extinction (Matsuda et al., 2010) suggests that SL327’s inability to impair extinction is not unique to procedures that involve repeated drug exposure.
Future studies should address whether the inability of systemically administered SL327 to impact extinction is unique to this MEK inhibitor or can be explained by insufficient reduction in ERK phosphorylation in critical brain areas. Additionally, it would be important to know whether site-specific injections of SL327 into hippocampus, medial prefrontal cortex or BLA impair fear extinction and if these effects diminish following repeated exposures.
4.2 ERK-independent EtOH-related acquisition learning
A more general explanation for the lack of an SL327 effect on extinction of EtOH-CPP might be that ERK activation is not required for formation of any ethanol-related learned memories. This hypothesis receives support from the results of Experiment 4, which showed that administration of SL327 during conditioning did not impair acquisition of EtOH-CPP. This idea is further supported by data showing no effect of SL327 on development of EtOH-induced sensitization (see Fig. 4b), which has previously been reported to involve an associative learning component in our procedure (Cunningham and Noble, 1992).
These findings contrast with previous studies showing that systemic pretreatment with similar doses of SL327 interfered with acquisition of cocaine-, THC- and MDMA-induced CPP (Valjent et al., 2000, 2001; Salzmann et al., 2003) and with development of cocaine- and d-amphetamine-induced locomotor sensitization (Valjent et al., 2006). Although the discrepancy between these findings and the present studies might be explained by differences in mouse genotype or various procedural variables, our overall pattern of findings suggests that the formation of ethanol-related memories, unlike those involving other drugs of abuse, occurs independently of the ERK signaling pathway. This conclusion is seemingly at odds with a recent study by Spina et al. (2010) who showed that intracerebroventricular (ICV) administration of the MEK inhibitor PD98059 impaired CPP induced by intragastric acetaldehyde, the primary metabolite of EtOH, in rats. However, although acetaldehyde and EtOH share some behavioral effects, studies suggest that the underlying pharmacological mechanisms are unique, and the presence of acetaldehyde in the central nervous system following EtOH consumption remains controversial (for review see Quertemont et al., 2005).
Additional evidence of the unique neurobiological mechanisms of EtOH-CPP comes from previously published data from our laboratory showing that acquisition of EtOH-CPP was not impaired by the NMDA-receptor antagonist MK-801 (Boyce-Rustay & Cunningham, 2004) despite reports that MK-801 prevented cocaine-CPP (Kim et al., 1996). Furthermore, Kim et al. (1996) also showed that MK-801 blocked cocaine-induced locomotor sensitization, whereas Meyer and Phillips (2003) reported no such effect on EtOH-induced sensitization. Thus, it appears that EtOH-CPP and other EtOH-related behaviors, including sensitization, may depend upon signaling mechanisms and receptor systems that are distinct from those involved with other drugs of abuse including cocaine.
4.3 Involvement of ERK in EtOH reward and reinforcement
Not only was SL327 unable to interfere with acquisition and extinction learning, but it also did not alter the direct rewarding effects of EtOH. Specifically, if ERK signaling were necessary for EtOH reward, mice that received SL327 before EtOH-conditioning trials would have shown a weaker CPP on Test 1 of Experiment 4. Because both groups showed equally strong CPP, it can be concluded that inhibition of ERK signaling with SL327 altered neither the learning-component of EtOH-CPP acquisition nor the rewarding effects of EtOH. Additionally, our results showed that the conditioned rewarding effects of EtOH do not require ERK signaling as SL327 did not impair expression of EtOH-CPP in Experiments 5 and 6. These results are in agreement with a previous study showing that intra-BLA MEK inhibition did not alter the expression of cocaine-CPP despite causing a 40–50% reduction in BLA-pERK levels (Lai et al., 2008).
Recently, it was observed that SL327 biphasically altered expression of EtOH-self administration in mice—a result that the authors suggested supported a role of ERK signaling in EtOH reinforcement (Faccidomo et al., 2009). Additionally, Radwanska et al. (2008) reported that presentation of relapse-inducing cues in an EtOH self-administration paradigm resulted in a significant increase in pERK levels in the BLA of rats. In contrast to these findings that link EtOH reinforcement with ERK signaling, Carnicella et al. (2008) showed that the MEK inhibitor U0126, when administered directly into the ventral tegmental area, failed to alter expression of EtOH self-administration in rats. Although these reports provide mixed support of our current data showing that MEK inhibition does not interfere with the direct- or conditioned-rewarding effects of EtOH, it is important to note that self-administration and CPP involve distinct neural mechanisms and learning processes while incorporating different aspects of drug reinforcement and reward (Bardo and Bevins, 2000). Thus, it remains possible that these two behaviors are differentially dependent upon the ERK pathway.
4.4 Reduction in subcortical and cortical pERK levels by SL327
Although the results of the current behavioral experiments show that SL327 was unable to impair acquisition, expression, and extinction of EtOH-CPP in mice, the doses and pre-treatment intervals used were sufficient to significantly reduce pERK levels in the two brain regions examined. Specifically, Experiment 7 showed that SL327 (50 mg/kg) caused a substantial (40%) reduction of pERK levels in both the dorsal striatum and motor cortex. Contrary to expectation, EtOH (2.5 g/kg) did not result in a significant increase in pERK levels in either the dorsal striatum or motor cortex, which is in disagreement with the study by Asyyed et al. (2006) that showed significant activation of the cAMP response element binding protein (CREB) pathway, a downstream effector of ERK, in both regions following an acute EtOH (3.2 g/kg) injection. Although it is not clear why this dose of EtOH did not alter pERK levels, it is clear that pre-treatment with SL327 significantly reduced pERK levels in these subcortical and cortical regions, regardless of whether or not animals received EtOH. Furthermore, these data are in complete agreement with those of Atkins et al. (1998) who showed that the same dose of SL327 (50 mg/kg) caused an approximately 40–50% reduction in hippocampal pERK levels at 60 min post-injection—a reduction that was sufficient to impair acquisition of conditioned fear. Additionally, a previous examination of the time-course of MEK inhibition showed that SL327 caused a significant, and equivalent, reduction of pERK levels at all time points between 30 and 100 min post injection (Selcher et al., 1999). Therefore, given the biochemical results of Experiment 7 in conjunction with the aforementioned published studies, we are confident that SL327 crossed the blood-brain-barrier and significantly reduced ERK activity during the extinction trials of Experiments 2 and 3 as well as the conditioning trials of Experiment 4 and preference tests of Experiments 5 and 6. This interpretation is further supported by the behavioral findings that SL327 pre-treatment resulted in a general depression of activity during extinction and preference tests, as well as a reduction in EtOH-induced locomotor activity during conditioning trials. Although we cannot rule out that the suppression of activity by SL327 was due to a peripheral effect of the drug, it is more likely that this effect corresponds to a reduction in pERK levels in the brain, in particular the dorsal striatum as this area has been shown to be involved in control of movement (Kreitzer & Malenka, 2008).
In conclusion, the findings of the behavioral and biochemical experiments of this study showed that SL327 had no affect on acquisition, expression, or extinction of EtOH-induced CPP in mice despite causing significant reduction of pERK levels in multiple brain regions. Additionally, although SL327 caused a generalized depression of locomotor activity, it did not prevent the development of EtOH-sensitization. In light of the current data, as well as previously published data from our laboratory and others, it appears that extinction-specific learning may be insensitive to inhibition of ERK-signaling via systemically administered SL327. Furthermore, our data also suggest that unlike other drugs of abuse, the EtOH-related associative learning components of EtOH-CPP, as well as the direct and conditioned rewarding properties of EtOH, may not require activation of the ERK pathway.
Figure 5. SL327 did not alter expression of EtOH-CPP following both 2- and 4-trial conditioning.
(A) SL327 (50 mg/kg), when administered prior to the CPP test did not alter expression of EtOH-CPP but did significantly reduce test activity (inset). (B) SL327 also failed to alter expression of EtOH-CPP following an extended conditioning (4-trials) procedure. As in 5A, SL327 significantly reduced test activity (inset). Error bars indicate standard error of the mean.
* denotes a significant decrease in activity as compared to the Vehicle-treated group (p < .05).
Acknowledgements
This research was supported by grants from the National Institutes of Health (AA018052, AA007702, DA07262), the American Psychological Association, and the N.L. Tartar Research Fund. We would like to thank Courtney Zerizef for her contributions to Experiment 6.
Abbreviations
- CPP
conditioned place preference
- DCS
d-cycloserine
- ERK
extracellular signal-regulated kinase
- EtOH
ethanol
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase-kinase
- NMDA
n-methyl d-aspartic acid
- pERK
phosphorylated extracellular signal-regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 2.Asyyed A, Storm D, Diamond I. Ethanol activates cAMP response element-mediated gene expression in select regions of the mouse brain. Brain Res. 2006;1106:63–71. doi: 10.1016/j.brainres.2006.05.107. [DOI] [PubMed] [Google Scholar]
- 3.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 4.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 5.Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- 6.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 7.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- 9.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chester JA, Cunningham CL. GABA(A) receptors modulate ethanol-induced conditioned place preference and taste aversion in mice. Psychopharmacology (Berl) 1999;144:363–372. doi: 10.1007/s002130051019. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham CL, Dickinson SD, Okorn DM. Naloxone facilitates extinction but does not affect acquisition or expression of ethanol-induced conditioned place preference. Experimental and Clinical Psychopharmacology. 1995;3:330–343. [Google Scholar]
- 12.Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham CL, Gremel CM. Proximal ethanol pretreatment interferes with acquisition of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2006a;85:612–619. doi: 10.1016/j.pbb.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006b;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CL, Patel P, Milner L. Spatial location is critical for conditioning place preference with visual but not tactile stimuli. Behav Neurosci. 2006c;120:1115–1132. doi: 10.1037/0735-7044.120.5.1115. [DOI] [PubMed] [Google Scholar]
- 18.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 19.Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204:135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gremel CM, Cunningham CL. Role of test activity in ethanol-induced disruption of place preference expression in mice. Psychopharmacology (Berl) 2007;191:195–202. doi: 10.1007/s00213-006-0651-5. [DOI] [PubMed] [Google Scholar]
- 22.Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gremel CM, Cunningham CL. Effects of disconnection of amygdala dopamine and nucleus accumbens N-methyl-d-aspartate receptors on ethanol-seeking behavior in mice. Eur J Neurosci. 2010;31:148–155. doi: 10.1111/j.1460-9568.2009.07044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herry C, Trifilieff P, Micheau J, Luthi A, Mons N. Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci. 2006;24:261–269. doi: 10.1111/j.1460-9568.2006.04893.x. [DOI] [PubMed] [Google Scholar]
- 27.Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- 28.Kim HS, Park WK, Jang CG, Oh S. Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. Brain Res Bull. 1996;40:201–207. doi: 10.1016/0361-9230(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 29.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai YT, Fan HY, Cherng CG, Chiang CY, Kao GS, Yu L. Activation of amygdaloid PKC pathway is necessary for conditioned cues-provoked cocaine memory performance. Neurobiol Learn Mem. 2008;90:164–170. doi: 10.1016/j.nlm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda S, Matsuzawa D, Nakazawa K, Sutoh C, Ohtsuka H, Ishii D, et al. d-serine enhances extinction of auditory cued fear conditioning via ERK1/2 phosphorylation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:895–902. doi: 10.1016/j.pnpbp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- 33.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 34.Mouledous L, Diaz MF, Gutstein HB. Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav. 2007;88:39–46. doi: 10.1016/j.pbb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog Neurobiol. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Radwanska K, Wrobel E, Korkosz A, Rogowski A, Kostowski W, Bienkowski P, et al. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33:1835–1846. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- 37.Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spina L, Longoni R, Vinci S, Ibba F, Peana AT, Muggironi G, et al. Role of Dopamine D Receptors and Extracellular Signal Regulated Kinase in the Motivational Properties of Acetaldehyde as Assessed by Place Preference Conditioning. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- 40.Szapiro G, Vianna MR, McGaugh JL, Medina JH, Izquierdo I. The role of NMDA glutamate receptors, PKA, MAPK, and CAMKII in the hippocampus in extinction of conditioned fear. Hippocampus. 2003;13:53–58. doi: 10.1002/hipo.10043. [DOI] [PubMed] [Google Scholar]
- 41.Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 45.Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]